Abstract
Background
Burkholderia cepacia complex (Bcc) are a group of multidrug-resistant gram-negative bacteria rarely reported in patients without cystic fibrosis (CF) or immunocompromising conditions. We investigated Bcc bloodstream infections (BSIs) in a cohort of non-CF patients from the US Veterans Health Administration (VHA).
Methods
Using VHA databases, we identified patients with Bcc BSI at facilities nationwide from 1999 through 2015. We ascertained clinical characteristics, treatments, and outcomes and identified factors associated with 30-day mortality in logistic regression analysis.
Results
We identified 248 patients with Bcc BSI, who were of advanced age (mean, 68 years), chronically ill, and had severe disease. The most common sources were central venous catheters (41%) and pneumonia (20%). Most cases were hospital-acquired (155 [62%]) or healthcare-associated (70 [28%]). Mortality at 14, 30, and 90 days was 16%, 25%, and 36%, respectively. Trimethoprim-sulfamethoxazole (TMP-SMX) and fluoroquinolones were active against 94% and 88% of isolates, respectively. Susceptibility to ceftazidime and meropenem occurred in approximately 70% of the isolates. The most prescribed antibiotics were fluoroquinolones (35%), followed by carbapenems (20%), TMP-SMX (18.5%), and ceftazidime (11%). In regression analysis, age (OR, 1.06 [95% confidence interval {CI}, 1.02–1.10], per added year) and the Pitt bacteremia score (OR, 1.65 [95% CI, 1.44–1.94], per unit increase) were associated with higher 30-day mortality.
Conclusions
In this large cohort of BSIs caused by Bcc, cases were mostly hospital-acquired and we observed high mortality, significant resistance to ceftazidime, and limited use of TMP-SMX. These observations add to our understanding of Bcc infection in non-CF patients and highlight the need for interventions to improve their outcome.
Keywords: Burkholderia cepacia complex, bacteremia, drug resistance, multidrug resistance, electronic health records
Summary
We assembled a large cohort of patients from the Veterans Health Administration with bloodstream infections caused by Burkholderia cepacia complex from 1999 through 2015. We observed high mortality, high resistance rates to ceftazidime, and limited use of trimethoprim-sulfamethoxazole and ceftazidime.
Burkholderia cepacia complex (Bcc) consist of gram-negative non-lactose-fermenting bacteria that are ubiquitous in water, soil, and plants. Organisms from this group, particularly Burkholderia multivorans and Burkholderia cenocepacia, are important opportunistic pathogens in patients with cystic fibrosis (CF) and chronic granulomatous disease. However, little is known about the epidemiology, clinical impact, and therapeutic approaches to Bcc infections in other populations [1].
Bcc pose formidable therapeutic and infection control challenges. They are intrinsically resistant to multiple classes of antibiotics, including aminoglycosides and polymyxins. They can harbor β-lactamase genes, such as the gene coding for PenA, an inhibitor-resistant class A carbapenemase that structurally resembles Klebsiella pneumoniae carbapenemase [2, 3]. Efflux pump systems contribute to multidrug resistance in Bcc, including resistance to chlorhexidine, a common disinfectant [4]. The importance of these mechanisms differs among Bcc species and their effect on clinical outcomes is not well understood [5]. Antibiotic treatment of Bcc in patients with CF and exacerbations of respiratory tract infection is difficult. Although trimethoprim-sulfamethoxazole (TMP-SMX) is often recommended, high-quality evidence to guide treatment in this and other settings is lacking [6].
In non-CF patients, Bcc are known to cause pneumonia, meningitis, urinary tract infections, and bloodstream infections (BSIs). Information about clinical characteristics and outcome of patients with Bcc BSI is mostly derived from small cohorts in the context of outbreaks. These indicate that patients with Bcc BSI have serious underlying diseases, receive intensive care, and have undergone invasive procedures [7, 8]. Outbreaks are often related to contaminated environmental sources, as well as oral, ophthalmic, and infusion solutions [9–11]. For instance, a multistate outbreak related to oral liquid docusate sodium, a stool softener, was recently reported [12, 13].
In this retrospective observational study, we aim to advance our understanding of Bcc BSI in non-CF patients by analyzing a large cohort from the US Veterans Health Administration (VHA). We describe the clinical epidemiology, summarize antibiotic susceptibility and treatment regimens, and identify predictors of mortality.
MATERIALS AND METHODS
Study Design and Setting
We collected data from VHA medical centers from 1 January 1999 to 31 December 2015. The VHA, one of the largest integrated healthcare systems nationwide, operates 152 hospitals, 800 community-based outpatient clinics, and 126 nursing homes caring for 6 million Veterans annually [14]. We obtained data from patients with positive blood cultures in the VHA’s Corporate Data Warehouse using the Veterans Informatics and Computing Infrastructure (VINCI) [15]. To complement, confirm, and correct data extracted within VINCI, we reviewed individual patients’ electronic health records (EHRs) using the Compensation and Pension Record Interchange (CAPRI) and the Veterans Health Information Systems and Technology Architecture (VistaWeb) [16]. We retained cases considered true BSI by the treating physicians and/or one of the investigators who reviewed clinical events (N. G. E.).
