Abstract
Electronic cigarettes (e-cigs) have fast increased in popularity but the physico-chemical properties and toxicity of the generated emission remain unclear. Reactive oxygen species (ROS) are likely present in e-cig emission and can play an important role in e-cig toxicity. However, e-cig ROS generation is poorly documented. Here, we generated e-cig exposures using a recently developed versatile exposure platform and performed systematic ROS characterization on e-cig emissions using complementary acellular and cellular techniques: 1) a novel acellular Trolox-based mass spectrometry method for total ROS and hydrogen peroxide (H2O2) detection, 2) electron spin resonance (ESR) for hydroxyl radical detection in an acellular and cellular systems and 3) in vitro ROS detection in small airway epithelial cells (SAEC) using the dihydroethidium (DHE) assay. Findings confirm ROS generation in cellular and acellular systems and is highly dependent on the e-cig brand, flavor, puffing pattern and voltage. Trolox method detected a total of 1.2–8.9 nmol H2O2 eq./puff; H2O2 accounted for 12–68 % of total ROS. SAEC cells exposed to e-cig emissions generated up to eight times more ROS compared to control. The dependency of e-cig emission profile on e-cig features and operational parameters should be taken into consideration in toxicological studies.
Keywords: Electronic cigarette, nicotine, reactive oxygen species, oxidative stress
1. INTRODUCTION
The use of electronic cigarettes (e-cig) have risen considerably in recent years, especially among teenagers [1,2]. E-cig global sales reached $7 billion in 2014, and continue to rise [3]. A great number of manufactures are competing for market shares, and a total of 466 brands and 7700 flavors were documented in 2014 [4]. Battery operated, e-cigs work by heating the e-liquid, which contains humectants (propylene glycol and glycerin), nicotine and flavor additives, to form emissions. Thus, e-cig emission is expected to be chemically complex, and likely strongly dependent on the e-liquid formulation and the design specifications of the e-cig that defines the heating/vaporization process.
There is a general perception that e-cigs are less harmful than tobacco cigarette [5,6]. However, large discrepancy has been reported in the published literature on e-cig emission physico-chemical properties and toxicity [7–19]. This is because e-cig exposure is not a single scenario but highly influenced by e-cig product type, e-liquid composition (flavor), operational parameters and user inhalation pattern [20]. The role of these parameters on both e-cig emission formation and chemical composition has not been studied systematically. Without an adequate understanding of e-cig emission properties and associated toxicological profile, one cannot establish whether e-cig can not cause any harm. For example, because e-liquid contains fewer chemicals and minimizes combustion processes [7], some studies indicate that e-cig emission contain significantly less particulate matter (PM) mass and organic species, therefore e-cig emissions are less cytotoxicity than regular cigarette smoke [11,21]. On the contrary, other studies state that e-cig emissions still poses potential health risks because it contains high numbers of particulate matter (a total of 109 particles/cm3 with peak between 100 nm–200 nm) [12–14] and a complex mixture of chemicals, including propylene glycol, glycerin, nicotine, carbonyls, volatile organic compounds (VOCs) and metals [15–18]. Such discrepancies in the literature are likely due to the differences in the exposure generation by randomly-selected parameters (type, brand, flavor, voltage etc.). Thus, there is real need for a systematic physico-chemical and toxicological characterization of e-cig emission as a function of product features (e.g. brand and flavor) and operational (e.g. voltage) parameters.
Reactive oxygen species (ROS) are a group of highly reactive and often short-lived radicals and include hydroxyl radicals, superoxide anions, singlet oxygen, alkoxyl, and alkylperoxy radicals [22]. Hydrogen peroxide (H2O2), in comparison to other ROS mentioned above, is relatively stable and in acellular systems it is formed from terminating reaction of various radicals, including hydroxyl radicals. ROS represent one important mechanism of emission-induced health effects because their presence can initiate pathological processes and greatly contribute to oxidative stress, damage of important biomolecules, including DNA, proteins, and lipids, and sustained pro-inflammatory responses. ROS and oxidative stress are involved in numerous diseases of the airways, cardiovascular system, neurological disorders and cancers [23,24]. The ability to generate ROS and induce oxidative stress by tobacco smoke has been categorized as a driving factor in smoking-related diseases [25–27]. Limited research already indicates that e-liquid and e-cig emissions induced oxidative stress in vitro [28–32] and in vivo [33], and this is indicative of the important role of ROS in e-cig induced cytotoxicity [34]. However, e-cig ROS characterization at present is limited and results are often contradictory in the literature. This is likely because research on e-cig is still an emerging area and, more importantly, e-cig emissions can be influenced by parameters such as e-liquid flavor and heating wire status [35–37] and user puffing patterns. For example, two studies found similar ROS generation from e-cig and tobacco cigarette, using both acellular [38] and cellular assays [39]. On the contrary, several other studies found e-cig induced less oxidative stress in vitro in bronchial epithelial cells [40–42] and endothelial cells [34], as well as from human blood biomarker analysis following controlled human exposures [43]. Thus, there is a need to investigate the influence of e-cig brand, e-liquid composition, operational parameters and puffing pattern on ROS generation in a systematic manner, using both acellular and cellular methodologies.
In this manuscript, we report for the first-time the results of a comprehensive e-cig ROS characterization as a function of the aforementioned influencing parameters, using complementary assays in acellular and cellular systems. The recently developed in our group versatile e-cig exposure generation system (Ecig-EGS) was used to precisely control the e-cig operational parameters for emission generation [20]. ROS was measured by collecting the emission in trapping reagents followed by liquid chromatography-electrospray ionization-tandem-mass spectrometry (LC-ESI-MS/MS), electron spin resonance (ESR) for ROS speciation, and cellular ROS utilizing the dihydrorthidum (DHE) fluorescent probe in human small airway epithelial cells (SAEC).
