Main point
Oral Polio Virus may cause nonspecific reductions in mortality by reducing etiology-specific diarrheal burden, specifically the number of days with diarrhea. This is likely driven by reductions in bacterial—especially Campylobacter, Shigella—diarrhea. Similar off-target effects were not identified for rotavirus vaccine.
Keywords: OPV, vaccines, enteropathogens, diarrhea, cross-protection
Abstract
Background.
As the global polio eradication initiative prepares to cease use of oral polio vaccine (OPV) in 2020, there is increasing interest in understanding if oral vaccination provides non-specific immunity to other infections so that the consequences of this transition can be effectively planned for and mitigated.
Methods.
Data were collected from infants in an urban slum in Bangladesh (Mirpur, Dhaka) as part of the performance of rotavirus and oral polio vaccines in developing countries (PROVIDE) study. Following vaccination with trivalent oral polio vaccine (tOPV) at 6, 10, and 14 weeks, infants were randomly assigned to receive tOPV (n = 315) or inactivated polio vaccine (IPV) (n = 299) at 39 weeks. Episodes of diarrhea were documented through clinic visits and twice-weekly house visits through 52 weeks. In sum, 14 pathogens associated with diarrhea were analyzed with TaqMan Array Cards.
Results.
Although the proportion of children experiencing diarrhea was not different between the tOPV and IPV groups (P = .18), the number of days with diarrhea (P = .0037) and the number of separate diarrheal episodes (P = .054) trended lower in the OPV arm. Etiological analysis revealed that male tOPV recipients were less likely to have diarrhea of bacterial etiology (P = .0099) compared to male IPV recipients but equally likely to experience diarrhea due to viruses (P = .57) or protozoa (P = .14). Among the 6 bacterial enteric pathogens tested, only Campylobacter jejuni/coli detection was significantly reduced in the OPV arm (P = .0048).
Conclusions.
Our results suggest that OPV may cause nonspecific reductions in mortality, as has been studied elsewhere, by reducing etiology-specific diarrheal burden. This is likely driven by reductions in bacterial diarrhea. Further study of nonspecific OPV effects before global cessation is supported.
Clinical Trials Registration.
(See the Editorial Commentary by Aaby and Benn on pages 420–1.)
Following the cessation of trivalent oral polio vaccine (tOPV) use in April 2016, the Global Polio Eradication Initiative (GPEI) plans to remove all OPV from routine immunization in 2020. OPV containing type 2 virus (OPV2) was removed from global use in April 2016 as an estimated 2200 to 3800 children have been paralyzed due to OPV2 vaccine-associated paralytic polio (VAPP) and circulating vaccine-derived poliovirus (cVDPV2) because wild poliovirus type 2 (WPV2) was eradicated in 1999 [1].
Although OPV possesses a small but direct risk to infants, there is growing awareness that live attenuated oral vaccines such as OPV may cause nonspecific enhancements in immune response [2]. A recent randomized trial found that a birth dose of OPV tended to reduced mortality, although reductions were only statistically significant in boys [3]. Importantly, this effect was primarily seen in the first several months after vaccination [3]. Nonspecific effects—reductions in mortality or disease incidence independent of the effect of the vaccine of the target disease—have also been found for both Bacille Calmette-Guérin (BCG) [4, 5] and measles vaccines [6]. An observational study of children in Denmark found recent OPV recipients had lower rates of hospital admissions for respiratory infections [7]. Before global OPV cessation, the importance of off-target immune enhancement needs to be better understood.
Mechanisms that could explain these nonspecific effects include trained innate immunity and T-cell mediated cross- reactivity [8, 9]. BCG vaccination has been associated with elevation in markers of innate immunity (cytokines interleukin 1β [IL-1β], interleukin 6 [IL-6], tumor necrosis factor α [TNF-α], interferon γ [IFN-γ]) [10, 11]. In these studies, enhanced activation of human monocytes was found to persist at least 3 months and up to 1-year post BCG vaccination, though at lower levels [10, 11]. Animal studies have uncovered epigenetic alterations—including histone trimethylation—in innate immune pathways associated with cross protection to various pathogens [12, 13].