Microbiological Data
We collected results of antibiotic susceptibility testing as reported by microbiology laboratories at each facility, selecting antibiotics recommended for testing against Bcc by the Clinical Laboratory and Standards Institute (CLSI): TMP-SMX, ceftazidime, meropenem, ticarcillin-clavulanate, and levofloxacin [17]. We defined polymicrobial BSI as ≥2 pathogens isolated from the same blood culture.
Clinical Data
We determined antibiotic use through review of patients’ records and data from the Bar-Code Medication Administration system, a VHA automated system that records inpatient medications [18]. We defined prior antibiotic use as antibiotics received within the 90-day period preceding the positive blood culture; empiric antibiotic therapy as antibiotic received within 24 hours prior to the time of blood culture collection and 72 hours after; and definitive antibiotic therapy as antibiotic received on the date the final blood culture result was released or in the following 7-day period. We confirmed this as the final, adapted choice of therapy by medical records review. We considered therapy, both empiric and definitive, appropriate if the microbiology laboratory reported the organism susceptible to the antibiotic used, and inappropriate if it reported the organism nonsusceptible. We also considered inappropriate the failure to initiate antibiotics or the use of antibiotics against which Bcc are intrinsically resistant (eg, aminoglycosides, polymyxins). We coded as “missing” the use of antibiotics to which Bcc are not intrinsically resistant but for which susceptibility data was unavailable.
We defined combination antibiotic therapy as the concomitant receipt of ≥2 antibiotics with activity against gram-negative bacteria within the timeframe of definitive therapy. Mode of acquisition included community-acquired infection, occurring as outpatient or <48 hours after admission without a known healthcare encounter in the preceding 90 days; hospital-acquired, occurring >48 hours after admission; and healthcare-associated, occurring <48 hours after admission with healthcare encounter within the preceding 90 days [19]. Healthcare encounters included VHA and non-VHA encounters as reported by providers in clinical notes and discharge summaries. We determined immunosuppression by records review and defined it as the presence of active malignancy, organ transplant, or receipt of immunosuppressive therapy. Immunosuppressive therapy included chemotherapy, chronic steroids (≥10 mg prednisone daily or equivalent for ≥4 weeks within 90 days preceding the infection), B-cell–depleting agents, tumor necrosis factor α inhibitors, tyrosine kinase inhibitors, and other cytokine inhibitors [20]. We calculated the Charlson index [21] and Pitt bacteremia score [22] on the day of blood culture collection, to assess chronic and acute severity of illness, respectively. We identified the source of infection by records review based on physician assessment and/or according to review of clinical events by one of the investigators (N. G. E.). We recorded infectious disease consultations and the date the consultation pertaining to the episode of BSI occurred.
Statistical Analysis
We summarized patient demographics and comorbidities, infection characteristics, and treatment regimens overall and by 30-day mortality. For unadjusted bivariable analyses, we performed comparisons using t tests or non parametric equivalents for continuous variables and the χ2 or Fisher exact test for categorical variables. We evaluated factors associated with 30-day mortality using multivariable logistic regression analysis. Given the number of deaths (n = 61), we sought to fit a parsimonious model. We initially included 9 candidate variables present at the time of culture that were of clinical interest or associated with 30-day mortality in bivariable analysis. We then used stepwise model selection based on the model’s Akaike information criterion to arrive at our final model. We checked all covariates for collinearity using the variance inflation factor and recorded final associations as odds ratio (OR) with 95% confidence interval (CI). Variables in the final model were age, Charlson index, Pitt bacteremia score, hemodialysis, and immunosuppression. We performed statistical analyses using R software version 3.2.4.
RESULTS
Demographics and Geographic and Time Distribution of Cases
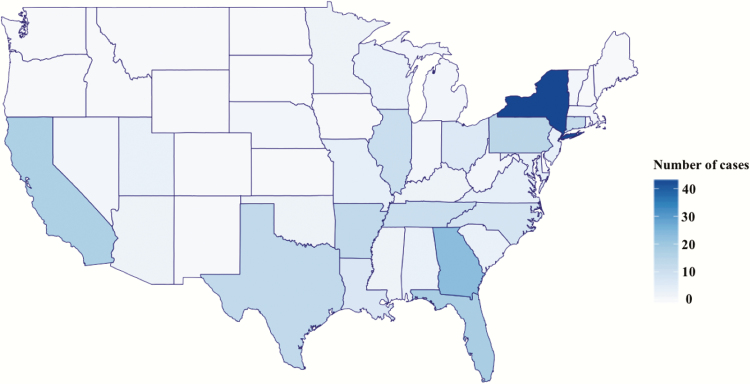
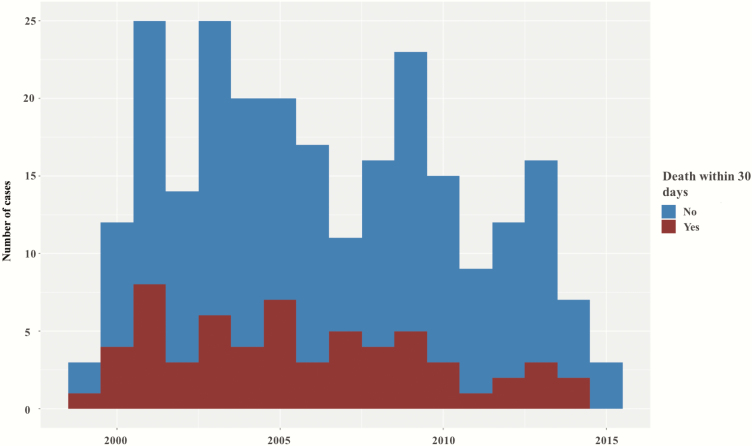
We identified 257 unique cases of bacteremia with Bcc at VHA facilities nationwide during the 17-year study period. After review, 248 cases of true BSI remained, from 62 facilities. Consistent with VHA demographics [23], patients were mostly older men (mean age, 68 years; 98% men), of whom 38 (15%) were nursing home residents. Cases were concentrated in the South (114/248 [46%]) and the Northeast (76/248 [31%]) US Census regions (Figure 1). We did not identify temporal variations or clustering throughout the study period (Figure 2).
