Abstract
Purpose of review
Dysregulated citrullination is a key element that drives the production and maintenance of antibodies to citrullinated proteins, a hallmark in rheumatoid arthritis (RA). This article reviews recent literature on the origin of citrullinated antigens in RA.
Recent findings
The study of synovial fluid (SF) from patients with RA has provide important insights into the identity of citrullinated proteins that accumulate in the RA joint (the RA citrullinome) and mechanisms that control their generation.
Summary
Citrullinating enzymes (peptidylarginine deiminases, PADs) are tightly controlled to limit their hyperactivation. Calcium and redox conditions are important regulators of PAD activity. Studies suggest that citrullination is dysregulated both intra- and extracellularly in RA. In neutrophils, host (i.e. perforin and the membrane attack complex) and bacterial (i.e. toxins) pore-forming proteins induce prominent calcium influx, cytolysis and hyperactivation of PADs, which likely maintain hypercitrullination in the RA joint and at extra-articular sites of disease initiation, respectively. Autoantibodies that bind and activate PAD4 have also been identified in the circulation of patients with severe RA. Since the extracellular environment is oxidizing, conditions that are known to inactivate PADs, efficient extracellular citrullination in RA probably requires the constant release of active enzymes from dying cells and may be accelerated by PAD-activating autoantibodies. Understanding how PADs are hyperactivated in patients with RA and the array of citrullinated proteins generated (i.e. the citrullinome), is important to identifying pathways responsible for the development and maintenance of anti-citrullinated protein immune responses.
Keywords: Rheumatoid arthritis, citrullination, peptidylarginine deiminase, leukotoxic hypercitrullination, ACPA, citrullinome
INTRODUCTION
The non-essential amino acid citrulline was isolated from the juice of the watermelon, citrullus vulgaris, by Koga and Ohtake in 1914 [1]. Wada established the structure of citrulline in 1930, and provided the first evidence that this amino acid can be found in proteins [2,3]. Further studies by Rogers et al. demonstrated that citrulline was enzymatically generated by side-chain conversion of peptidylarginine to peptidylcitrulline, in a calcium-dependent process known as deimination or citrullination [4–6]. The enzyme responsible for this reaction was partially purified by Fujisaki and Sugawara in 1981 and named peptidylarginine deiminase (PAD) [7]. Five PADs have since been identified in humans (PAD1-4 and 6) [8–13] and are located in a cluster on chromosome 1p36.1 [12]. While the discovery of citrullination generated interest in different areas of research, the finding by Schellekens and colleagues that citrullinated proteins are major targets of antibodies in patients with rheumatoid arthritis (RA) [14] sparked major interest in understanding the role of citrullination in RA pathogenesis. Here, we discuss the most recent evidence regarding the causes and significance of dysregulated production of citrullinated proteins in RA, collectively referred to as the RA citrullinome.
MECHANISMS THAT REGULATE PHYSIOLOGIC CITRULLINATION
In order to discuss pathways that may lead to dysregulated protein citrullination in RA, it is important to first review what is known about the physiologic mechanisms regulating protein citrullination and the significant gaps in knowledge that still exist. In contrast to many other posttranslational modification (PTM), citrullination appears to be an irreversible process. “De-citrullinating” enzymes that convert citrullinated proteins back to their native peptidylarginine containing forms have not been discovered. The mechanisms involved in the clearance or turn-over of citrullinated proteins in cells also remain unknown. As citrullination reduces the net charge of proteins through the loss of one positive charge per modified arginine residue, it can increase protein hydrophobicity, lead to protein unfolding, and alter intra- and inter-molecular interactions [15]. These structural changes can lead to either gain or more likely, loss of protein function [15–21]. Given that citrullination can have important and potentially irreversible consequences on protein function, the role of citrullination as a mechanism to control cellular processes must be tightly regulated to avoid excessive citrullination of physiologic targets or citrullination of non-physiologic substrates.
One component that controls PAD activation is calcium [6,7]. Binding to calcium induces conformational changes that generate the active form of the enzymes [22]. While full activation of PADs requires millimolar amounts of calcium in vitro [7,23], PAD activation in cells is observed under physiologic conditions where intracellular calcium does not exceed nanomolar concentrations [19,24–27]. It is possible that these suboptimal calcium concentrations induce a PAD conformation that selects for only high efficiency substrates, thereby limiting aberrant citrullination events. Additionally, it is suspected that intracellular protein co-factors may be responsible for modulating calcium sensitivity and specificity of the PAD enzymes, but such binding partners have not been identified. Considering the importance of calcium in PAD activation, pathways that dysregulate calcium binding to PADs (as discussed below) are likely relevant to RA pathogenesis.