2. METHODS
2.1 Generation and sampling of e-cig exposure
E-cig exposure generation system (Ecig-EGS)
The recently developed by the authors Ecig-EGS platform was used to generate real world e-cig exposure for ROS characterization as indicated in Figure 1 [20]. In brief, a single port e-cig generator (ECAG, e~Aerosols, LLC, Central Valley, NY), which is fully programmable and enables precise control of the puffing pattern and e-cig operational voltage, was connected to an e-cig (Figure 1). The cylindrical mixing chamber connected to ECAG had a volume of 7 L. Generated e-cig emission and the dilution air were introduced into the mixing chamber through two separate ports and thoroughly mixed in there. The Ecig-EGS was connected with real time instrumentation and time integrated sampling for physico-chemical characterization of emission [44,45].
Figure 1.
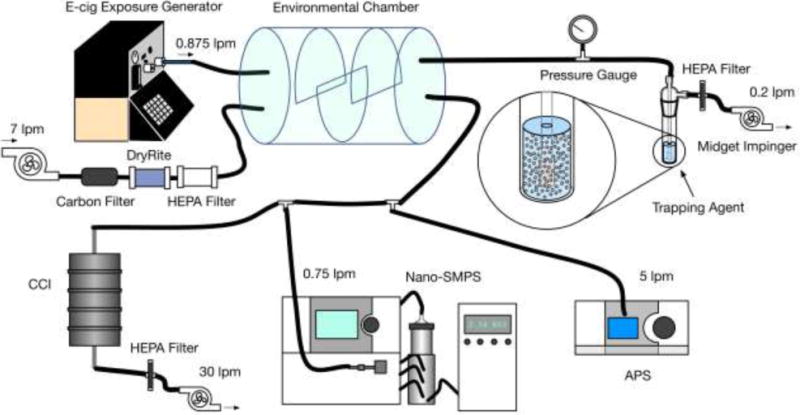
E-cig exposure generation system (Ecig-EGS, adapted from Zhao et al. (2016)) for ROS characterization. This schematic summarizes all tests and individual flow rates required for each test. CCI, SMPS, APS and the impinger were not used at the same time. For example, when impinger was used for sampling, it was used alone (CCI, SMPS and APS were not operated). A balance pump was used to balance the total input and output flow rate, when necessary.
In this study, a commonly used advanced e-cig with refillable tank was used [46,47]. The residence time of the mixing chamber was set at 60s to mimic the “washout time” of a smoker’s lungs in active smoking [48].
Influence of e-cig brand, e-liquid flavor, puffing protocol and operational voltage
For baseline experiments, tobacco flavor (10 mg/mL nicotine) e-liquid from a popular e-cig brand A was used. The e-cig was operated at 3.7V, a standard voltage according to the manufacturer. A modified puffing protocol (MPP), which reflected real world e-cig smoking behavior [10,49] was applied. MPP defines puffing regime as: puff volume, 55 mL; puff duration, 4 s; and puffing interval, 30 s.
To systematically investigate the influence of the aforementioned parameters, one out of these four parameters was modified each time and its effect on ROS content was then compared with the baseline experiment using an array of acellular and cellular techniques as described below. In total, two popular e-cig brands (brand A and B), two flavors (tobacco flavor, fruit flavor-10 mg/ml nicotine), two puffing protocols (MPP and the standardized Federal Trade Commission protocol (FTC): puff volume, 35 mL; puff duration, 2 s; and puffing interval, 60 s [50] and three voltage scenarios (3.7, 4.8 and 5.7 V) were used.
E-cig emission sampling
For ROS characterization: Generated e-cig emissions were bubbled through fritted head impingers (porosity A (145–174 μm) tip; Ace glass Inc., NJ) containing trapping regents corresponding to the analytical methods (Figure 1). For Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) method (LC-ESI-MS/MS), 10 mL of 100 μM trolox in 1 mM phosphate buffer (pH = 7.4) was used. For ESR analysis, a 5 mL vial was placed inside the impinger containing 1.7mL of 500 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO; Sigma Aldrich, MO). The samples were immediately frozen post-sampling until analysis. For the two cellular assays (DHE and MTS), 20 mL of small airway basal medium (SABM, Lonza Inc., Allendale, NJ) was used. The samples were stored at 4 °C until cell exposure experiments. The sampling duration was 30 minutes.
Blanks were collected in a similar fashion by bubbling only pretreated room air (cleaned through charcoal and high-efficiency particulate filters) through the impinger for 30 minutes containing the same trapping reagents as above.
For nicotine characterization: Nicotine was measured in the SABM sample as a way to normalize the dose. The medium was refrigerated to −80 °C prior to analysis.
2.2 E-cig emission characterization
Particle size and concentration were measured using both real time and integrated instrumentation as described in previous publications by the authors [20,51]. More detailed information can be found in the supplemental material.
2.3 ROS characterization
Trolox method (LC-ESI-MS/MS)
Trolox, a water-soluble form of vitamin E, is an excellent antioxidant. In the presence of ROS, Trolox is oxidized to its corresponding Trolox quinone (TQ) (Figure S1). After sampling, two 1mL aliquots of Trolox solution were transferred into two separate 1.8 mL liquid chromatography vials. One unit of horseradish peroxidase (HRP) was added into one of the vials that was used for H2O2 quantification. The other vial was not modified. Subsequently, both vials were incubated at 37 °C for 30 min and then subjected to LC-ESI-MS/MS analysis for TQ quantitation (Figure S2). The amount of TQ from the unmodified vial (no HRP) was used to quantify short-lived ROS species. The H2O2 amount was quantified as the difference between TQ amount from the vial containing HRP and the vial without HRP. More details on the methodology are provided in the supplemental material.