The performance of rotavirus and oral polio vaccines in developing countries (PROVIDE) study was designed to evaluate the performance of oral vaccines (OPV and rotavirus vaccine) in the low-income communities of Mirpur, Dhaka, and Kolkata [14–17]. Detailed data were collected on diarrheal episodes of enrolled children through 1 year of age. We explored PROVIDE data to assess for the possible contribution of oral vaccines to nonspecific reductions in mortality by focusing on reductions in the burden of diarrhea—the second leading cause of mortality in children under 5 in developing countries [18]. We therefore evaluated the nonspecific effects of OPV versus inactivated polio vaccine (IPV) on total and etiology-specific diarrheal incidence during the 3-month window following vaccination.
DATA AND METHODS
Study Design
The PROVIDE study was conducted in the Mirpur area of Dhaka, Bangladesh, and ran from May 2011 to November 2014 [15]. A full description of PROVIDE study design is available in reference [16]. All enrolled children received tOPV at 6, 10, and 14 weeks of age. Children were randomly assigned to receive rotavirus vaccine (Rotarix) scheduled at 10 and 17 weeks. At 39 weeks, children were again randomly assigned to receive tOPV or IPV, and at 52 weeks all children received a tOPV booster. Infants were then equally distributed across from groups depending on receipt of rotavirus vaccine or not at 10 and 17 weeks and receipt of IPV or OPV at 39 weeks. All children received other vaccines according to the Bangladesh Expanded Program on Immunizations, including mealses-rubella bivalent vaccine at 40 weeks. Infants enrolled in the study did not participate in national OPV vaccination campaigns. Diarrheal episodes were recorded through clinic visits and twice-weekly household surveillance visits. For analysis, diarrheal episodes were counted beginning 7 days postvaccination—to attempt to exclude infections that were acquired prior to vaccination—through 52 weeks of age. A parallel analysis of rotavirus vaccine (RV) effects was conducted using surveillance from week 18 through 39 (before supplemental OPV or IPV dose). Separate diarrheal episodes were defined as loose, watery stools more than 72 hours apart. Ethics approval for this study was obtained from the University of Virginia, VA, United States, and the International Center for Diarrheal Disease Research, Bangladesh.
Molecular Diagnostics
Diarrheal stool samples were collected and analyzed for enteric pathogens using custom-developed TaqMan array cards (TAC) [14, 17]. Nucleic acid was extracted from diarrheal stools using the QIAamp Fast DNA Stool mini kit. Two external controls, MS2 bacteriophage and phocine herpes virus, were spiked in the samples during extraction to monitor nucleic acid extraction and amplification efficiency. Enteric pathogens analyzed included 14 pathogens identified through etiological studies to be significantly associated with diarrhea [19]. (Bacteria: enterotoxigenic Escherichia coli (ETEC), Vibrio cholerae, enteroinvasive E. coli (EIEC)/Shigella, Campylobacter jejuni/coli, enteropathogenic E. coli (EPEC), and Salmonella spp. Virus: Adenovirus 40/41, Astrovirus, norovirus GII, Sapovirus, Rotavirus. Protazoa: Cryptosporidium hominus/parvum, Entamoeba histolytica, Cyclospora cayetanensis.) A quantification cycle (Cq) of 35 was used as the analytical limit of detection. For these pathogens, quantitative thresholds could be discerned that were highly predictive of diarrhea in a case-control analysis (Table S1). Using these etiological Cq thresholds, we classified episodes of diarrhea as having a bacterial, viral, or protozoan etiology.
Statistical Analysis
The association between the type of vaccine received at 39 weeks and the number of diarrheal episodes given at least 1 episode, and the number of diarrheal days given at least 1 day with diarrhea were evaluated using zero-truncated Poisson regression. We used generalized estimating equations (GEEs) to fit a logistic regression model to evaluate associations between vaccine type and presence/absence of enteric pathogens to adjust for multiple samples per individual. First, associations were tested between vaccine received and general diarrheal etiologies (bacterial, viral, protozoan). If a general class of pathogen was significantly associated, specific pathogens of that type were individually analyzed. For significant etiological pathogens identified in this analysis, Cox regression was used to assess the relationship of time to occurrence of pathogen-specific diarrhea with the type of vaccine received; cumulative risk curves were drawn using the Kaplan-Meier method. The same method was used to assess impact of rotavirus vaccine on diarrheal burden and etiology between weeks 18 and 39. All P-values were considered statistically significant at a level of .05 using 2-sided tests. Bonferroni correction was used to adjust for multiple hypothesis testing. All analyses were performed in R 3.1.2.