Another important component for efficient PAD activity is the presence of a reducing environment [6,7], which is necessary to maintain the active site free thiol cysteine required for catalysis [28]. The oxidizing nature of the extracellular environment, which contrasts with the reducing environment inside cells [29], may provide the conditions needed to protect against aberrant extracellular citrullination by PADs that may leak from activated or dying cells. The importance of reactive oxygen species (ROS) in controlling PAD activity has been recently underscored by the finding that ROS generated by NADPH oxidase inhibits the catalytic activity of PAD2 and PAD4 [30]. The activation of NADPH oxidase as a mechanism to limit PAD activation may play an important role in preventing hypercitrullination in cells suffering a form of cell death termed NETosis [31], in which citrullination may have deleterious effects on the antimicrobial activity of neutrophil extracellular traps (NETs) [32].
DEFINING THE RA CITRULLINOME
A key sustaining component in autoimmune rheumatic diseases is the autoantigen, which fuels the ongoing immune response. Synovial fluid (SF) from patients with RA contains a unique pattern of citrullination that includes proteins spanning the range of molecular weights, termed hypercitrullination [33]. Proteomic analysis of the cellular and soluble components in RA SF have identified more than 100 citrullinated proteins [34–36], which include both intra- and extracellular substrates and together comprise the RA citrullinome. The significance of this citrullinome to RA pathogenesis and the generation of anti-citrullinated protein antibodies (ACPAs), however, is not fully understood.
Despite the considerable number of citrullinated proteins found in RA SF, only few have been identified as being targets of ACPAs (e.g. vimentin, α-enolase and fibrinogen). Whether unique ACPAs exist for each protein in the RA citrullinome or whether only few citrullinated proteins drive the complete ACPA response is still unknown. Given that citrullination is a physiologic process, it is unclear why this PTM becomes a target of an abnormal immune response in RA. Interestingly, while citrullination is clearly an important process for the physiologic function of proteins such as trichohyalin, filaggrin, histones and transcription factors [6,24,26,27,37–44], it is unknown whether the majority of proteins that comprise the RA citrullinome are physiologic or accidental pathologic targets of PADs. In the case of well-defined citrullinated autoantigens like α-enolase and vimentin, for example, hypercitrullination in vitro inactivates their function [16,20]. However, it is unknown whether this modification is part of the normal regulation of these proteins or only occurs in pathologic conditions. If non-selective citrullination occurs accidentally as a consequence of uncontrolled PAD activation, it may lead to the generation of neo-citrullinated proteins not previously tolerized by the immune system and consequently trigger an autoimmune response in susceptible individuals. Alternatively, it is possible that hyperactivation of PADs may accidentally target novel sites in proteins, generating non-tolerized neo-epitopes in abnormally hypercitrullinated molecules. In this regard, recent work demonstrated that while fibrinogen is more extensively citrullinated by PAD2 compared to PAD4 [45], distinct partially citrullinated forms of fibrinogen induced by PAD4, are preferentially recognized by ACPAs [46]. This supports the hypothesis that the generation of unique citrullination sites in proteins may drive their immunogenicity. The production of neo-citrullinated proteins or epitopes may occur and propagate specifically in RA target tissues, such as the joints, due to the establishment of amplification cycles in which damage to PAD-expressing cells by immune components leads to further hypercitrullination (discussed below). This may explain why other tissues highly enriched with physiologically citrullinated proteins, such as the skin, are not pathologic targets for ACPAs.
THE ORIGIN OF THE RA CITRULLINOME
The RA citrullinome is comprised of intra- and extracellular proteins, suggesting that PADs are dysregulated in both compartments. Any PAD-expressing cell present in the RA joint could contribute to the RA citrullinome, including immune cells as well as resident fibroblast-like synoviocytes (FLSs) [47,48]. Recent studies have revealed neutrophils, the most abundant immune cells in RA SF [49], as major sources of intracellular citrullination and soluble PADs for extracellular citrullination [33,50–52]. FLSs and monocytes have also been shown to generate citrullinated α-enolase and vimentin, known RA autoantigens, following treatment with specific stimuli [53,54]. Mechanisms implicated in generating the RA citrullinome will be discussed in detail below.