Indirect confirmation of H2O2 measurements: Catalase treatment
Catalase, a 240 kDa protein, specializes in degradation of H2O2 to water and oxygen. Catalase is highly efficient at this conversion at room temperature and neutral pH. In order to verify H2O2 results from the Trolox/HRP approach, an additional set of experiments were performed. In these experiments, reference concentrations of Trolox and H2O2, as well as e-cig emissions from various test conditions described earlier were incubated with catalase at 100 units/mL for 10 minutes at room temperature, followed by filtration/centrifugation of the medium with a centrifugal 3000 Da filter at 4000 rpm for 10 min. HRP (1 unit/mL) was then added to the filtrate as in earlier experiments and the medium was incubated as previously described for HRP (37 °C for 30 min). The residual Trolox and TQ were measured by LC-ESI-MS/MS. The difference in TQ intensity between experiments with and without catalase treatment was considered as an indirect measure of the H2O2 concentration. The H2O2 values of e-cig samples calculated directly by HRP and catalase/HRP treatment agreed within 15% of the original values and for this reason these data have been omitted.
ESR measurements
Electron spin resonance (ESR) spin trapping was used to detect short-lived free radical intermediates. Hydroxyl radicals (·OH) were measured using the addition-type reaction of a short-lived radical with a compound (spin trap) to form a relatively long-lived free radical product (spin adduct), which can then be studied using conventional ESR. ESR measurements were conducted using a Bruker EMX spectrometer (Bruker Instruments Inc. Billerica, MA 01821, USA) and a flat cell assembly. Hyperfine couplings were measured (to 0.1 G) directly from magnetic field separation using potassium tetraperoxochromate and 1,1-diphenyl-2-picrylhydrazyl as reference standards [52,53]. The Acquisit program (Bruker Instruments Inc.) was used for data acquisitions and analyses.
Macrophages attack and engulf foreign bodies (including particles) that invade the lung where they can react with H2O2 as one of their defense mechanisms. Thus H2O2 can react with particles in a Fenton-like manner and generate damaging hydroxyl radicals. Modeling this respiratory burst defense system with H2O2 provides a source of preliminary data on the biological reactivity of the particles involved [54]. Therefore, E-cigarette emissions were first reacted with H2O2 in order to measure their potential to generate reactive radical species when inhaled and exposed to an organism’s defense systems. Acellular E-cigarette samples were mixed with H2O2 [1mM] in distilled, purified H2O and 500 mM of 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) (Sigma-Aldrich, St Louis, MO. 63103, USA) then measured 3 min after reaction initiation. Cellular samples were exposed to E-cigarette emissions mixed with 2 × 106 RAW 264.7 mouse monocyte macrophages suspended in phosphate-buffered saline (PBS) in the presence of 500 mM DMPO and incubated at 37°C for 3 minutes [55]. This system allows the monocyte macrophages to react to the particle exposure and measures respiratory burst and free radical generation.
After incubation both sample types were transferred to a quartz flat cell (Wilmad glass, Vineland, NJ 08360, USA) and placed in the ESR cavity for measurement. ESR settings for both sample types were: center field 3510 ± 100, frequency 9.75 GHz, power 126.6 mW, gain 1 × 104, modulation frequency 100 kHz, modulation amplitude 1.0 G, time constant 40 ms. The intensity of the ESR signal was used to measure the amount of short-lived radicals trapped, and the hyperfine couplings and peak spacing of the spin adduct were characteristic of the original trapped radicals.
Biological assays: DHE and MTS
SAEC were gifted by Dr. Tom K. Hei (Columbia University, New York, NY) [56]. SAEC were cultured in SABM with the addition of the following growth factors: bovine pituitary extract, hydrocortisone, human epidermal growth factor, epinephrine, transferrin, insulin, retinoic, triiodothyronine, gentimicin amphotericin-B, and bovine serum albumin-fatty acid free (BSA-FAF) from the manufacturer (Lonza Inc., Allendale, NJ).
Cytotoxicity (MTS): SAEC were plated in a 96-well plate (BD Biosciences, NJ, USA) at 15,000 cells per well. SAEC were exposed for 24 h to the following doses: 0.025, 0.05, 0.075, 0.1 puffs/mL. To dilute the e-cig sample, the exposed media was diluted in supplement free SABM media. Manufacturer’s guidelines were followed while using the Cell Titer 96® Aqueous One Solution Cell Proliferation Assay Kit (Promega, WI).
Confocal Microscopy: To analyze superoxide production SAEC cells were seated at a confluency on coverslips at 150,000 cells per well in a 6-well plate. SAEC were exposed to either 0.05 or 0.1 puffs/mL e-cig sampled media for 24 h and DHE (Invitrogen, NY) was added at a final concentration of 5 μM for the last 30 min of the e-cig emission treatment. The cells on the coverslips after exposure were fixed with 3.6% paraformaldehyde and permeabilized with 0.1% triton x-100/PBS for 10 min and then washed three times with 1 × PBS. Coverslips were then mounted and imaged with a confocal microscope (Zeiss Germany). Scale bar and quantification of DHE per sample were generated and processed using LSM software (Zeiss Germany). The reason we used confocal microscopy was because based on our experience measuring superoxide formation in adherent cells (both human microvascular endothelial cells and human small airway epithelial cells) exposed to particles using a fluorescence plate reader, the existence of the particles in the cells interfered the results of fluorescence plate reader reading. It is worth noting that the experiments were designed to complement each other and provide compelling and solid data on ROS generation by e-cig emissions and it is not the purpose of this manuscript to compare ROS methods and their limitations.
Nicotine analysis
Fifty μL of the impinger solution (containing SABM media) was transferred post-sampling into a LC vial, spiked with 10 ng of nicotine-D4 internal standard, diluted to 1 mL with deionized water, vortexed for 15 s and subjected to analysis by the LC-ESI-MS/MS. Method details are provided in the supplementary information.
3. RESULTS
3.1 E-cig emission characterization
Particle size and concentration
Bi-modal size distribution, with peaks at approximately 200 nm and 1 μm, was observed for e-cig emissions in all the scenarios. The modal diameters varied by up to 40% depending on the scenario (Figure S3). A total of 106 – 107 particles/cm3 were generated in e-cig emission for all the scenarios, except for FTC (4.32×105 particles/cm3). E-cig Brand B generated 40% more particles compared to Brand A (Figure S3-A). Tobacco flavor e-liquid generated 50% more particles than fruit flavor (Figure S3-B). The longer and more frequent puffing protocol MPP generated six times more particles (#/cm3) compared to the FTC protocol (Figure S3-C). Finally, increasing operational voltage first increased particle number concentration (from 3.7 V to 4.8 V) and then decreased at 5.7 V (Figure S3-D) indicative of possible changes in formation and chemistry of the emissions.