Figure 1.
Geographical distribution of cases of Burkholderia cepacia complex bloodstream infection in facilities from the US Veterans Health Administration, 1999–2015.
Figure 2.
Time distribution of cases of Burkholderia cepacia complex bloodstream infection and trends in 30-day mortality.
Clinical and Microbiological Characteristics
Patients with Bcc BSI at the VHA were mostly chronically and acutely ill older adults, with elevated mean Charlson indices and Pitt bacteremia scores of 4.5 and 2.3, respectively. Diabetes mellitus was present in 108 patients (44%), and 58 (23%) were on hemodialysis. Three patients had human immunodeficiency virus infection and none had AIDS. As many as 102 (41%) patients received mechanical ventilation during the index hospitalization and 39 (16%) were in septic shock the day the cultures were drawn, defined as hypotension necessitating pressor support. The most frequent identified source was a central venous catheter (101 cases [41%]), followed by pneumonia (49 [20%]). Most infections were hospital acquired (155 [62%]) or healthcare associated (70 [28%]). Notably, 23 cases (9%) were community acquired and in patients without known immunosuppression. Among these, the most common source was pneumonia (12/23), and 2 instances were related to intravenous drug use. Polymicrobial BSI occurred in 78 cases (31%), most commonly with Staphylococcus species (n = 29; 10 methicillin-resistant S. aureus) and Enterococcus species (n = 21; 12 vancomycin-resistant Enterococcus).
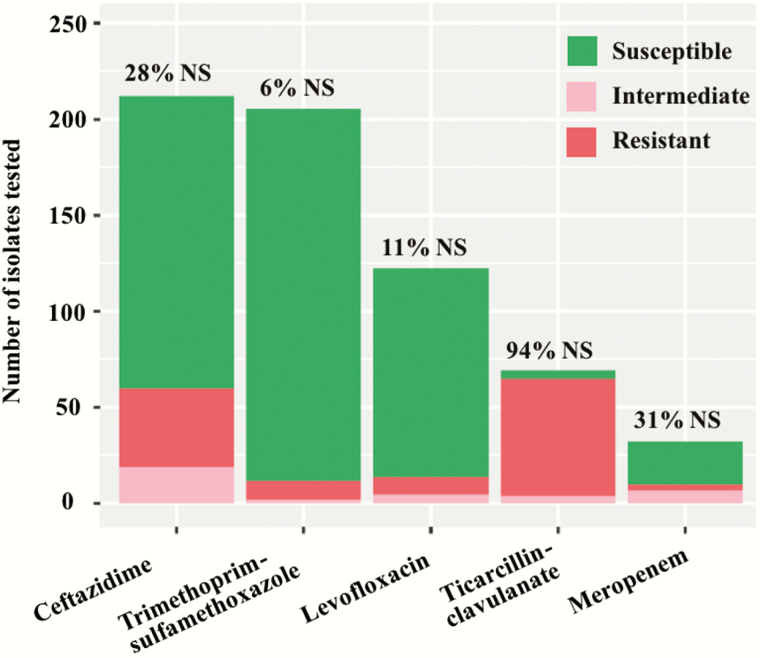
Reporting of susceptibility to antibiotics was not uniform among facilities. TMP-SMX and ceftazidime susceptibilities were available in 83% and 85% of cases, respectively. TMP-SMX was the most active with 94% susceptibility (193 of 205 isolates tested), followed by levofloxacin with 88% susceptibility (108/123). Susceptibility to ceftazidime was 72% (152/212) and 69% to meropenem, although the latter is based on a limited number of isolates (22/32). Ticarcillin-clavulanate displayed poor activity (6% susceptibility [4/69]) (Figure 3).
Figure 3.
Results of antimicrobial susceptibility testing of isolates of Burkholderia cepacia complex causing bloodstream infection. Abbreviation: NS, nonsusceptible.