MEMBRANOLYTYC PATHWAYS AS DRIVERS OF THE INTRACELLULAR RA CITRULLINOME
The finding that cells in RA SF have marked citrullination has focused attention on understanding mechanisms of hypercitrullination that can reproduce the RA citrullinome. Analysis of a broad range of stimuli that induce neutrophil activation and death identified that perforin and the membrane attack complex (MAC), two immune-mediated membranolytic pathways, have the unique capacity to reproduce similar patterns of hypercitrullination observed in RA SF [33]. Perforin and MAC are pore forming cytolytic proteins that induced the influx of ions, particularly calcium, and osmotic lysis [55–57]. The form of cell death induced in neutrophils by these pore-forming mechanisms has recently been named leukotoxic hypercitrullination (LTH), to distinguish it from other forms of neutrophil death that do not induce hypercitrullination [32]. However, even nonlethal amounts of perforin and MAC can lead to significant increases of intracellular calcium to the micromolar range [58,59], so may also support hypercitrullination. The abrupt and prominent influx of calcium resulting from pore-induced membranolytic damage likely overcomes regulatory pathways that control PAD activation in cells, leading to hyperactivation of the enzymes and subsequent hypercitrullination.
The ability to trigger calcium flux-induced LTH is not limited to host immune-pore forming pathways, but also includes bacterial calcium ionophores (i.e. ionomycin and calcimycin from Streptomyces species) and pore-forming toxins [32,51]. In this regard, the tantalizing idea that periodontal disease (PD) may initiate RA has been recently strengthened by the finding that the periodontal pathogen Aggregatibacter actinomycetemcomitans (Aa), activates cellular hypercitrullination in neutrophils [51] via the secretion of the pore-forming protein leukotoxin A (LtxA). Like host pore-forming proteins, LtxA induces target cell death by membrane destabilization, influx of extracellular calcium, and osmotic lysis [60,61]. Importantly, both the citrullinome induced by LtxA as well as the citrullinome present in the periodontium in PD have high similarity to the citrullinome found RA SF [51]. Remarkably, the association of ACPAs with HLA-DRB1 alleles linked to RA appears to be significant only in patients with RA that had evidence of a history of infection with leukotoxic strains of Aa [51], as measured by the presence of antibodies to LtxA. This suggests that Aa may play a role in ACPA development in individuals with a genetic predisposition to develop RA.
Interestingly, bacteria that colonize and infect other mucosal surfaces implicated in RA, such as the lung, gut and urothelium [62,63], also rely on the production of pore-forming toxins as virulence factors to target neutrophils [64–66]. Initial characterization of some of these toxins has confirmed that other pathogenic bacteria, such as Staphylococcus aureus (via Panton-Valentine Leukocidin) and Streptococcus pyogenes (via Streptolysin O), also have the capacity to induce neutrophil hypercitrullination [32]. This suggests that membranolytic damage induced by several different bacterial pathobionts may stimulated chronic hypercitrullination in neutrophils, ACPA production, and RA development in susceptible individuals. Once an ACPA response is initiated at an extra-articular site against bacterial toxin-induced hypercitrullination, host pore-forming pathways (i.e. perforin and MAC) may be responsible for sustaining antigen production in the RA joints. In this scenario, bacteria may be necessary for disease initiation, but are not required to sustain ongoing immune-mediated damage in the articular compartment.
CELL DEATH PATHWAYS AS DRIVERS OF THE INTRA- AND EXTRACELLULAR RA CITRULLINOME
Several mechanisms of cell death have been implicated in generating citrullinated autoantigens in RA, including autophagy, NETosis, necrosis, and more recently LTH (discussed in detail above). These forms of cell death could induce intracellular PAD activation and de novo citrullinated protein generation, as well as the release of transiently active PADs into the extracellular environment [32,52,53,67]. NETosis has gained attention as a mechanism to generate and release citrullinated autoantigens extracellularly, thereby triggering ACPA-associated experimental arthritis [67,68]. However, this antimicrobial form of neutrophil death has not been demonstrated to reproduce the magnitude or breadth of the RA citrullinome that is induced by LTH [33]. While some citrullinated proteins can be detected in NETs by mass spectrometry [52,68], including histones, is unclear whether this citrullination is greater than the background citrullination found in control unstimulated neutrophils. In this regard, several citrullinated peptides reported to be in NETs (such as actin, actin related protein 2/3 complex subunit 1B, coronin, and leukocyte elastase inhibitor, among others) [52,68] are also found in control unstimulated neutrophils [33], raising the possibility that NETosis is redistributor of an existent steady state citrullinome in neutrophils, rather than a generator of de novo pathogenic citrullination in RA.
