In East Africa, data on the risk and predictors of visceral leishmaniasis relapse in HIV coinfected patients are scarce. This study shows high risk of relapse, particularly in those not on antiretroviral therapy or with a high tissue parasite load.
Keywords: visceral leishmaniasis, HIV, relapse, risk, predictors
Abstract
Background
East Africa, where Leishmania donovani is prevalent, faces the highest burden world-wide of visceral leishmaniasis (VL) and human immunodeficiency virus (HIV) coinfection. However, data on the risk and predictors of VL relapse are scarce. Such information is vital to target medical follow-up and interventions to those at highest risk.
Methods
We conducted a retrospective cohort study at a Médecins Sans Frontières−supported health center in northwest Ethiopia. We included adult VL-HIV coinfected patients treated for VL and discharged cured between February 2008 and February 2013. The risk of relapse was calculated using Kaplan-Meier methods, and predictors were determined using Cox regression models.
Results
Of the 146 patients included, 140 (96%) were male and the median age was 31 years. At the index VL diagnosis, 110 (75%) had primary VL, 57 (40%) were on antiretroviral therapy (ART), and the median CD4 count was 149 cells/µL. The median follow-up time after cure was 11 months, during which 44 (30%) patients relapsed. The risk of relapse was 15% at 6 months, 26% at 12 months, and 35% at 24 months. Predictors of relapse were: not being on ART at VL diagnosis, ART not initiated during VL treatment, and high tissue parasite load (parasite grade 6+) at VL diagnosis.
Conclusions
The risk of VL relapse in coinfected patients was high, particularly in those not on ART or presenting with a high tissue parasite load. These patients should be preferentially targeted for secondary prophylaxis and/or regular medical follow-up. Timely ART initiation in all coinfected patients is crucial.
Visceral Leishmaniasis (VL) is a vector borne protozoan disease caused by species of the Leishmania donovani complex that predominantly target the reticuloendothelial cells [1]. Annually, an estimated 200000−400000 new VL cases occur within approximately 70 countries [2]. Whereas L. infantum is the causative species in the Mediterranean region and South America, L. donovani is prevalent in East Africa and the Indian subcontinent [1].
Human immunodeficiency virus (HIV) infection is a risk factor for developing symptomatic VL, and the HIV epidemic led to the re-emergence of VL in the endemic countries of southern Europe [3]. Prior to antiretroviral therapy (ART) scale up, VL-HIV coinfection was common in southern Europe [4]. It is now a major burden in low resource settings, where access to ART is limited. The highest burden worldwide is reported in northwest Ethiopia, where an estimated 20% of VL patients are HIV coinfected [5]. Young, male, migrant workers from the highlands, who travel for seasonal work in this VL endemic area, are the most at risk [5].
Coinfected patients pose a challenge in clinical management: they present atypically, have higher rates of drug toxicity, treatment failure, mortality, and relapse [4, 5]. VL relapse is a new VL episode that occurs after an initial cure. Coinfected patients are not only at high risk of relapse but often have multiple relapses, which progressively become less responsive to therapy eventually causing death [4].
In East Africa, the region with the highest burden of coinfection and where L. donovani is prevalent, data on the risk and predictors of VL relapse are scarce. A systematic review reported relapse rates varying between 20 and 70%, but all except one of the studies included were from Europe, where L. infantum is prevalent [6]. The only included study relating to L. donovani was conducted in Ethiopia [7]. Moreover, this study had a relatively short patient follow-up time, and more than half of the patients were excluded from analysis, mainly due to loss to follow-up. Furthermore, this study was also conducted around 10 years ago, before the wide availability of ART [7]. Currently, with the scaling up of ART, patient survival has increased, and gradually the patient profile is shifting from primary VL cases not on ART to relapse VL cases on ART.
The current lack of reliable information on the risk and predictors of VL relapse has 2 important implications. First, there are no benchmark data to which interventions to decrease relapse can be compared. Second, identification of predictors is vital to improve patient management in a rational manner. Risk stratification is required to target preventive strategies to those most likely to benefit. This will avoid unnecessary follow-up visits thereby reducing the burden on the healthcare providers and patients. In this study, we aimed to identify the risk and predictors of VL relapse in HIV coinfected patients in Ethiopia.
METHODS
Study Setting
The study was conducted in northwest Ethiopia, at Abdurafi Health center supported by Médecins Sans Frontières (MSF). The health center is located in a remote town. Coinfected patients discharged from VL treatment, receive health education about VL, the risk of relapse, are advised to return if symptoms recur, and are referred to the HIV clinic where follow-up continues. Since the program onset, clinical data were collected using standardized data collection tools and stored in electronic databases.