Antibiotic Treatment and Outcomes
Overall, 232 (94%) patients received empiric antibiotic therapy, 48 (21%) with 2 agents. The most frequently used antibiotics were piperacillin-tazobactam (75 patients), cefepime (28 patients), and imipenem (28 patients). Two hundred thirty-six patients (95%) received definitive antibiotic therapy. This included fluoroquinolones in 35% (ciprofloxacin [22%] and levofloxacin [13%]), carbapenems in 20% (meropenem [11%] and imipenem [9%]), TMP-SMX in 18.5%, piperacillin-tazobactam in 15%, and ceftazidime in 11% of cases. Definitive antibiotic therapy was inappropriate in only 27 patients (11%). Using the same definition, empiric therapy was inappropriate in 86 patients (35%). Combination therapy was used in 29% (73/248), with 39 different combinations. Of the 73 patients who received combination therapy, 43 (59%) were given fluoroquinolone-containing regimens, 25 (34%) carbapenem-containing regimens, 16 (22%) TMP-SMX–containing regimens, and 13 (18%) ceftazidime-containing regimens. Fluoroquinolones were most commonly combined with carbapenems (12/43), piperacillin-tazobactam (8/43), cefepime (8/43), and TMP-SMX (7/43). Ceftazidime and TMP-SMX were coadministered in 5 cases. Infectious disease consultation was obtained in 56% (138/248) of cases. Notably, patients evaluated by an infectious disease consultant were significantly more likely to receive appropriate definitive antibiotic therapy (75 vs 57%, P = .003). Mortality at 14, 30, and 90 days was 16%, 25%, and 36%, respectively. Table 1 shows clinical characteristics by outcome.
Table 1.
Characteristics of Patients With Burkholderia cepacia Complex Bloodstream Infection at US Veterans Health Administration Facilities, 1999–2015
| Characteristic | Survived at 30 d (n = 187) | 30-d Mortality (n = 61) | P Value |
|---|---|---|---|
| Demographic characteristics and comorbidities | |||
| Age, y, mean (SD) | 66.1 (12.3) | 72.41 (11.2) | <.001 |
| Male sex | 182 (97.3) | 60 (98.4) | 1 |
| Region | |||
| Northeast | 48 (25.7) | 28 (45.9) | .02 |
| Midwest | 24 (12.8) | 5 (8.2) | |
| South | 94 (50.3) | 20 (32.8) | |
| West | 18 (9.6) | 5 (8.2) | |
| Puerto Rico | 3 (1.6) | 3 (4.9) | |
| Nursing home resident | 28 (15.0) | 10 (16.4) | .95 |
| Comorbidities | |||
| Dialysis | 48 (25.7) | 10 (16.4) | .19 |
| Immunosuppressiona | 28 (15.0) | 14 (23.0) | .21 |
| HIV infection | 1 (0.5) | 2 (3.3) | .30 |
| Diabetes mellitus | 83 (44.4) | 25 (41.0) | .75 |
| Chronic kidney disease | 74 (39.6) | 19 (31.1) | .30 |
| Coronary artery disease | 66 (35.3) | 27 (44.3) | .27 |
| Chronic pulmonary disease | 66 (35.3) | 24 (39.3) | .68 |
| Congestive heart failure | 54 (28.9) | 24 (39.3) | .17 |
| Cerebrovascular accident | 37 (19.8) | 17 (27.9) | .25 |
| Spinal cord injury | 21 (11.2) | 3 (4.9) | .23 |
| Charlson index scoreb, mean (SD) | 4.3 (2.3) | 5.0 (2.6) | .08 |
| Clinical characteristics | |||
| Maximal temperature, °C, mean (SD) | 38.2 (1.2) | 38.0 (1.3) | .39 |
| Systolic blood pressure, mm Hg, mean (SD) | 128.3 (25.1) | 117.5 (27.8) | .005 |
| Heart rate, beats/min, mean (SD) | 87.2 (18.9) | 97.00 (23.9) | .001 |
| Respiratory rate, breaths/min, mean (SD) | 19.9 (4.4) | 21.8 (6.2) | .001 |
| Mechanical ventilation | 58 (31.0) | 44 (72.1) | <.001 |
| Septic shock | 14 (7.5) | 25 (41.0) | <.001 |
| Intensive care unit admission | 37 (19.8) | 19 (31.1) | .09 |
| Pitt bacteremia score, mean (SD) | 1.4 (1.8) | 5.0 (3.7) | <.001 |
| Microbiological and infection characteristics | |||
| Polymicrobial bacteremia | 53 (28.3) | 25 (41.0) | .09 |
| Source of infection | |||
| Central venous catheter | 82 (43.9) | 19 (31.1) | .049 |
| Pneumonia | 37 (19.8) | 12 (19.7) | |
| Other | 27 (14.4) | 6 (9.8) | |
| Unknown | 41 (21.9) | 24 (39.3) | |
| Mode of acquisitionc | |||
| Community acquired | 21 (11.2) | 2 (3.3) | <.001 |
| Hospital acquired | 103 (55.1) | 52 (85.2) | |
| Healthcare associated | 63 (33.7) | 7 (11.5) | |
| Antibiotic use within prior 90 d | 144 (77.0) | 56 (91.8) | .019 |
| Hospitalization within prior 90 d | 98 (52.4) | 36 (59.0) | .45 |
| Treatment and outcome | |||
| Appropriate definitive therapy | |||
| Appropriate | 132 (70.6) | 35 (57.4) | .071 |
| Inappropriate | 16 (8.6) | 11 (18.0) | |
| Missing | 39 (20.9) | 15 (24.6) | |
| Appropriate empiric therapy | |||
| Appropriate | 58 (31.0) | 18 (29.5) | .069 |
| Inappropriate | 71 (38.0) | 15 (24.6) | |
| Missing | 58 (31.0) | 28 (45.9) | |
| Definitive therapy | |||
| Trimethoprim-sulfamethoxazole | 36 (19.3) | 10 (16.4) | .76 |
| Ceftazidime | 22 (11.8) | 6 (9.8) | .86 |
| Meropenem | 22 (11.8) | 5 (8.2) | .59 |
| Imipenem | 15 (8.0) | 7 (11.5) | .57 |
| Levofloxacin | 28 (15.0) | 5 (8.2) | .26 |
| Ciprofloxacin | 43 (23.0) | 12 (19.7) | .72 |
| Combination definitive therapy | 52 (27.8) | 21 (34.4) | .41 |
| Infectious disease consultation | 109 (58.3) | 29 (47.5) | .19 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
aImmunosuppression includes patients with solid organ or hematologic malignancies not in remission, organ transplant recipients, and those receiving immunosuppressive therapy.