Although autophagy does not induce hypercitrullination in neutrophils [33], a recent study demonstrated that this programmed cell death mechanism does lead to the citrullination of α-enolase and vimentin in monocytes and FLSs [53]. These citrullinated antigens are known targets of ACPAs, and increased autophagy markers were reported to correlate with ACPA titers, suggesting that autophagy may contribute to generation of citrullinated proteins in patients with RA [53].
Although many forms of cell death may be responsible for releasing PADs extracellularly in RA, only NETosis and necrosis in neutrophils have been studied in detail [52]. Spontaneous release of nuclear material, described as NETosis, has been reported to occur in neutrophils from patients with RA, resulting in the extracellular release of PAD4 [69], and increased nuclear material and PAD activity is present in the SF from patients with RA compared to OA [52]. In in vitro studies measuring extracellular PAD activity following cell death, significantly more active PAD enzyme was released following necrosis, compared to NETosis [52]. While the relative contributions of NETosis, necrosis, and other forms of cell death to extracellular PAD enzyme release in RA are unknown, these findings indicate that the release of extracellular PADs in RA is not representative of a single form of cell death.
AUTOANTIBODIES AS DRIVERS OF THE EXTRACELLULAR CITRULLINOME IN RA
Irrespective of the mechanism, increased soluble extracellular PAD2 and PAD4 is observed in the SF from patients with RA [52,70]. Once released, these PADs have the potential to citrullinate extracellular substrates and interact with anti-PAD autoantibodies. Autoantibodies that enhance the catalytic activity of PAD4 by lowering the amount of calcium required for catalysis were recently described in RA [71]. These antibodies distinguish a unique subgroup of patients with the most erosive disease and pulmonary involvement [71–73], supporting their potentially pathogenic role in RA. Interestingly, a recent study found that SF is unable to sustain PAD activity unless reducing agents, such as dithiothreitol or reduced glutathione were present [74], but the assays that demonstrated the agonistic effect of PAD4-activating autoantibodies were performed in the absence of reducing reagents [71]. Since PADs released from activated neutrophils are transiently active [74], despite these non-permissive conditions, it is possible that PAD4-activating antibodies may act by binding to PAD continually released from activated and dying cells, thus enhancing dysregulated extracellular protein citrullination. Defining the citrullinated proteins generated in the presence of PAD4-activating antibodies will be important for understanding their contribution to the RA citrullinome.
CONCLUSION
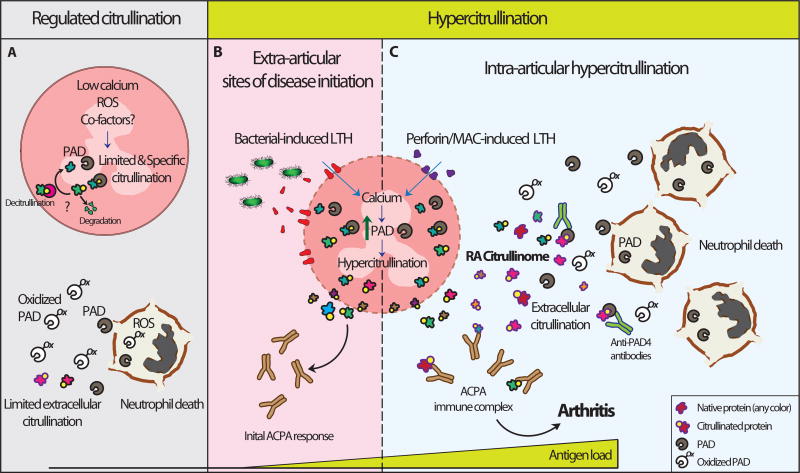
The prominent accumulation of intra- and extracellular citrullinated proteins in the rheumatoid joint strongly suggests that citrullination is dysregulated in RA (Figure 1). The experimental replication of the cellular RA citrullinome requires stimuli that produce damage to the cell membrane, a prominent increase in intracellular calcium concentrations, and cytolysis (e.g. LTH). As such, bacterial pore-forming pathways may be important in generating non-tolerized neo-citrullinated epitopes at extra-articular sites of disease initiation (Figure 1B). Once tolerance is broken to the citrullinated products of hypercitrullination, host pore-forming proteins can sustain ongoing citrullinated autoantigen generation in the joints of patients with RA (Figure 1C). The production of the extracellular RA citrullinome likely requires a constant release of PADs from activated and dying cells, as well as the presence of co-factors that increase PAD activity (such as autoantibodies) to maintain efficient citrullination in the inhospitable oxidizing extracellular environment. Thus, inhibiting pathways that lead to PAD enzyme hyperactivation could suppress ongoing generation of the RA citrullinome, and provide therapeutic benefit to patients with RA.