Study Design and Population
We conducted a retrospective cohort study using routine program data. We included VL-HIV coinfected patients diagnosed between February 2008 and February 2013, aged ≥18 years, cured at the end of VL treatment, remained VL free for >28 days after cure, and were in HIV care/follow-up. We excluded patients if they died, defaulted, or were transferred out during treatment and if the final treatment outcome was a positive parasitological test result (treatment failure). We also excluded patients if they died, were lost to follow-up, or received pentamidine secondary prophylaxis (PSP) within 28 days following cure. PSP was offered as part of a clinical trial [8] at the end of the study period. We followed up all patients until December 31, 2013, or until the last date of contact for patients that died, defaulted, were transferred out, lost to follow-up, or started PSP.
Visceral Leishmaniasis Diagnosis
All patients with VL clinical suspicion (prolonged fever, splenomegaly, and wasting), underwent further diagnostic evaluations [9]. Patients without prior VL treatment history (primary VL) were first screened using the rK39 rapid diagnostic test (IT-Leish®, Bio-Rad laboratories, USA) [10]; a positive result was diagnostic of VL. Those testing negative were tested with the leishmania direct agglutination test (DAT, Royal Tropical Institute, Amsterdam, The Netherlands) [11]; a high titer (≥1:3200) was diagnostic. Those with an intermediate DAT titer (1:800–1:1600) underwent tissue aspiration (spleen, bone marrow, or lymph node); a positive result was diagnostic. Patients with prior VL treatment history (relapse VL) underwent tissue aspiration and a positive result was diagnostic. A clinical diagnosis was made in patients contraindicated for spleen aspirate (e.g., severe anemia, bleeding tendency, pregnancy, or collapse), who refused bone marrow aspirate, and didn’t have palpable lymph nodes, or in whom bone marrow aspirate results were negative despite persistent strong clinical suspicion in the absence of differential diagnoses [12, 13].
Human Immunodeficiency Virus Diagnosis and Treatment
A positive test was defined by 2 positive results of serological tests performed in parallel [KHB (Shanghai Kehua Bio-engineering Co-Ltd, Shanghai, China) and STAT-PAK TM (Chembio HIV1/2, Medford, New York, USA)] and confirmed by the ELISA test [ImmunoComb (Orgenics ImmunoComb® II, HIV 1&2 Combfirm)]. ART prescription was according to the national guidelines and tenofovir, lamivudine, and efavirenz combination was the most common first-line regimen.
Visceral Leishmaniasis Treatment
In 2008 to 2010, the first-line treatment was liposomal amphotericin B (AmBisome, Gilead Sciences) at a total dose of 30 mg/kg divided into 6 infusions of 5 mg/kg on alternate days. Patients failing on this treatment received sodium stibogluconate (Albert David Ltd., Kolkata) at a dose of 20 mg/kg/day by intramuscular injection for a minimum of 30 days. From 2011, the first-line treatment was changed to combination therapy of AmBisome at the above dosage and miltefosine (Impavido, Paladin Labs, Montreal, Canada) administered orally for 28 days (100 mg/day in patients >25 kg and 50 mg/day in those ≤25 kg) [13].
Visceral Leishmaniasis and Human Immunodeficiency Virus Treatment Outcomes
VL cure was defined as improvement in VL symptoms and signs, approximately 4 weeks after treatment initiation (i.e., absence of fever, decrease in spleen size, increase in hemoglobin level, weight gain) and a negative parasitological test result. From 2011 onward, parasitological tests were performed at the end of treatment for all coinfected patients. Prior to that, a parasitological test of cure was done for VL relapse patients or those with poor clinical treatment response. For patients without palpable spleen or lymph nodes at the end of treatment and who refused bone marrow aspirate, cure was assessed clinically. Treatment failure was defined as a positive parasitological test result at the end of final VL treatment. A relapse episode was defined as a new VL episode diagnosed ≥4 weeks after VL cure. A new VL episode occurring within 4 weeks after cure was considered treatment failure and treated accordingly. Transfer-out was defined as referral to another health facility. Lost to follow-up was defined as failure to attend scheduled HIV clinic appointments for ≥2 months and having no further follow-up records available. Defaulting was defined as absconding from treatment. Death from all causes was documented.
Data Collection and Measurement of Variables
The following data were collected at admission: age, sex, residential status, VL history (primary, relapse), weight, height, body mass index [BMI], spleen size, tissue parasite grade [0 (no parasites/1000 microscopic fields) to 6 (>100 parasites/microscopic field)] [14], hemoglobin level, HIV serostatus, World Health Organization (WHO) stage, tuberculosis, CD4 count, VL, and ART regimens. Weight, spleen size, and hemoglobin level were also collected at the end of treatment. Hemoglobin level was measured using a hematology analyzer–Beckman Coulter AcT diff, Beckman Coulter Inc., 2003, USA. Tuberculosis was diagnosed according to WHO guidelines [15]. CD4 count was measured at baseline and every 6 months after ART initiation using the FACS counter (BD FACS Calibur flow cytometer, 2009, USA).