bCharlson index does not include age as a factor.
cCommunity acquired: infection occurring as outpatient or <48 hours after admission and no healthcare encounter within the preceding 90 days; hospital acquired: infection occurring >48 hours after admission; healthcare associated: infection occurring <48 hours after admission plus healthcare encounter within the preceding 90 days.
Risk Factors for Mortality
Bivariable analysis revealed that patients who did not survive at 30 days were older and sicker (reflected by older age, higher Charlson index, Pitt bacteremia score, mechanical ventilation, and shock). Previous antibiotic use was significantly higher in those who died. In the multivariable logistic regression analysis of factors affecting 30-day mortality, age (OR, 1.06 [95% CI, 1.02–1.10], per added year) and Pitt bacteremia score (OR, 1.65 [95% CI, 1.44–1.94], per unit increase) were associated with higher mortality (Table 2).
Table 2.
Multivariable Logistic Regression Model for 30-Day Mortality
| Variables | Bivariable Analysisa | Multivariable Analysisa |
|---|---|---|
| Ageb | 1.01 (1.00–1.01) | 1.06 (1.02–1.10) |
| Pitt bacteremia scoreb | 1.09 (1.07–1.10) | 1.65 (1.44–1.94) |
| Charlson indexb,c | 1.02 (.99–1.04) | 1.14 (.98–1.33) |
| Dialysis | 0.91 (.80–1.03) | 0.51 (.18–1.28) |
| Immunosuppression | 1.11 (.96–1.28) | 2.03 (.80–5.01) |
| Mode of acquisition | ||
| HAI vs CAI | 1.28 (1.07–1.54) | … |
| HCAI vs CAI | 1.01 (.83–1.23) | … |
| Polymicrobial bacteremia | 1.11 (.99–1.25) | … |
| Antibiotic use within prior 90 d | 1.19 (1.04–1.36) | … |
| Inappropriate empiric therapy | ||
| Inappropriate vs appropriate | 0.94 (.82–1.07) | … |
| Inappropriate vs missing | 1.09 (.96–1.25) | … |
Abbreviations: CAI, community-acquired infection; HAI, hospital-acquired infection; HCAI, healthcare-associated infection.
aOdds ratio (95% confidence interval).
bOdds ratio for every single unit increase.
cCharlson index does not include age as a factor.
DISCUSSION
In this study, we assembled a cohort of patients with Bcc BSI to investigate the epidemiology, treatment, and outcomes of such infections. The use of nationwide data curated from the VHA from 1999 through 2015 afforded us unique insights. The VHA is one of the largest integrated healthcare systems in the nation and uses EHRs at all its sites. Additionally, VHA databases include well-structured microbiology data [24]. In this study, we were able to confirm and complement this information through careful examination of individual patients’ EHRs. As a result, we gathered one of the largest cohorts of patients with Bcc BSI in non-CF patients. Although several studies describe the treatment and impact of Bcc infections in CF patients [5], most reports from non-CF patients are in the context of outbreaks (Table 3) [7, 8, 25–27].
Table 3.