Figure 1.
Normal and dysregulated citrullination in RA. (A) PAD function is limited by transient nanomolar fluctuations in intracellular calcium, oxidizing environments and potentially protein co-factors. By maintaining a suboptimal activity of PADs, these components may increase enzyme specificity, avoiding the abnormal citrullination of non-physiological substrates. Moreover, efficient mechanisms of clearance of citrullinated proteins (likely by degradation and less likely by re-conversion of citrulline residues to arginine residues) are important to prevent their abnormal accumulation in cells. During physiologic forms of cell death, such as NETosis, PADs are likely inhibited by ROS to prevent hyperactivation as result of calcium influx in dying cells. Similarly, oxidation appears to protect against extracellular citrullination by PADs released from activated and dying cells. Through regulated citrullination, the load of immunogenic proteins is insufficient to drive an ACPA response under physiologic conditions. (B, C). Hypercitrullination results from mechanisms that over-activate the PAD enzymes. Membranolytic damage induced by host and bacterial pore-forming proteins are potent inducers of leukotoxic hypercitrullination (LTH). (B) Bacterial pore-forming toxins are potential triggers of LTH and ACPA production in extra-articular sites of diseases initiation (e.g. gums, gut, and lungs, others). (C) Immune-mediated membranolytic pathways, such as perforin and MAC, likely sustain hypercitrullination in the rheumatoid joint. The large number of dying neutrophils in the articular compartment in RA likely maintains a constant release of active PADs for extracellular citrullination. The presence of agonistic antibodies to PADs may enhance extracellular citrullination before the enzymes are inactivated by oxidation. Together, citrullinated proteins from intra- and extracellular sources constitute the RA citrullinome. Uncontrolled hypercitrullination generates suprathreshold amounts of non-tolerized antigens that may initiate an ACPA response and RA in genetically susceptible individuals.
KEY POINTS.
The RA citrullinome comprises a unique set of intra- and extracellular citrullinated proteins that are highly enriched in the rheumatoid joint, and suggests that mechanisms controlling citrullination are dysregulated in RA.
Calcium and reducing conditions are necessary for efficient PAD activation. Oxidizing environments present extracellular or induced intracellular by ROS inhibit PAD activity.
Pathways that promote suprathreshold amounts of intracellular calcium, such as host (i.e. perforin and MAC) and bacterial (i.e. toxins) pore-forming proteins, are potent activators of PADs and inducers of hypercitrullination.
Bacterial and host pore-forming pathways likely sustain citrullinated autoantigen production at extra-articular sites of disease initiation and in the RA joint, respectively.
Since PADs are inactivated extracellularly, efficient extracellular citrullination in RA likely requires PAD activating co-factors, such as anti-PAD activating antibodies, and the continual release of active PADs enzymes from dying and activated cells.
Acknowledgments
Financial support and sponsorship
The work was supported by the Rheumatology Research Foundation, Jerome L. Greene Foundation, Department of Defense (DoD) office of the Congressionally Directed Medical Research Programs (CDMRP) grant number W81XWH-15-1-0159, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)/National Institutes of Health (NIH) grant R01 AR069569. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAMS or the National Institutes of Health.