RESULTS
Study Population
Initially 700 infants were enrolled during the week following birth. In total, 299 children received IPV and 315 received OPV at 39 weeks. A total number of 661 diarrheal episodes were recorded in the study period, of which 468 (71%) had stools samples collected and molecular analysis conducted. These 468 samples were collected from 293 different participants; 82 participants had 2 samples tested, 27 had 3 samples, 7 had 4 samples and 4 had 5+ samples tested.
Summary statistics of infants receiving either IPV or OPV at 39 weeks of age are presented in Table 1. There was a lower proportion of females in the OPV arm, though this difference was not statistically significant.
Table 1.
Characteristics of Infants Randomized to Receive tOPV or IPV at Week 39 After Birth, Performance of Rotavirus and Oral Polio Vaccines in Developing Countries Study, Mirpur, Dhaka, Bangladesh, May 2011–November 2014
| Variable | OPV Arm | IPV Arm | P-valuea |
|---|---|---|---|
| N | 315 | 299 | |
| Female | 44.4% | 51.1% | .11 |
| Received RV at 10 + 17 wk | 47.9% | 53.2% | .22 |
| Polio neutralizing Ab at 18 wk | 9.86 ± 1.36 | 9.88 ± 1.36 | .83 |
| Breastfed 1 wk prior | 96.8% | 96.7% | >.99 |
| Height (cm) | |||
| At birth | 48.8 ± 1.7 | 48.6 ± 1.8 | .14 |
| At 39 wk | 68.3 ± 2.6 | 68.3 ± 2.4 | .91 |
| Weight (kg) | |||
| At birth | 2.80 ± 0.35 | 2.75 ± 0.38 | .09 |
| At 39 wk | 7.71 ± 1.12 | 7.68 ± 1.03 | .71 |
| SES, water, sanitation | |||
| Income (1000s Tk) | 13.3 ± 10.5 | 12.8 ± 8.7 | .51 |
| Mother uneducated | 28.3% | 28.8% | .96 |
| Open drain by house | 40.0% | 40.1% | >.99 |
| Drinking water filtered/boiled | 57.8% | 61.5% | .39 |
| Toilet/septic tank | 53.7% | 53.5% | >.99 |
| Pre-39 wk diarrhea | |||
| Any diarrhea | 87.0% | 88.6% | .63 |
| Bacterial etiology | 16.4% | 13.0% | .17 |
| C. jejuni/coli presence | 26.9% | 27.2% | .97 |
| Shigella/EIEC etiology | 6.7% | 4.2% | .11 |
Abbreviations: Ab, antibody; IPV, inactivated polio vaccine; RV, rotavirus vaccine; SES, socioeconomic status; tOPV, trivalent oral polio vaccine.
aNo sex-specific differences were identified across any variables above.
Diarrhea Surveillance
In the 12-week period following vaccination, 57% (170/299) of children in the IPV group and 63% (197/315) of children in the OPV group were recorded as having any diarrhea (P = .18). In those who did report diarrhea, children receiving OPV experienced an average of 5.9 days of diarrhea compared to 6.7 days in the IPV group (P = .0037). Similarly, children receiving OPV experienced an average of 1.7 distinct diarrheal episodes, compared to 1.9 in the IPV group (P = .054). When genders were analyzed separately, male infants alone had significantly reduced days with diarrhea in the OPV arm: 6.6 days compared to 7.7 days in the IPV arm (P = .0025) (Table 2).
Table 2.