The total particle mass concentration for all scenarios was in the range of 0.1 – 1.8 g/m3, except for the FTC protocol (0.01 g/m3). The majority of particle mass (88% – 96%) was collected on the PM0.1–2.5 size-fraction. Similar to particle number concentration, particle mass concentration was also influenced by e-cig brand and flavor (Figure S4-A and S4-B). The longer and more frequent puffing protocol MPP generated 60 times more particle mass than the FTC protocol (Figure S4-C). It is also worth noting that increasing operational voltage from 3.7 V to 5.7 V increased particle mass concentration more than three times (Figure S4-D).
3.2 Nicotine characterization
Table 1 shows nicotine concentration in the collected cell culture media. No nicotine was detected in the blanks. Higher nicotine concentration was observed for the 5.7 V condition (0.46 μmol/puff) compared to the 3.7 V (0.12 μmol/puff).
Table 1.
Nicotine concentration in collected SAEC cell culture media as determined by LC-ESI-MS/MS.
| Sample | Concentration (μmol/puff) |
|---|---|
|
| |
| Blank | nd |
| 3.7V | 0.12 ± 0.02 |
| 5.7V | 0.46 ± 0.04 |
3.3 ROS characterization
Trolox method
Figure 2 summarizes the total ROS and H2O2 measured with the Trolox method, normalized per puff of e-cig emission. The ROS amount varied between 1.2 - 8.9 nmol H2O2 eq./puff. H2O2 concentration varied between 0.2 - 6.1 nmol/puff, which accounted for 12 -68 % of the total ROS. Results from the Trolox method show the ROS amount produced is dependent on the e-cig brand, flavor and puffing regime (Figure 2.A–C). ROS concentration increased eight times as the operational voltage increased from the 3.7 V baseline value to 5.7 V, with the H2O2 amount and its relative percentage of the total ROS increasing from 46 to 68% (Figure 2D).
Figure 2.
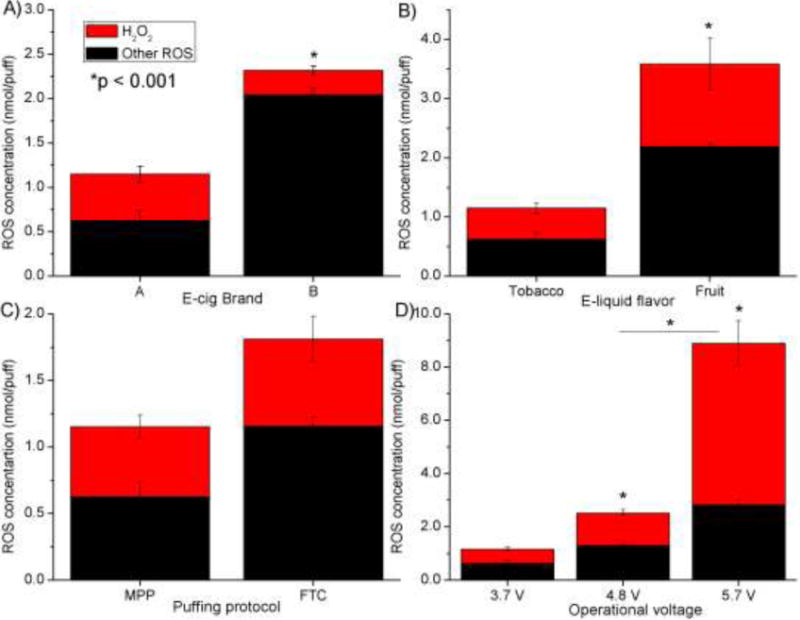
Trolox LC-ESI-MS/MS analysis: ROS (H2O2 and other species) generation reported as H2O2 equivalent unit as a function of the following parameters A) e-cig brand, B) e-liquid flavor, C) puffing protocol, D) operational voltage. One parameter was changed at a time from baseline scenario (Brand A, tobacco flavor, refillable tank, MPP puffing protocol, 3.7 V) to investigate influence of various parameters. For example, in the e-cig brand analysis, everything but the brand was kept constant. Brand A was changed to Brand B.
ESR method
Figure 3A documents the generation of hydroxyl radicals as a function of voltage. The 1:2:2:1 peak splitting and separation of 15 gauss are indicative of the hydroxyl radical spin adduct with DMPO. In the acellular assay, the blank sample showed no peaks corresponding to hydroxyl radicals, however the e-cig samples showed radical production with and without the presence of exogenous H2O2 (Figure 3B), which is added to model the reaction of particles phagocytosed by alveolar macrophages. Without the addition of H2O2, the 3.7V sample showed 40% more hydroxyl radical generation compared to 5.7V sample. After addition of H2O2, increased radical generation was seen for both voltage conditions. In the cellular assay with RAW 264.7 macrophages, the blank sample showed no radical production; however, RAW 264.7 cells exposed to e-cig samples corresponding to both voltages showed comparable amounts of radical production (Figure 3C), with the 5.7V having slightly higher ROS values.
Figure 3.