Conflicts of interest
ED and FA are authors on issued patent no. 8,975,033, entitled "Human autoantibodies specific for pad3 which are cross-reactive with pad4 and their use in the diagnosis and treatment of rheumatoid arthritis and related diseases”. ED previously served on the scientific advisory board for Padlock Therapeutics, Inc. FA and ED received a grant from Medimmune. FA serves as consultant for Bristol-Myers Squibb.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Koga Y, Ohtake R. Study report of the constituents of squeezed watermelon. J Tokyo Chem Soc. 1914;35:519–528. [Google Scholar]
- 2.Wada M. Über Citrullin, eine neue Aminosäure im Presssaft der Wassermelone, Citrullus vulgaris Schrad. Biochem Z. 1930;224:420–429. [Google Scholar]
- 3.Wada M. Isolierung des Citrullins (ä-carbamido-Ornithin) aus tryptischen Verdauungsprodukten des Caseins. Biochem Z. 1933;257:1. [Google Scholar]
- 4.Rogers GE, Simmonds DH. Content of citrulline and other amino-acids in a protein of hair follicles. Nature. 1958;182:186–187. doi: 10.1038/182186a0. [DOI] [PubMed] [Google Scholar]
- 5.Rogers GE. Occurrence of citrulline in proteins. Nature. 1962;194:1149–1151. doi: 10.1038/1941149a0. [DOI] [PubMed] [Google Scholar]
- 6.Rogers GE, Harding HW, Llewellyn-Smith IJ. The origin of citrulline-containing proteins in the hair follicle and the chemical nature of trichohyalin, an intracellular precursor. Biochim Biophys Acta. 1977;495:159–175. doi: 10.1016/0005-2795(77)90250-1. [DOI] [PubMed] [Google Scholar]
- 7.Fujisaki M, Sugawara K. Properties of peptidylarginine deiminase from the epidermis of newborn rats. J Biochem. 1981;89:257–263. doi: 10.1093/oxfordjournals.jbchem.a133189. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima K, Hagiwara T, Ishigami A, et al. Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3) J Biol Chem. 1999;274:27786–27792. doi: 10.1074/jbc.274.39.27786. [DOI] [PubMed] [Google Scholar]
- 9.Kanno T, Kawada A, Yamanouchi J, et al. Human peptidylarginine deiminase type III: molecular cloning and nucleotide sequence of the cDNA, properties of the recombinant enzyme, and immunohistochemical localization in human skin. J Invest Dermatol. 2000;115:813–823. doi: 10.1046/j.1523-1747.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishigami A, Ohsawa T, Asaga H, et al. Human peptidylarginine deiminase type II: molecular cloning, gene organization, and expression in human skin. Arch Biochem Biophys. 2002;407:25–31. doi: 10.1016/s0003-9861(02)00516-7. [DOI] [PubMed] [Google Scholar]
- 11.Guerrin M, Ishigami A, Mechin MC, et al. cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type I. Biochem J. 2003;370:167–174. doi: 10.1042/BJ20020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavanas S, Mechin MC, Takahara H, et al. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene. 2004;330:19–27. doi: 10.1016/j.gene.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Dai J, Zhao E, et al. cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type VI. Acta Biochim Pol. 2004;51:1051–1058. [PubMed] [Google Scholar]
- 14.Schellekens GA, de Jong BA, van den Hoogen FH, et al. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarcsa E, Marekov LN, Mei G, et al. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki M, Takahara H, Nishi Y, et al. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem. 1989;264:18119–18127. [PubMed] [Google Scholar]
- 17.Wang Y, Wysocka J, Sayegh J, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 18.Proost P, Loos T, Mortier A, et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J Exp Med. 2008;205:2085–2097. doi: 10.1084/jem.20080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuthbert GL, Daujat S, Snowden AW, et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Jang B, Jeon YC, Choi JK, et al. Peptidylarginine deiminase modulates the physiological roles of enolase via citrullination: links between altered multifunction of enolase and neurodegenerative diseases. Biochem J. 2012;445:183–192. doi: 10.1042/BJ20120025. [DOI] [PubMed] [Google Scholar]
- 21.Fert-Bober J, Giles JT, Holewinski RJ, et al. Citrullination of myofilament proteins in heart failure. Cardiovasc Res. 2015;108:232–242. doi: 10.1093/cvr/cvv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arita K, Hashimoto H, Shimizu T, et al. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11:777–783. doi: 10.1038/nsmb799. [DOI] [PubMed] [Google Scholar]