Effect of OPV vs IPV at 39 Weeks on Different Diarrheal Outcomes in Subsequent 12 Weeks
| Outcome | OPV Group Avg | IPV Group Avg | |||
|---|---|---|---|---|---|
| Est | n | Est | n | P-Value | |
| All infants | |||||
| Any occurrence of diarrhea | 62.5% | 315 | 56.9% | 299 | .18 |
| Number of days with diarrhea | 5.9 | 197 | 6.7 | 170 | .0037a |
| Number of diarrheal episodes | 1.7 | 197 | 1.9 | 170 | .054a |
| Females | |||||
| Any occurrence of diarrhea | 65.0% | 140 | 53.6% | 153 | .062 |
| Number of days with diarrhea | 5.2 | 91 | 5.5 | 82 | .23a |
| Number of diarrheal episodes | 1.6 | 91 | 1.7 | 82 | .26a |
| Males | |||||
| Any occurrence of diarrhea | 60.6% | 175 | 60.3% | 146 | .99 |
| Number of days with diarrhea | 6.6 | 106 | 7.7 | 88 | .0025a |
| Number of diarrheal episodes | 1.8 | 106 | 2.1 | 88 | .10a |
Abbreviations: IPV, inactivated polio vaccine; OPV, oral polio vaccine.
a P-value from zero-truncated Poisson model.
Diarrheal Pathogens
Next, we examined if OPV affected the general class of pathogen found in diarrheal samples. Episodes were categorized as bacterial, viral, or protozoan using the TAC diagnostic test. In sum, 230 of 468 (49%) diarrheal samples had at least 1 pathogen identified at or above the quantitative polymerase chain reaction (PCR) threshold for a significant diarrheal pathogen: 178 had 1 pathogen, 43 had 2, 7 had 3, and 2 had 4 or more pathogens. Only male children receiving OPV at 39 weeks had a smaller proportion of diarrheal episodes due to bacterial etiologies (P = .0099) (Table 3).
Table 3.
Effect of OPV vs IPV at 39 Weeks on Different Pathogen Classes of Diarrheal Etiology
| Likely Etiology | OPV Group Avg | IPV Group Avg | P-Valuea | |||
|---|---|---|---|---|---|---|
| Est | n | Est | n | Standard | Adjusted | |
| All infants | ||||||
| Viral | 25.9% | 251 | 29.5% | 217 | .41 | >.99 |
| Bacterial | 21.9% | 251 | 27.2% | 217 | .19 | .57 |
| Protozoan | 4.0% | 251 | 6.0% | 217 | .37 | >.99 |
| Females | ||||||
| Viral | 22.0% | 100 | 35.6% | 90 | .041 | .12 |
| Bacterial | 30.0% | 100 | 23.3% | 90 | .30 | .90 |
| Protozoan | 6.0% | 100 | 4.4% | 90 | .67 | >.99 |
| Males | ||||||
| Viral | 28.5% | 151 | 25.2% | 127 | .57 | >.99 |
| Bacterial | 16.6% | 151 | 30.0% | 127 | .0099b | .030 |
| Protozoan | 2.6% | 151 | 7.1% | 127 | .14 | .42 |
Abbreviations: IPV, inactivated polio vaccine; OPV, oral polio vaccine.
a P-values from GEE binomial model with robust standard errors
bStatistically significant after Bonferroni adjustment for multiple hypothesis testing
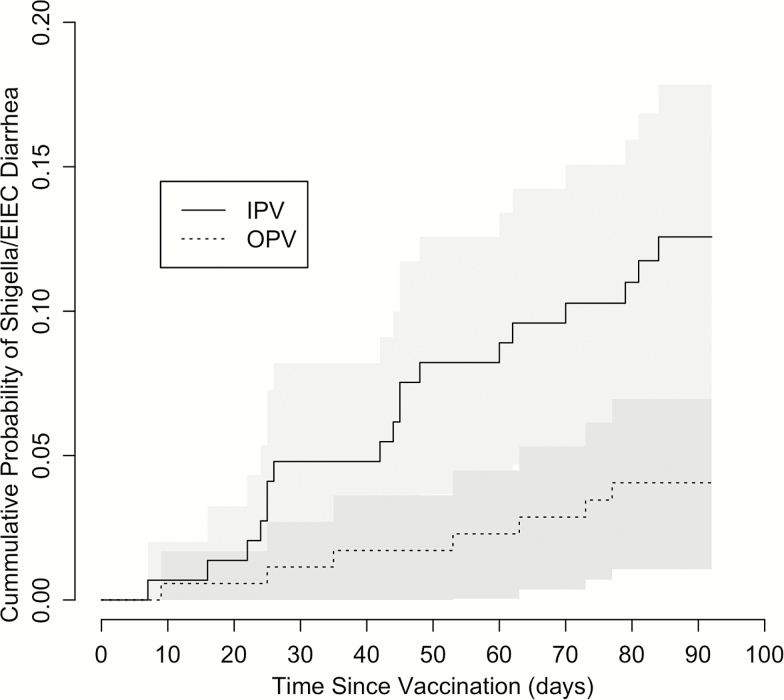
Next, we assessed the individual associations of bacterial pathogens with OPV vaccination at 39 weeks, using the PCR threshold to identify pathogens likely causing diarrhea. Salmonella spp., C. Jejuni/coli were not included as there were no positives. EPEC was also excluded because of a very small number of positives (4) resulted in model failure. This left 3 bacterial pathogens that were tested. In males, OPV was associated with fewer episodes of Shigella/EIEC diarrhea: those receiving IPV were 3.3 times (95% confidence interval [CI]: 1.3–8.6) more likely to have Shigella/EIEC diarrhea than children receiving OPV (adjusted P = .038). When analyzed from an individual perspective, males receiving OPV had a significantly longer time to first Shigella/EIEIC diarrhea (P = .009, Figure 1). No other pathogens were significantly related to vaccine received after adjusting for multiple testing (Table S2).