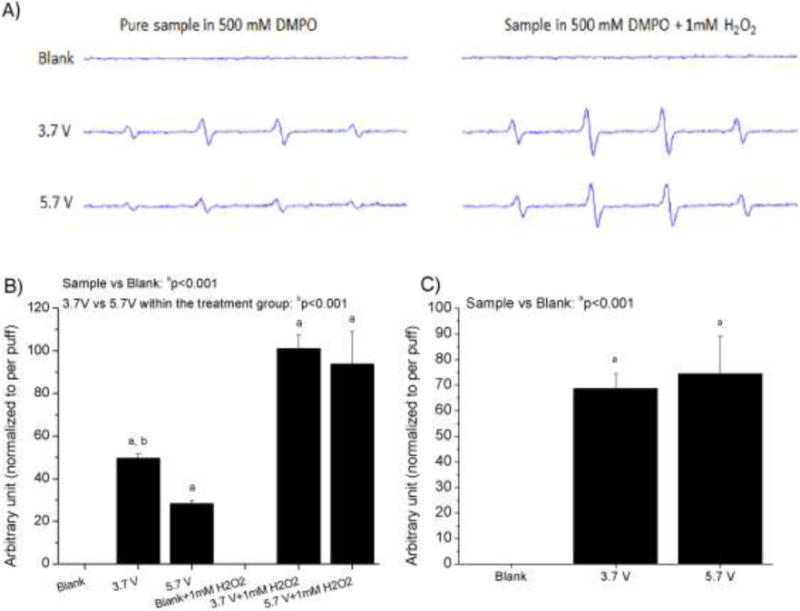
ESR analysis after spin trapping with DMPO: Acellular: A) representative ESR spectra, peaks represent hydroxyl radicals. The settings were: receiver gain, 1.0 × 104; time constant 0.40 s; modulation amplitude, 1 G; scan time, 40 s; magnetic field, 3510 ± 100 G. The data represents mean ± SEM values of 4 independent experiments. B) ROS generation as a function of voltage (adding H2O2 mimics the process of foreign nano/particle digestion by lung macrophages inside the lysosomes, in part by producing more H2O2). Cellular: C) ROS generation as a function of voltage, incubated with RAW 264.7 mouse macrophages
MTS Assay
To determine e-cig cellular toxicity, SAEC were dosed with the following e-cig emission concentrations: 0.025, 0.05, 0.075, and 0.1 puffs/mL at both 3.7 and 5.7 V. No cellular toxicity was seen at any of the test doses (Figure S5). There was, however, an increase in cellular proliferation at the higher doses of 0.075 and 0.1 puffs/mL of the 3.7 V compared to control. No significant differences were seen between the two conditions (3.7 V and the 5.7 V) at all doses.
DHE Assay
Confocal images of SAEC exposed to 0.05 and 0.1 puffs/mL e-cig emission induced ROS production at both 3.7 and 5.7 V compared to control (Figure 4A). ROS production was significantly increased at both 0.05 and 0.1 puffs/mL compared to control and also 5.7 V induced significantly more ROS compared to 3.7 V at both the 0.05 and 0.1 puffs/mL (Figure 4B).
Figure 4.
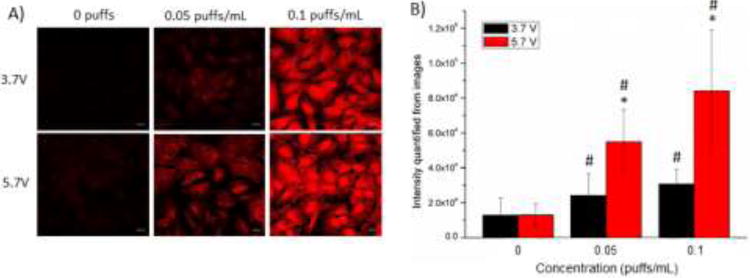
ROS induction in SAEC after exposure to e-cig emission. SAEC were dosed with 0.05 and 0.1 puffs/mL for 24 h and with 5 μM DHE for the last 30 min to measure ROS production. (A) SAEC were imaged and (B) quantified. Images are a representation of n = 3 images. * indicates p < 0.05 compared between 3.7 and 5.7 V and # indicates p < 0.05 compared to control.
4. DISCUSSION
Our results clearly demonstrate ROS generation in the e-cig emissions for both the acellular and cellular assessment approaches. In acellular tests, a total of 1.2 – 8.9 nmol H2O2 eq./puff ROS was quantified with the Trolox assay. This result was confirmed by the observation of hydroxyl radicals in ESR. Acellular ESR experiments detected hydroxyl radicals in e-cig emissions. Furthermore, upon addition of H2O2 to the acellular ESR sample, ROS production increased significantly, probably from a Fenton-like reaction (Figure 3) [57]. This indicates e-cig emission may contain ROS precursors, which can generate more ROS after deposition in the lung and phagocytosis by macrophages. Previous work has found small amounts of transition metals, such as Fe, Cu, Ni, in e-cig emission [18,58], which can catalyze ROS generation. Analytical methods were carefully selected to achive meaningful quantitation of ROS species. Our choice of Trolox and ESR takes into account two major issues: (i) interferences commonly present in spectrophotometric methods; and (ii) presence of multiple ROS species requires employment of complementary assays that provide both specificity towards various species and absolute quantitation of ROS. It is important to note that no single method exists that meets both these requirements towards ROS species. Hence our selection of two complementary methods: ESR and Trolox by LC-MS/MS. ESR is arguably one of the most selective methods for ROS speciation, but it provides only semi-quantitative information of ROS. Trolox -LC-MS/MS is an accurate and interference-free method for total ROS quantitation. As our data show, these methods complement each-other very well.
In cellular systems, the generation of hydroxyl radicals was also observed by ESR in the RAW 264.7 murine macrophages. SAEC had induced ROS production at both 0.05 and 0.1 puffs/mL, confirming that e-cig emission can cause oxidative stress in vitro and could potentially lead to lung inflammation. Limited literature also reports ROS generation measured by acellular fluorescent assay [38] and oxidative stress after e-cig exposure in vitro [59] and in vivo [28].
Based on available literature, ROS generation may be related to pyrolysis of organic compounds, nicotine, humectants (propylene glycol and glycerin) and flavoring agents, catalytic activity of metals sublimating from the heating elements, and metal-organic and emission-cell interaction [33,38,42,60,61]. Our results clearly documented high level of nicotine present in concentrated e-cig emission. Moreover, e-cig emissions contain large amount of particles (in millions particles/cm3 and g/m3), with the majority of particles being very small, < 200 nm. It is well established in particle toxicology that particles with smaller size (especially nanoparticles) have larger surface area, which can provide greater interface for reactions to generate more ROS [62–65]. Of note also is the relatively high proportion of H2O2 in the collected e-cig samples. H2O2 is formed from radical chain reactions (between hydroxyl radicals and other terminal reactions), conditions that are more favorable kinetically in rich aerosols with high surface areas, which catalyze free radical production and other ROS-sustaining reactions.