- 23.Damgaard D, Senolt L, Nielsen MF, et al. Demonstration of extracellular peptidylarginine deiminase (PAD) activity in synovial fluid of patients with rheumatoid arthritis using a novel assay for citrullination of fibrinogen. Arthritis Res Ther. 2014;16:498. doi: 10.1186/s13075-014-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christophorou MA, Castelo-Branco G, Halley-Stott RP, et al. Citrullination regulates pluripotency and histone H1 binding to chromatin. Nature. 2014;507:104–108. doi: 10.1038/nature12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherrington BD, Morency E, Struble AM, et al. Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones. PLoS One. 2010;5:e11768. doi: 10.1371/journal.pone.0011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Gamble MJ, Stadler S, et al. Genome-wide analysis reveals PADI4 cooperates with Elk-1 to activate c-Fos expression in breast cancer cells. PLoS Genet. 2011;7:e1002112. doi: 10.1371/journal.pgen.1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denis H, Deplus R, Putmans P, et al. Functional connection between deimination and deacetylation of histones. Mol Cell Biol. 2009;29:4982–4993. doi: 10.1128/MCB.00285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bicker KL, Thompson PR. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers. 2013;99:155–163. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottaviano FG, Handy DE, Loscalzo J. Redox regulation in the extracellular environment. Circ J. 2008;72:1–16. doi: 10.1253/circj.72.1. [DOI] [PubMed] [Google Scholar]
- 30**.Damgaard D, Bjørn ME, Nielsen CH. Reactive oxigen species inhibit catalytic activity of peptidylarginine deiminase. Ann Rheum Dis. 2017;76:1075. doi: 10.1080/14756366.2017.1368505. This study provides evidence that PADs are inactivated by oxidation in the extracellular environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps (NETs) and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. This study stresses that distinct forms of neutrophil activation and death have been mistakenly clasified as NETosis and demonstrates that bacterial pore-forming toxins are potent inducers of hypercitrullination in neutrophils. The study also introduces the term leukotoxic hypercitrullination (LTH) to distinguish cytolysis induced by pore-forming pathways from NETosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero V, Fert-Bober J, Nigrovic PA, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5:209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, et al. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum. 2013;65:69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- 35.Tutturen AE, Fleckenstein B, de Souza GA. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J Proteome Res. 2014;13:2867–2873. doi: 10.1021/pr500030x. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Chen FF, Gao WB, et al. Identification of citrullinated peptides in the synovial fluid of patients with rheumatoid arthritis using LC-MALDI-TOF/TOF. Clin Rheumatol. 2016;35:2185–2194. doi: 10.1007/s10067-016-3247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding CR, Scott IR. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983;170:651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
- 38.Ghari F, Quirke AM, Munro S, et al. Citrullination-acetylation interplay guides E2F-1 activity during the inflammatory response. Sci Adv. 2016;2:e1501257. doi: 10.1126/sciadv.1501257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slade DJ, Horibata S, Coonrod SA, et al. A novel role for protein arginine deiminase 4 in pluripotency: the emerging role of citrullinated histone H1 in cellular programming. BioEssays. 2014;36:736–740. doi: 10.1002/bies.201400057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stadler SC, Vincent CT, Fedorov VD, et al. Dysregulation of PAD4-mediated citrullination of nuclear GSK3beta activates TGF-beta signaling and induces epithelial-to-mesenchymal transition in breast cancer cells. Proc Natl Acad Sci U S A. 2013;110:11851–11856. doi: 10.1073/pnas.1308362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li P, Wang D, Yao H, et al. Coordination of PAD4 and HDAC2 in the regulation of p53-target gene expression. Oncogene. 2010;29:3153–3162. doi: 10.1038/onc.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolodziej S, Kuvardina ON, Oellerich T, et al. PADI4 acts as a coactivator of Tal1 by counteracting repressive histone arginine methylation. Nat Commun. 2014;5:3995. doi: 10.1038/ncomms4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma P, Azebi S, England P, et al. Citrullination of histone H3 interferes with HP1-mediated transcriptional repression. PLoS Genet. 2012;8:e1002934. doi: 10.1371/journal.pgen.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deplus R, Denis H, Putmans P, et al. Citrullination of DNMT3A by PADI4 regulates its stability and controls DNA methylation. Nucleic Acids Res. 2014;42:8285–8296. doi: 10.1093/nar/gku522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama-Hamada M, Suzuki A, Kubota K, et al. Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem Biophys Res Commun. 2005;327:192–200. doi: 10.1016/j.bbrc.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 46*.Blachere NE, Parveen S, Frank MO, et al. High-Titer Rheumatoid Arthritis Antibodies Preferentially Bind Fibrinogen Citrullinated by Peptidylarginine Deiminase 4. Arthritis Rheumatol. 2017;69:986–995. doi: 10.1002/art.40035. This study shows that ACPAs have preferential binding to unique patterns of citrullination induced by distinct PAD isotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foulquier C, Sebbag M, Clavel C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 48.Chang X, Yamada R, Suzuki A, et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 2005;44:40–50. doi: 10.1093/rheumatology/keh414. [DOI] [PubMed] [Google Scholar]