Studies Describing Burkholderia cepacia Complex Bloodstream Infections in Patients Without Cystic Fibrosis
| Characteristics | This Study | Liao et al [27] | Bressler et al [25], Woods et al [26] | Lu et al [12] | Huang et al [13] |
|---|---|---|---|---|---|
| Study population | |||||
| Time frame | 1999–2015 | 2004–2007 | 1996–2002 | 1982–1995 | 1997–1999 |
| No. of cases | 248 | 95 | 53, 9 with cystic fibrosis | 70 | 42 |
| Age, y, mean (range) | 68 (22–94) | 69 (55–87) | 46 (24–82) | 34 (median, <1–83) | 70 (2–92) |
| Source | |||||
| Catheter-related infection | 155/248 (63) | 21/95 (22) | … | 11/70 (16) | 8/42 (19) |
| Respiratory tract infection | 49/248 (20) | 47/95 (49) | … | 17/70 (24) | 20/42 (48) |
| Nosocomial | 225/248 (91) | All cases | All cases | 64/70 (91) | 40/42 (95) |
| Susceptibility pattern | |||||
| Trimethoprim-sulfamethoxazole | 193/205 (89) | 0/73 (0) | 44/53 (83) | … | 36/42 (86) |
| Ceftazidime | 152/212 (72) | 71/73 (97) | 37/40 (92) | 41/43 (95) | 40/42 (95) |
| Meropenem | 22/32 (69) | 73/73 (100) | … | … | … |
| Imipenem | 54/136 (40) | … | 5/40 (13) | 23/38 (61) | 32/42 (76) |
| Recorded outcomes | |||||
| 14-d mortality | 40/248 (16) | 16/95 (17) | 25/53 (47) | 8/70 (11) | … |
| 30-d mortality | 61/248 (25) | … | … | … | … |
| In-hospital mortality | … | 51/95 (54) | … | … | 27/42 (64) |
Data are presented as No. (%) unless otherwise indicated.
We found that Bcc BSIs in the VHA were mostly hospital acquired or healthcare associated, and occurred in severely ill older adults with high mortality (25% at 30 days). We identified central venous catheters and pneumonia as the 2 most common sources, consistent with other reports [8, 27]. Bcc BSI occurred in 23 patients without immunocompromising conditions or documented healthcare exposures, which is uncommon [8, 28, 29]. Multivariable regression analysis showed that age and the Pitt bacteremia score were associated with increased 30-day mortality. Although potential unmeasured predictors limit the interpretation of this result, our data align with evidence demonstrating that severity of illness captured by the Pitt bacteremia score is a determinant of mortality in patients with gram-negative BSI [22, 30–32].
Antibiotic therapies consisted mainly of fluoroquinolones (36%), followed by carbapenems (21%), TMP-SMX (18.5%), and ceftazidime (11%). While TMP-SMX and ceftazidime are often considered treatments of choice for Bcc infections due to their reliable activity (Table 3), the evidence to support that recommendation is limited [6]. In our cohort of Bcc BSIs in non-CF patients from VHA facilities, most patients were treated with an active agent and we did not observe a difference in outcome between patients who received TMP-SMX or ceftazidime and those who received other agents (Table 1).
We observed reliable in vitro activity of TMP-SMX (94% susceptibility) in our cohort but a high rate of nonsusceptibility to ceftazidime (28%). Similarly, resistance to meropenem and ticarcillin-clavulanate was high, although only a limited number of isolates were tested. The use of ceftazidime-avibactam, a novel β-lactam β-lactamase inhibitor combination with potent activity against the PenA β-lactamase present in Bcc, may serve as an option in cases where “first-line” agents are not active. In vitro assays demonstrate that the addition of avibactam to ceftazidime resulted in a ≥4-fold reduction in minimum inhibitory concentration among 35 of 49 ceftazidime-resistant Bcc strains [33]. In another study, addition of avibactam to ceftazidime also restored susceptibility to ceftazidime in all ceftazidime-resistant B. multivorans isolates from CF patients [34].
The therapeutic challenge posed by infections caused by multidrug-resistant gram-negative bacteria has led to renewed interest in combination antibiotic therapy. In infections caused by carbapenem-resistant Enterobacteriaceae, there is evidence from observational studies in favor of combination [35], and randomized controlled trials are being conducted to test the hypothesis that combination is superior to monotherapy (ClinicalTrials.gov identifiers NCT01597973, NCT01732250). In this cohort of patients with Bcc BSI, combination regimens were very heterogeneous and the observational nature of our study precludes any conclusions regarding the causality of observed associations between treatment and outcomes.
This is a retrospective cohort consisting mostly of male Veterans. Nevertheless, given that our observations come from a large nationwide cohort comprising a prolonged period, they likely can be extended to a general population of adult non-CF patients. Several investigations have documented the comprehensiveness and reliability of VHA databases to study other bacterial infections [24, 36]. Although the reliability of the data extracted from VHA databases in the early period of this study are not guaranteed, our approach of confirming and complementing administrative data through review of individual EHRs aimed to ensure accuracy. That same approach using EHR review to complement and verify administrative data has been used in other studies [37–39].
Due to the prolonged duration and the large geographical reach of this study, there are likely variations in the identification and antimicrobial susceptibility testing of Bcc isolates. We derive our observations from clinical microbiology reports from facilities where testing is performed according to standard practices at each site, which may vary. Notably, although CLSI offers interpretive breakpoints for Bcc, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) does not provide guidelines for antimicrobial susceptibility testing in Bcc [40]. Since we did not have access to the isolates, we could not confirm the identification of Bcc nor perform antimicrobial susceptibility testing with a uniform methodology. We also could not undertake molecular typing of Bcc, and therefore cannot rule out intra- and interfacility clonal dissemination. We did not detect any significant clustering in time or space to suggest outbreaks, but this remains a possibility. Additionally, molecular characterization of isolates would have allowed identification at the species level, permitting insights into the clinical impact of different species. For instance, Woods et al demonstrated different virulence potentials in 2 strains of B. cenocepacia (genomovar III) responsible for a prolonged outbreak of Bcc BSI associated with high mortality [26].