It is worthwhile noting that ROS has been detected in other earlier studies of e-cig emissions, as well as of conventional tobacco cigarette smoke. Liu et al (2012) reported 0.1–10 μg H2O2 e.q./cigarette, which corresponded to 29–294 nmol/puff, and at least an order of magnitude higher than the 0.1–10 nmol/puff range that we detected from e-cig [66]. However, Zhao and Hopke (2012) and Miljevic et al. (2010) reported 12.7–14.7 nmol/puff ROS from tobacco cigarette, which was comparable to e-cig [22,67]. Lerner et al. (2015) also observes similar acellular oxidant reactivity from e-cig and tobacco cigarette in the range of 11.8 – 33.3 μM of H2O2 equivalent [38]. Moreover, Rubenstein et al. (2015) found similar amounts of H2O2 production from tobacco smoke and e-cig exposure in vitro [39]. The above studies indicate that the merit in switching from tobacco cigarette to e-cig is limited in the view of ROS production. However, other in vitro studies find e-cig emission induces less oxidative stress [40–42,68] and cytotoxicity [19]. Additionally, Carnevale et al. (2016) measures oxidative stress biomarkers in the blood of human adults after a single e-cig use and found less oxidative stress, compared to tobacco cigarette [43]. Again, this discrepancy is probably due to the difference in e-cig exposure generation because of the different parameters applied. One notable strength of our study is that we applied complementary analytical methods to quantify and speciate ROS from the same e-cig emission and clearly observed production of ROS in both acellular and cellular systems. Therefore, ROS in e-cig emissions is real and may play important roles in initiating pro-inflammatory responses in the airways of humans, and should be included in subsequent health evaluations of e-cig exposures.
We have documented for the first-time substantial influence of e-cig brand, e-liquid flavor, puffing pattern and operational voltage on e-cig ROS and emission concentrations. A two-fold difference was observed in the ROS concentration from the same flavor e-liquids, manufactured by different e-cig brands. The content and relative percentage of H2O2 is also influenced by the brand. The observed ROS concentration differences are likely related to the different manufacturers’ recipes. The ‘fruit flavor’ generated more than three times the amount of ROS compared to the tobacco flavor. This is likely related to the presence of aldehydes, ketones, esters, and unsaturated double bonds in flavoring agents, which enhance or sustain formation of various free radicals, including alkoxyl, and organic peroxyl radicals. Although there are no previous studies on the effects of flavoring agents on e-cig ROS generation, limited literature demonstrates that flavoring e-liquid induces greater cytotoxicity [37,69,70]. We also demonstrated that higher voltage generated a higher amount of total ROS, as well as hydroxide and superoxide radicals. Higher voltage leads to higher filament temperature, which enhances the e-liquid vaporization process, increased concentration of e-cig emissions, pyrolysis, and other chemical reactions, including chain free radial reactions [20]. Two previous studies also observe an increase in the amount of carbonyls concentration under higher voltage, which may also lead to the increase in ROS production seen in our study [71,72]. Research on the influence of these parameters on the physico-chemical properties of e-cig emissions and their toxicological properties is imperative, because it is the variation of such e-cig product features and operational parameters that leads to the different properties in generated e-cig emission, which in turn may drive large discrepancies in the published e-cig literature. Given that e-cig can produce comparable ROS levels to tobacco cigarette, on this ground alone it cannot be promoted as a “safe” alternative to tobacco cigarette.
5. CONCLUSIONS
In summary, the systematic acellular and cellular characterizations of ROS in e-cig emissions using complementary techniques provide conclusive evidence that ROS is generated from e-cig exposure. It was also shown that total ROS, H2O2, and free radicals (such as hydroxyl and superoxide) concentrations and the overall particle concentration were highly affected by the e-cig brand, e-liquid flavor, puffing protocol and operational voltage. These parameters have undoubtedly a measurable effect on the e-cig emission physico-chemical properties and toxicity. This study contributes towards our understanding of e-cig emission profiles and potential toxicological effects on users and provides important data needed for risk assessment purposes. Furthermore, it is noteworthy to mention the strengths and flexibility provided by the versatile exposure generation platform, which has been used to investigate e-cig physico-chemical properties, as well as toxicity in vitro and in vivo as a function of these parameters.
Supplementary Material
Highlights.
Both acellular and cellular systems found ROS generation in e-cig emissions.
E-cig features (brand, flavor) highly influence ROS formation.
Operational parameters (puffing and voltage) highly influence on ROS formation.
E-cig emission can contain comparable level of ROS compared to tobacco cigarette.
Influence of parameters should be considered in e-cig toxicological studies.