- 49.Malinin TI, Pekin TJ, Jr, Zvaifler NJ. Cytology of synovial fluid in rheumatoid arthritis. Am J Clin Pathol. 1967;47:203–208. doi: 10.1093/ajcp/47.2.203. [DOI] [PubMed] [Google Scholar]
- 50.Darrah E, Rosen A, Giles JT, et al. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis. 2012;71:92–98. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Konig MF, Abusleme L, Reinholdt J, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. This study demonstrates that the periodontal pathogen Aggregatibacter actinomycetemcomitans (Aa) is prevalent in RA, and has the capacity to generate citrullinated autoantigens by inducing neutrophil hypercitrullination. This process is mediated by leukotoxin A (LtxA), an Aa toxin that induces cytolysis, calcium influx, and PAD activation. LtxA-mediated neutrophil lysis is followed by extracellular release of DNA, which is consistent with leukotoxic hypercitrullination that is induced by other host and bacterial pore-forming pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spengler J, Lugonja B, Jimmy YA, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67:3135–3145. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorice M, Iannuccelli C, Manganelli V, et al. Autophagy generates citrullinated peptides in human synoviocytes: a possible trigger for anti-citrullinated peptide antibodies. Rheumatology (Oxford) 2016;55:1374–1385. doi: 10.1093/rheumatology/kew178. [DOI] [PubMed] [Google Scholar]
- 54.Vossenaar ER, Radstake TR, van der HA, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–381. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;51:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 57.Morgan BP, Luzio JP, Campbell AK. Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium. 1986;7:399–411. doi: 10.1016/0143-4160(86)90042-4. [DOI] [PubMed] [Google Scholar]
- 58.Jones J, Hallett MB, Morgan BP. Reversible cell damage by T-cell perforins. Calcium influx and propidium iodide uptake into K562 cells in the absence of lysis. Biochem J. 1990;267:303–307. doi: 10.1042/bj2670303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan BP, Campbell AK. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985;231:205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taichman NS, Iwase M, Lally ET, et al. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J Immunol. 1991;147:3587–3594. [PubMed] [Google Scholar]
- 61.Fong KP, Pacheco CM, Otis LL, et al. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brusca SB, Abramson SB, Scher JU. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheumatol. 2014;26:101–107. doi: 10.1097/BOR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grace LE, Bukhari M, Lauder RM, et al. The Presence of Staphylococcal Toxins in The Urine of Patients with Rheumatoid Arthritis. Ann Rheum Dis. 2016;75:930. [Google Scholar]
- 64.Linhartova I, Bumba L, Masin J, et al. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev. 2010;34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Los FC, Randis TM, Aroian RV, et al. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dal PM, van der Goot FG. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 67.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carmona-Rivera C, Carlucci PM, Moore E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2:eaag3358. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sur CC, Giaglis S, Walker UA, et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16:R122. doi: 10.1186/ar4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinloch A, Lundberg K, Wait R, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58:2287–2295. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- 71.Darrah E, Giles JT, Ols ML, et al. Erosive Rheumatoid Arthritis Is Associated with Antibodies That Activate PAD4 by Increasing Calcium Sensitivity. Sci Transl Med. 2013;5:186ra65. doi: 10.1126/scitranslmed.3005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giles JT, Darrah E, Danoff S, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One. 2014;9:e98794. doi: 10.1371/journal.pone.0098794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Navarro-Millan I, Darrah E, Westfall AO, et al. Association of anti-peptidyl arginine deiminase antibodies with radiographic severity of rheumatoid arthritis in African Americans. Arthritis Res Ther. 2016;18:241. doi: 10.1186/s13075-016-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damgaard D, Bjorn ME, Steffensen MA, et al. Reduced glutathione as a physiological co-activator in the activation of peptidylarginine deiminase. Arthritis Res Ther. 2016;18:102. doi: 10.1186/s13075-016-1000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]