Statistical Methods
The primary outcome was the risk of VL relapse at 3, 6, 12, and 24 months after cure. The cumulative incidence of relapse was calculated as the complementary of the Kaplan-Meier estimates. In secondary analysis, the risk of relapse or death was calculated. The predictors of relapse were determined using multivariate Cox regression. The predictors analyzed were: age, sex, VL history, ART regimen, timing of ART initiation, tuberculosis infection, and tissue parasite grade; and (both before and after VL treatment): hemoglobin level, BMI, and spleen size. To overcome the problem of missing values in a substantial proportion of the CD4 counts, a composite marker for advanced HIV infection was created, defined as having either additional WHO stage IV disease or a CD4 count <50 cells/µL at VL diagnosis [16]. To visualize the association between CD4 counts and the risk of relapse, the nonparametric LOWESS smoothing method was used (lowess’ command in STATA). Parasite grading was categorized as: (1) 6+ (high); (2) <6+; (3) not done: (serological/clinical diagnosis). Timing of ART initiation was categorized as: (1) being on ART at VL diagnosis; (2) ART initiation during VL treatment; (3) ART initiation after VL treatment; (4) ART never initiated; included as a time-varying covariate.
All predictors were first assessed in a univariate analysis, then those with a significant effect on a 10% significant level in the multivariate analysis. The following predictors: timing of ART initiation, VL history, and advanced HIV infection were included a priori in all multivariate analyses based on existing knowledge [4, 6]. The multiple models were reduced by backward elimination. To evaluate the effect of missing CD4 count data, a sensitivity analysis was conducted with multiple imputations, using the methodology of chained equations. The imputed data replaced “advanced HIV infection” in the above analysis [17]. The cumulative incidence of relapse, with death as a competing risk, and its predictors were also calculated, because in this case standard survival methods could lead to biased estimates [18, 19]. The proportional hazard assumption was assessed graphically and tested formally using Schoenfeld residuals. Colinearity was evaluated by calculating the variance inflation factors. All tests were performed at a 5% level of significance. All statistical analyses were performed with Stata version 14.
Ethics Approval
Ethics approval was received from the Institutional Review Board of the Institute of Tropical Medicine, Antwerp, Belgium, the Ethical Review Committee of the Institute of Public Health, Gondar University, Ethiopia; this research fulfilled the exemption criteria set by the MSF Ethical Review Board (ERB) for a posteriori analyses of routinely collected clinical data and thus did not require MSF ERB review. It was conducted with permission from the Medical Director of the MSF Operational Centre Amsterdam.
RESULTS
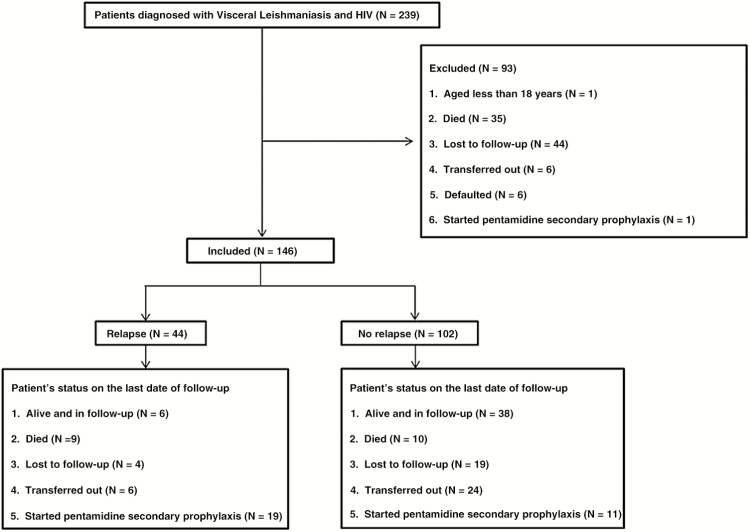
Between February 2008 and February 2013, 239 VL-HIV coinfected patients were diagnosed and treated at Abdurafi health center. One patient was aged <18 years, 35 died during treatment, 44 were lost to follow-up, 6 were transferred out, 6 defaulted, and 1 received PSP. These 93 patients (38.9%) were excluded from the study. A total of 146 patients were included in the analysis (Figure 1). A comparison between those included and excluded is shown in supplementary Table S1.
Figure 1.