Figure 1.
Cumulative probability of experiencing Shigella/EIEC diarrhea among male infants receiving OPV or IPV at 39 weeks over subsequent 12-week period. Shaded regions represent 95% confidence interval. Abbreviations: EIEC, enteroinvasive E. coli; IPV, inactivated polio vaccine; OPV, oral polio vaccine.
Lastly, we evaluated whether OPV at 39 weeks was associated with differences in the simple presence of bacterial pathogens in stool (i.e., at any detectable concentration). Salmonella spp. was not detected at any level in any samples so it was excluded here. OPV vaccination at 39 weeks was associated with a reduction in the detection of any C. jejuni/coli in diarrheal stool in all infants: children receiving IPV were 1.9 times (95% CI: 1.2–2.9) more likely to have the bacteria in their diarrheal stool at any quantity (adjusted P = .024) (Table S2).
Rotavirus Vaccine
Infants who received Rotarix vaccine experienced an average of 1.5 fewer days of diarrhea in the 21 weeks following receipt of vaccine compared to those who did not (P < .0001, Table S3). The reduction in diarrheal days was greater for males that received RV compared to females that received RV, though reduction was statistically significant for both groups (Table S3). Infants who received RV demonstrated a significant decrease in viral diarrhea (P = .004) but not bacterial or protozoan diarrhea (Table S4). When viral pathogens were analyzed separately, RV was associated with a reduction in rotavirus-attributable diarrhea (P < .0001) and the presence of rotavirus in diarrhea (P = .0001)—however, no other effects were detected (Table S5).
DISCUSSION
The most important finding of this study is that receiving OPV was associated with significantly fewer days of diarrhea in the 12 weeks after vaccination compared to receiving IPV. Although this difference is small (~ 1 day), we would expect the measured protective effect to be amplified when comparing an IPV-only routine vaccination schedule to an OPV-only one. Furthermore, a longer period of follow-up may also uncover a larger effect of OPV on diarrheal burden.
The work therefore supports the hypothesis that vaccination with the live attenuated Sabin virus may induce off-target immunity toward a subset of diarrheal pathogens [3]. The active diarrheal surveillance in this study allowed such a difference to be detected for the first time. Similar to other studies, we found that male infants experienced greater protective effects that females, possibly due to their higher baseline burden of disease [20]. Although no results regarding female infants were significant after controlling for multiple testing, some parameters suggested females fared worse with OPV (in the case of occurrence of any diarrhea or shigella diarrhea specifically) or better with OPV (in the case of diarrhea of viral etiology). A recent study uncovered sex-specific differences in nonspecific immunomodulation following DTP and measles vaccine [21], but further studies of the mechanism underlying these differences is needed.