In summary, this large cohort of non-CF patients with Bcc BSI at VHA facilities nationwide reveals that these occurred in severely ill older adults, often with central venous catheters, with high rates of mortality. Treatment frequently consisted of fluoroquinolones, which demonstrated activity against Bcc in this cohort. Despite the extensive activity of TMP-SMX, most patients were treated with other active agents without differences in mortality. We also observed unexpectedly high resistance to ceftazidime, likely mediated by β-lactamases. Experimental models and clinical studies comparing novel β-lactamase inhibitor combinations with established therapies are needed to advance management strategies. We consider that the approach to improve survival in non-CF patients with Bcc BSI should include controlling the source of infection and prompt initiation of effective antibiotic therapy, with TMP-SMX and fluoroquinolones offering the best probability of activity. The participation of infectious disease consultants may facilitate those goals. Interventions to improve the diagnosis and treatment of Bcc BSI using determined channels (eg, EHR clinical decision tools) and planned strategies (eg, education to modify prescribing practices) may also help improve outcomes. Lastly, we advance that Bcc serve as a paradigm of the challenge posed by multidrug-resistant gram-negative bacteria, where prospective observations are urgently needed to discern correlations between strain types, mechanisms of resistance, treatment choices, and clinical outcomes.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) through the Clinical and Translational Science Collaborative of Cleveland (grant number UL1TR000439 to F. P.); National Institute of Allergy and Infectious Diseases (grant numbers AI100560, AI114508) to R. A. B.; in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, VA Research and Development Office (grant number BX001974 to R. A. B. and grant number BX002872 to K. M. P.-W.) from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service; and the Veterans Integrated Service Networks-10 Geriatrics Research, Education and Clinical Center.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. In addition, the contents do not represent the views of the US Department of Veterans Affairs or the United States government.
Potential conflicts of interest. R. A. B. receives research grants from Merck, Allergan, Wockhart, and the NIH in addition to a Merit Review from the US Department of Veterans Affairs. F. P. receives research grants from Pfizer and Merck and consulting fees from Allergan and Achaogen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Abbott IJ, Peleg AY. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: antimicrobial resistance and therapeutic strategies. Semin Respir Crit Care Med 2015; 36:99–110. [DOI] [PubMed] [Google Scholar]
- 2. Papp-Wallace KM, Taracila MA, Gatta JA, Ohuchi N, Bonomo RA, Nukaga M. Insights into β-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem 2013; 288:19090–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papp-Wallace KM, Becka SA, Taracila MA, et al. Exploring the role of the Ω-loop in the evolution of ceftazidime resistance in the PenA β-lactamase from Burkholderia multivorans, an important cystic fibrosis pathogen. Antimicrob Agents Chemother 2017; 61:e01941–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Podnecky NL, Rhodes KA, Schweizer HP. Efflux pump-mediated drug resistance in Burkholderia. Front Microbiol 2015; 6:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect 2010; 16:821–30. [DOI] [PubMed] [Google Scholar]
- 6. Horsley A, Jones AM, Lord R. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 2016; 1:CD009529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu DC, Chang SC, Chen YC, Luh KT, Lee CY, Hsieh WC. Burkholderia cepacia bacteremia: a retrospective analysis of 70 episodes. J Formos Med Assoc 1997; 96:972–8. [PubMed] [Google Scholar]
- 8. Huang CH, Jang TN, Liu CY, Fung CP, Yu KW, Wong WW. Characteristics of patients with Burkholderia cepacia bacteremia. J Microbiol Immunol Infect 2001; 34:215–9. [PubMed] [Google Scholar]
- 9. De Smet B, Veng C, Kruy L et al. Outbreak of Burkholderia cepacia bloodstream infections traced to the use of Ringer lactate solution as multiple-dose vial for catheter flushing, Phnom Penh, Cambodia. Clin Microbiol Infect 2013; 19:832–7. [DOI] [PubMed] [Google Scholar]
- 10. Romero-Gómez MP, Quiles-Melero MI, Peña García P et al. Outbreak of Burkholderia cepacia bacteremia caused by contaminated chlorhexidine in a hemodialysis unit. Infect Control Hosp Epidemiol 2008; 29:377–8. [DOI] [PubMed] [Google Scholar]
- 11. Lalitha P, Das M, Purva PS et al. Postoperative endophthalmitis due to Burkholderia cepacia complex from contaminated anaesthetic eye drops. Br J Ophthalmol 2014; 98:1498–502. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Multistate outbreak of Burkholderia cepacia infections Available at: https://www.cdc.gov/hai/outbreaks/b-cepacia/index.html. Accessed 2 May 2017.
- 13. Marquez L, Jones KN, Whaley EM et al. An outbreak of Burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol 2017; 38:567–73. [DOI] [PubMed] [Google Scholar]
- 14. Department of Veterans Affairs. Unique veteran users report, FY 2014 Available at: https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Unique_Veteran_Users_2014.pdf. Accessed 27 January 2017.