Acknowledgments
The authors would like to thank Dr. Georgios Pyrgiotakis for the constructive discussions. Research funding was provided by NIEHS Grant (ES-000002). Jiayuan Zhao gratefully acknowledges the Swiss National Science Foundation for the Early Postdoc Mobility Fellowship (P2LAP3_161808).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER
The findings and conclusion in the report are of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17:219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh T. Morb Mortal Wkly Rep. 2015;65:1425–1429. doi: 10.15585/mmwr.mm655051a2. [DOI] [PubMed] [Google Scholar]; Kennedy ara, SSingh T, Kennedy S, Marynak K, Persoskie A, Melstrom P, King BA. Characteristics of Electronic Cigarette Use Among Middle and High School Students — United States. 2016 doi: 10.15585/mmwr.mm655051a2. [DOI] [PubMed] [Google Scholar]; Marynak K, Persoskie A, Melstrom P, King BA. Characteristics of Electronic Cigarette Use Among Middle and High School Students — United States, 2015. Morb Mortal Wkly Rep. 2016;65:1425–1429. doi: 10.15585/mmwr.mm655051a2. [DOI] [PubMed] [Google Scholar]
- 3.Evans P. E-Cigarette Makers Face Rise of Fakes. E-cigarette global sales hit $7 billion at the end of 2014. Wall Str J. 2015 [Google Scholar]
- 4.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3–9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011–2012. Am J Prev Med. 2014;46:409–412. doi: 10.1016/j.amepre.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. E-cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn J, Monakhova YB, Hengen J, Kohl-himmelseher M, Schüssler J, Hahn H, Kuballa T, Lachenmeier DW. Electronic cigarettes : overview of chemical composition and exposure estimation. Tob Induc Dis. 2014:1–12. doi: 10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrino RM, Tinghino B, Mangiaracina G, Marani a, Vitali M, Protano C, Osborn JF, Cattaruzza MS. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM) Ann Ig. 2012;24:279–288. [PubMed] [Google Scholar]
- 9.Saffari A, Daher N, Ruprecht AA, De Marco C, Pozzi P, Boffi R, Hamad SH, Shafer M, Schauer JJ, Westerdahl D, Sioutas C. Particulate Metals and Organic Compounds from Electronic and Tobacco-containing Cigarettes: Comparison of Emission Rates and Secondhand Exposure. Environ Sci Process Impacts. 2014:2259–2267. doi: 10.1039/C4EM00415A. [DOI] [PubMed] [Google Scholar]
- 10.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (Vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, Smokeless tobacco and nicotine replacement therapy products: E-liquids, Extracts and collected aerosols. Int J Environ Res Public Health. 2014;11:11325–11347. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicol Appl Pharmacol. 2014;278:9–15. doi: 10.1016/j.taap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Sumner W, Chen D, Ph D, Louis S, Author C, Sumner W, Louis S. In Vitro Particle Size Distributions in Electronic and Conventional Cigarette Aerosols Suggest Comparable Deposition Patterns. 2013;15:501–508. doi: 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
- 15.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, Christiani DC. Flavoring chemicals in e-cigarettes: Diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124:733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418:192–199. doi: 10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 17.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob PI, Benowitz N. Levels of selected carcinogens and toxicants in vapor from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859.Levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzopardi D, Patel K, Jaunky T, Santopietro S, Camacho OM, McAughey J, Gaça M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol Mech Methods. 2016;26:477–491. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Pyrgiotakis G, Demokritou P. Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal Toxicol. 2016;0:1–12. doi: 10.1080/08958378.2016.1246628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10:5146–5162. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao JY, Hopke PK. Concentration of Reactive Oxygen Species (ROS) in Mainstream and Sidestream Cigarette Smoke. Aerosol Sci Technol. 2012;46:191–197. doi: 10.1080/02786826.2011.617795. [DOI] [Google Scholar]
- 23.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011 doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14(Suppl 1):90–96. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- 26.Gould NS, Min E, Gauthier S, Martin RJ, Day BJ. Lung glutathione adaptive responses to cigarette smoke exposure. Respir Res. 2011;12:133. doi: 10.1186/1465-9921-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Toorn M, Smit-de Vries MP, Slebos D-J, de Bruin HG, Abello N, van Oosterhout AJM, Bischoff R, Kauffman HF. Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1156–L1162. doi: 10.1152/ajplung.00081.2007. [DOI] [PubMed] [Google Scholar]
- 28.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodas M, Van Westphal C, Carpenter-Thompson R, Mohanty DK, Vij N. Nicotine exposure induces bronchial epithelial cell apoptosis and senescence via ROS mediated autophagy-impairment. Free Radic Biol Med. 2016;97:441–453. doi: 10.1016/j.freeradbiomed.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Shivalingappa PC, Hole R, Van Westphal C, Vij N. Airway exposure to e-cigarette-vapors impairs autophagy and induces aggresome-formation. Antioxid Redox Signal. 2016;24:186–204. doi: 10.1089/ars.2015.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Golli N, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, Dallagi Y, El May MV, El Fazaa S. Impact of e-cigarette refill liquid exposure on rat kidney, Regul. Toxicol Pharmacol. 2016;77:109–116. doi: 10.1016/j.yrtph.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Golli NE, Rahali D, Jrad-Lamine A, Dallagi Y, Jallouli M, Bdiri Y, Ba N, Lebret M, Rosa JP, El May M, El Fazaa S. Impact of electronic-cigarette refill liquid on rat testis. Toxicol Mech Methods. 2016;26:417–424. doi: 10.1016/j.yrtph.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Investig. 2016;20:477–483. doi: 10.1007/s00784-015-1537-x. [DOI] [PubMed] [Google Scholar]
- 34.Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci. 2016:1–9. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 35.Putzhammer R, Doppler C, Jakschitz T, Heinz K, F??rste J, Danzl K, Messner B, Bernhard D. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:1–26. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol Vitr. 2014;28:198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, Rahman I. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015;198:100–107. doi: 10.1016/j.envpol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubenstein DA, Hom S, Ghebrehiwet B, Yin W. Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Mol Immunol. 2015;67:652–660. doi: 10.1016/j.molimm.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Taylor M, Carr T, Oke O, Jaunky T, Breheny D, Lowe F, Gaça M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol Mech Methods. 2016;26:465–476. doi: 10.1080/15376516.2016.1222473. [DOI] [PubMed] [Google Scholar]