Nonspecific effects of OPV on diarrhea appear to be limited to diarrhea caused by bacterial pathogens, and in males specifically. When bacterial pathogens were analyzed individually, we found that OPV use was associated with reductions in C. jejuni/coli diarrheal prevalence in all infants, and Shigella/EIEC diarrheal prevalence at etiological levels when males were analyzed separately. C. jejuni/coli and Shigella are similar in that they cause inflammatory diarrhea, including dysentery—they are responsible for the vast majority of bacillary dysentery. If OPV given at birth primes an immune response that it is protective against mortality,3 and that mortality benefit is seen primarily in the first few months, then it is likely that the protection is against invasive bacterial infection, that is, neonatal sepsis.
Exposure to viral antigens has previously been associated with nonspecific protection from bacterial pathogens. Vaccination with herpes simplex virus type 1 can provide protection from Listeria monocytogenes via induction of more robust CD8 T-cell cytotoxicity, prolonged production of IFN-γ, and systemic activation of macrophages in mouse models [22, 23]. OPV can induce long-term cytotoxic CD8 T cell responses and CD4 memory; however, cross-reactivity with bacterial pathogens has not been well explored [24]. As nonimpaired CD4 and T-cell immunity is important for protection of patients from infection with C. jejuni perhaps OPV provides for a more robust cross-reactive T-cell response that helps in clearance of C. jejuni [25]. Alteration of T-cell responses might be a downstream effect of changes in responsiveness of innate population such as myeloid cells or dendritic cells as observed with BCG vaccination. Increased cytokine production from innate cells in OPV vaccinated patients may help support more robust T-cell responses. OPV vaccination is known to induce type I interferon production from human mononuclear blood cells, and these responses are important for protection from C. jejuni [26, 27].
We found no evidence for nonspecific effects of rotarix vaccine on diarrheal burden or etiology. Although RV was associated with a reduction in days with diarrhea, the only pathogen reduced by RV was rotavirus [28]. This may be due to weaker replication of rotavirus vaccine in the gastrointestinal tract and a resulting weakened immune response [29]. The period studied for RV effects was earlier than that for OPV (18 to 39 vs 40 to 52 weeks), so differences in overall immune system development make direct comparison more complicated.
There are several limitations to our study. First, the relatively short period of follow-up (12 weeks) did not allow for analysis of longer term nonspecific effects. Second, the small sample size in this study prevented a formal study of the impact of OPV on all-cause mortality. Third, analysis of diarrheal etiology relied on case-control derived thresholds that are not equally discriminatory for all pathogens, especially C. jejuni [19]. This could explain why no association with C. jejuni was found at etiological levels. Additionally, we were unable to analyze the effect of OPV on respiratory infections, which has been examined elsewhere and is another leading cause of infant mortality worldwide [7, 18]. Finally, our study compared the impact of OPV versus IPV in infants with a background of 3 OPV doses. This may result in a smaller estimated treatment effects compared to a study in OPV naive children.
We find nonspecific effects of OPV on diarrheal burden, which appear to be limited to diarrhea caused by bacterial pathogens in males. Previous studies of OPV have also examined nonspecific effects; however, these have examined OPV relative to no OPV as opposed to OPV versus IPV [3], as we do here. This comparison is most relevant question for GPEI policy decisions as when OPV is withdrawn from global usage in routine immunization, IPV will be used in its place. Although we find protective effects of OPV relative to IPV here, this study only begins to address the nonspecific trade-offs of OPV compared to IPV. Further research is necessary to understand the differences in OPV-only versus IPV-only routine vaccination schedules in order to fully appreciate the consequences of OPV cessation, currently planned for 2020. Given the risk of reverting to wild-type virus, global usage of OPV must be stopped in order to complete polio eradication. However, better understanding of the unintended negative consequences of OPV withdrawal is necessary so that these can be mitigated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Authors’ Contributions. A. U. B. led data analysis and writing of the report. M. T. led data acquisition. J. A. P.-M. contributed to data acquisition and data analyses. R. H., B. K., E. H., and W. A. P. designed the original protocol and contributed to data interpretation and writing of the report. M. T., J. A. P.-M., S. L. B., M. S. O., W. W., and K. Z. contributed to interpretation and writing of the report.
Acknowledgements. We thank the children and their families who participated in the PROVIDE study in Mirpur and the Parasitology Lab at icddr,b for sample collection and processing.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of CDC (Centers for Disease Control and Prevention) and other contributing agencies. The funding sources were not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication.