- 15. Department of Veterans Affairs. VA Informatics and Computing Infrastructure Available at: http://www.hsrd.research.va.gov/for_researchers/vinci/. Accessed 8 September 2016.
- 16. Brown SH, Lincoln MJ, Groen PJ, Kolodner RM. VistA—U.S. Department Of Veterans Affairs national-scale HIS. Int J Med Inform 2003; 69:135–56. [DOI] [PubMed] [Google Scholar]
- 17. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. CLSI Supplement M-100. 24th ed. Wayne, PA: CLSI, 2014. [Google Scholar]
- 18. Schneider R, Bagby J, Carlson R. Bar-Code Medication Administration: a systems perspective. Am J Health Syst Pharm 2008; 65:2216, 8–9. [DOI] [PubMed] [Google Scholar]
- 19. Friedman ND, Kaye KS, Stout JE et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 20. Shorr AF, Zilberberg MD, Reichley R et al. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis 2012; 54:193–8. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 22. Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 1989; 87:540–6. [DOI] [PubMed] [Google Scholar]
- 23. RAND Corporation for the US Department of Veterans Affairs. Assessment A: demographics Available at: http://www.va.gov/opa/choiceact/documents/assessments/Assessment_A_Demographics.pdf. Accessed 10 August 2016.
- 24. Goto M, O’Shea AMJ, Livorsi DJ et al. The effect of a nationwide infection control program expansion on hospital-onset gram-negative rod bacteremia in 130 Veterans Health Administration medical centers: an interrupted time-series analysis. Clin Infect Dis 2016; 63:642–50. [DOI] [PubMed] [Google Scholar]
- 25. Bressler AM, Kaye KS, LiPuma JJ et al. Risk factors for Burkholderia cepacia complex bacteremia among intensive care unit patients without cystic fibrosis: a case-control study. Infect Control Hosp Epidemiol 2007; 28:951–8. [DOI] [PubMed] [Google Scholar]
- 26. Woods CW, Bressler AM, LiPuma JJ et al. Virulence associated with outbreak-related strains of Burkholderia cepacia complex among a cohort of patients with bacteremia. Clin Infect Dis 2004; 38:1243–50. [DOI] [PubMed] [Google Scholar]
- 27. Liao CH, Chang HT, Lai CC et al. Clinical characteristics and outcomes of patients with Burkholderia cepacia bacteremia in an intensive care unit. Diagn Microbiol Infect Dis 2011; 70:260–6. [DOI] [PubMed] [Google Scholar]
- 28. Pujol M, Corbella X, Carratala J, Gudiol F. Community-acquired bacteremic Pseudomonas cepacia pneumonia in an immunocompetent host. Clin Infect Dis 1992; 15:887–8. [DOI] [PubMed] [Google Scholar]
- 29. Waterer GW, Jones CB, Wunderink RG. Bacteremic community-acquired pneumonia in an immunocompetent adult due to Burkholderia cepacia. Chest 1999; 116:1842–3. [DOI] [PubMed] [Google Scholar]
- 30. Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Predictive scoring model of mortality in gram-negative bloodstream infection. Clin Microbiol Infect 2013; 19:948–54. [DOI] [PubMed] [Google Scholar]
- 31. Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E et al. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2016; 60:4159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palacios-Baena ZR, Gutiérrez-Gutiérrez B, De Cueto M et al. ; REIPI/ESGBIS/INCREMENT Group Development and validation of the INCREMENT-ESBL predictive score for mortality in patients with bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 2017; 72:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 2010; 65:2376–81. [DOI] [PubMed] [Google Scholar]
- 34. Papp-Wallace KM, Becka SA, Zeiser ET et al. Overcoming an extremely drug resistant (XDR) pathogen: avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from cystic fibrosis patients. ACS Infect Dis 2017. doi:10.1021/acsinfecdis.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother 2016; 17:761–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gentry CA, Williams RJ 2nd. Trends in susceptibility rates and extended-spectrum β-lactamase production of Klebsiella pneumoniae in bloodstream infections across the United States Veterans Affairs healthcare system. Microb Drug Resist 2015; 21:590–9. [DOI] [PubMed] [Google Scholar]
- 37. Mittal S, Kanwal F, Ying J et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol 2016; 65:1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goetz MB, Hoang T, Kan VL, Rimland D, Rodriguez-Barradas M. Development and validation of an algorithm to identify patients newly diagnosed with HIV infection from electronic health records. AIDS Res Hum Retroviruses 2014; 30:626–33. [DOI] [PubMed] [Google Scholar]
- 39. Xiao DY, Luo S, O’Brian K et al. Longitudinal body composition changes in diffuse large B-cell lymphoma survivors: a retrospective cohort study of United States veterans. J Natl Cancer Inst 2016; 108. doi:10.1093/jnci/djw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. European Committee on Antimicrobial Susceptibility Testing. Antimicrobial susceptibility testing of Burkholderia cepacia complex (BCC) Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/BCC_susceptibility_testing_130719.pdf. Accessed 2 May 2017.