- 41.Scheffler S, Dieken H, Krischenowski O, Förster C, Branscheid D, Aufderheide M. Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health. 2015;12:3915–3925. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moses E, Wang T, Corbett S, Jackson GR, Drizik E, Perdomo C, Perdomo C, Kleerup E, Brooks D, O’Connor G, Dubinett S, Hayden P, Lenburg ME, Spira A. Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicol Sci. 2016:kfw198. doi: 10.1093/toxsci/kfw198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco versus electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Demokritou P, Lee SJ, Ferguson ST, Koutrakis P. A compact multistage (cascade) impactor for the characterization of atmospheric aerosols. J Aerosol Sci. 2004;35:281–299. [Google Scholar]
- 45.Pal AK, Watson CY, Pirela SV, Singh D, Chalbot MCG, Kavouras I, Demokritou P. Linking exposures of particles released from nano-enabled products to toxicology: An integrated methodology for particle sampling, extraction, dispersion, and dosing. Toxicol Sci. 2015;146:321–333. doi: 10.1093/toxsci/kfv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawkins L, Turner J, Roberts A, Soar K. “Vaping” profiles and preferences: An online survey of electronic cigarette users. Addiction. 2013;108:1115–1125. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 47.Giovenco DP, Lewis MJ, Delnevo CD. Factors associated with e-cigarette use: A national population survey of current and former smokers. Am J Prev Med. 2014;47:476–480. doi: 10.1016/j.amepre.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Invernizzi G, Ruprecht A, De Marco C, Paredi P, Boffi R. Residual tobacco smoke: measurement of its washout time in the lung and of its contribution to environmental tobacco smoke. Tob Control. 2007;16:29–33. doi: 10.1136/tc.2006.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav. 2015;48:1–4. doi: 10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.I.O.F. Standardization. ISO 3308: routine analytical cigarette-smoking machine - definitions and standard conditions. Geneva, Switzerland: 2012. [Google Scholar]
- 51.Pirela SV, Pyrgiotakis G, Bello D, Thomas T, Castranova V, Demokritou P. Development and characterization of an exposure platform suitable for physico-chemical, morphological and toxicological characterization of printer-emitted particles (PEPs) Inhal Toxicol. 2014;26:400–8. doi: 10.3109/08958378.2014.908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buettner G. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3:259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 53.Janzen EG, Blackburn BJ. Detection and identification of short-lived free radicals by an electron spin resonance trapping technique. J Am Chem Sac. 1968;90:5909–5910. [Google Scholar]
- 54.Dahlgren C, Karksson A, Bylund J. Measurement of respiratory burst products generated by professional phagocytes, in. Neutrophil Methods Protoc. 2007;412:349–363. doi: 10.1007/978-1-59745-467-4_23. [DOI] [PubMed] [Google Scholar]
- 55.Pyrgiotakis G, McDevitt J, Bordini A, Diaz E, Molina R, Watson C, Deloid G, Lenard S, Fix N, Mizuyama Y, Yamauchi T, Brain J, Demokritou P. A chemical free, nanotechnology-based method for airborne bacterial inactivation using engineered water nanostructures. Environ Sci Nano. 2014;1:15–26. doi: 10.1039/C3EN00007A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piao CQ, Liu L, Zhao YL, Balajee AS, Suzuki M, Hei TK. Immortalization of human small airway epithelial cells by ectopic expression of telomerase. Carcinogenesis. 2005;26:725–731. doi: 10.1093/carcin/bgi016. [DOI] [PubMed] [Google Scholar]
- 57.Leonard SS, Chen BT, Stone SG, Schwegler-Berry D, Kenyon AJ, Frazer D, Antonini JM. Comparison of stainless and mild steel welding fumes in generation of reactive oxygen species. Part Fibre Toxicol. 2010;7:32. doi: 10.1186/1743-8977-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob Res. 2016:1895–1902. doi: 10.1093/ntr/ntw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A, Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun. 2016;477:620–625. doi: 10.1016/j.bbrc.2016.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan Z-Z, Yu W-F, Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem Int. 2003;43:243–249. doi: 10.1016/S0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 61.Mosbah R, Yousef A. Mokhtar Ibrahim Mantovani, Nicotine-induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: the protective effects of green tea extract. Exp Toxicol Pathol. 2015;67:253–259. doi: 10.1016/j.etp.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Riediker M. Detecting the oxidative reactivity of nanoparticles: A new protocol for reducing artifacts. J Nanoparticle Res. 2014;16 doi: 10.1007/s11051-014-2493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pirela SV, Miousse IR, Lu X, Castranova V, Thomas T, Qian Y, Bello D, Kobzik L, Koturbash I, Demokritou P. Effects of Laser Printer–Emitted Engineered Nanoparticles on Cytotoxicity, Chemokine Expression, Reactive Oxygen Species, DNA Methylation, and DNA Damage: A Comprehensive in Vitro Analysis in Human Small Airway Epithelial Cells, Macrophages, and Lymphobla. Environ Health Perspect. 2016;124:210–219. doi: 10.1289/ehp.1409582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sisler JD, Pirela SV, Friend S, Farcas M, Schwegler-Berry D, Shvedova A, Castranova V, Demokritou P, Qian Y. Small airway epithelial cells exposure to printer-emitted engineered nanoparticles induces cellular effects on human microvascular endothelial cells in an alveolar-capillary co-culture model. Nanotoxicology. 2015;9:769–79. doi: 10.3109/17435390.2014.976603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson CY, DeLoid GM, Pal A, Demokritou P. Buoyant Nanoparticles: Implications for Nano-Biointeractions in Cellular Studies. Small. 2016:3172–3180. doi: 10.1002/smll.201600314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu H, Sun S, Zong Y, Li P, Xie J. Analysis of hydrogen peroxide in cigarette smoke from selected Chinese cigarette brands under conventional and intense machine smoking conditions. Eur Food Res Technol. 2012;235:1107–1115. doi: 10.1007/s00217-012-1840-6. [DOI] [Google Scholar]
- 67.Miljevic B, Fairfull-Smith KE, Bottle SE, Ristovski ZD. The application of profluorescent nitroxides to detect reactive oxygen species derived from combustion-generated particulate matter: Cigarette smoke - A case study. Atmos Environ. 2010;44:2224–2230. doi: 10.1016/j.atmosenv.2010.02.043. [DOI] [Google Scholar]
- 68.Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. ToxSci Adv Access. 2016 doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 69.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol Vitr. 2014;28:999–1005. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jensen P, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in E-Cigarette Aerosols. N Engl J Med. 2015;372:392–394. doi: 10.1056/NEJMc1414731. [DOI] [PubMed] [Google Scholar]
- 72.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