Financial support. This work was supported by grants to W.A.P. from the Bill and Melinda Gates Foundation [OPP# 1017093] and National Institutes of Health [R501 A1043596].
Potential conflict of interests. All authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Patel M, Orenstein W. A world free of polio—the final steps. N Engl J Med 2016; 374:501–3. [DOI] [PubMed] [Google Scholar]
- 2. Fish EN, Flanagan KL, Furman D et al. . Changing oral vaccine to inactivated polio vaccine might increase mortality. Lancet 2016; 387:1054–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lund N, Andersen A, Hansen ASK et al. . The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin Infec Dis 2015; 61:1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biering-Sørensen S, Aaby P, Napirna BM et al. . Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr Infect Dis J 2012; 31:306–8. [DOI] [PubMed] [Google Scholar]
- 5. Aaby P, Roth A, Ravn H et al. . Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011; 204:245–52. [DOI] [PubMed] [Google Scholar]
- 6. Aaby P, Martins CL, Garly ML et al. . Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ 2010; 341:c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis 2015; 3:ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Netea MG, Joosten LAB, Latz E et al. . Trained immunity: a program of innate immune memory in health and disease. Science 2016; 352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benn CS, Netea MG, Selin LK, Aaby P. A small jab—a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013; 34:431–9. [DOI] [PubMed] [Google Scholar]
- 10. Kleinnijenhuis J, Quintin J, Preijers F et al. . Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 2012; 109: 17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen KJ, Larsen N, Biering-Sorensen S et al. . Heterologous immunological effects of early BCG vaccination in low-birth weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 2014; 211:956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quintin J, Saeed S, Martens JHA et al. . Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012; 12:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burgess SL, Buonomo E, Carey M et al. . Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. mBio 2014; 5:e01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taniuchi M, Platts-Mills JA, Begum S et al. . Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine 2016; 34:3068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mychaleckyj JC, Haque R, Carmolli M et al. . Effect of substituting IPV for tOPV on immunity to poliovirus in Bangladeshi infants: an open-label randomized controlled trial. Vaccine 2016; 34:358–66. [DOI] [PubMed] [Google Scholar]
- 16. Kirkpatrick BD, Colgate ER, Mychaleckyj JC et al. . The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg 2015; 92:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Gratz J, Amour C et al. . A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 2013; 51:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naghavi M, Wang H, Lozano R et al. . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Platts-Mills JA, Juma J et al. . Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a re-analysis of the GEMS case-control study. Lancet 2016; 388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muenchhoff M, Goulder PJR. Sex differences in pediatric infectious diseases. J Infect Dis 2014; 209(suppl 3):S120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noho-Konteh J, Adetifa JU, Cox M et al. . Sex-differential non-vaccine specific immunological effects of diphtheria-tetanus-pertussis and measles vaccination. Clin Infect Dis 2016; 63:1213–26. [DOI] [PubMed] [Google Scholar]
- 22. Lauterbach H, Kerksiek KM, Busch DH et al. . Protection from bacterial infection by a single vaccination with replication-deficient mutant herpes simplex virus type 1 protection from bacterial infection by a single vaccination with replication-deficient mutant herpes simplex virus type 1. J Virol 2004; 78:4020–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barton ES, White DW, Cathelyn JS et al. . Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007; 447:326–29. [DOI] [PubMed] [Google Scholar]
- 24. Wahid R, Cannon MJ, Chow M. Cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J Virol 2005; 79:5988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janssen R, Krogfelt KA, Cawthraw SA, Van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev 2008; 21:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palmer P, Charley B, Rombaut B, Daëron M, Lebon P. Antibody-dependent induction of type I interferons by poliovirus in human mononuclear blood cells requires the type II fcgamma receptor (CD32). Virology 2000; 278(1):86–94. [DOI] [PubMed] [Google Scholar]
- 27. Klaas M, Oetke C, Lewis LE et al. . Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J Immunol 2012; 189:2414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Colgate ER, Haque R, Dickson DM et al. . Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clin Infect Dis 2016; 63:634–41. [DOI] [PubMed] [Google Scholar]
- 29. Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 2012; 30:A30–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.