Flow diagram showing the number of patients in the study and their outcomes.
Demographic and Clinical Characteristics
At the index VL diagnosis, the majority of patients were male, 140 (95.9%), and young (median age of 31 years). The number of migrant workers and residents were similar, 70 each (49.3%). The majority (n = 110; 75.3%) had primary VL, most patients (n = 110; 82.7%) had advanced HIV infection, and 57 (39.6%) were on ART prior to VL diagnosis. The median CD4 count was 149 cells/µL. The median follow-up time was 11.4 months (Table 1). At the end of the study period, 23 (15.8%) patients were lost to follow-up (Figure 1), occurring after a median of 7.4 months (interquartile range [IQR], 2.6−29.4).
Table 1.
Demographic and Clinical Characteristics of Patients With Visceral Leishmaniasis and HIV Coinfection Treated by Médecins Sans Frontières in Ethiopia From February 2008 to December 2013, by Visceral Leishmaniasis Relapse Status
| Characteristics at Admission | Total (N = 146) | VL Relapse (N = 44) | No VL Relapse (N = 102) | P |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 140 (95.9) | 43 (97.7) | 97 (95.1) | .67a |
| Female | 6 (4.1) | 1 (2.3) | 5 (4.9) | |
| Residential status, n (%); n = 142 | ||||
| Migrant worker | 70 (49.3) | 25 (59.5) | 45 (45.0) | .24a |
| Resident | 70 (49.3) | 17 (40.5) | 53 (53.0) | |
| Settler | 2 (1.4) | 0 (0.0) | 2 (2.0) | |
| Age (years), median (IQR) | 31 (27–38) | 30.5 (27−39) | 31.5 (27–37) | .61b |
| VL history, n (%) | ||||
| Primary VL | 110 (75.3) | 35 (79.6) | 75 (73.5) | .44c |
| Relapse VL | 36 (24.7) | 9 (20.4) | 27 (26.5) | |
| Spleen size (cm), median (IQR); n = 144 | 5 (3−8) | 5 (3−7) | 5 (3−8) | .72b |
| Body mass index (kg/m 2 ), median (IQR); n = 134 | 16 (15−18) | 16 (15−17) | 16 (15−18) | .96b |
| Hemoglobin (g/dL), median (IQR); n = 144 | 8.7 (7.2–10.1) | 9.2 (7.5−10.2) | 8.5 (6.9−9.8) | .14b |
| Parasitological test result at VL diagnosis, n (%)d | ||||
| <6+ | 84 (57.5) | 26 (59.1) | 58 (56.9) | .11c |
| 6+ | 22 (15.1) | 10 (22.7) | 12 (11.7) | |
| Not done: serological/clinical diagnosis | 40 (27.4) | 8 (18.2) | 32 (31.4) | |
| Tuberculosis, n (%); n = 145 | ||||
| Yes | 32 (22.1) | 10 (23.3) | 22 (21.6) | .82c |
| No | 113 (77.9) | 33 (76.7) | 80 (78.4) | |
| WHO stage, n (%); n = 111 | ||||
| III | 5 (4.5) | 1 (2.9) | 4 (5.3) | 1.00a |
| IV | 106 (95.5) | 34 (97.1) | 72 (94.7) | |
| CD4 count (cells/µL)e, median (IQR); n = 84 | 149 (65−256) | 139 (56−209) | 180 (74−278) | .19b |
| CD4 count (cells/µL), n (%)e; n = 84 | ||||
| ≤100 | 32 (38.1) | 11 (42.3) | 21 (36.2) | .75a |
| 101−199 | 21 (25.0) | 7 (26.9) | 14 (24.2) | |
| 200−349 | 19 (22.6) | 6 (23.1) | 13 (22.4) | |
| ≥350 | 12 (14.3) | 2 (7.7) | 10 (17.2) | |
| Advanced HIV infection, n (%)f; n = 133 | ||||
| Yes | 110 (82.7) | 36 (83.7) | 74 (82.2) | .83c |
| No | 23 (17.3) | 7 (16.3) | 16 (17.8) | |
| ART regimen, n (%)g | ||||
| Tenofovir-based regimen | 64 (43.8) | 15 (34.1) | 49 (48.0) | .01a |
| Non–tenofovir-based regimen | 76 (52.1) | 24 (54.5) | 52 (51.0) | |
| None | 6 (4.1) | 5 (11.4) | 1 (1.0) | |
| Timing of ART initiation, n (%); n = 144 | ||||
| ART initiated before VL episode | 57 (39.6) | 13 (29.6) | 44 (44.0) | .002a |
| Duration on ART (months) at VL diagnosis, median (IQR) | 6.8 (1.9−15.9) | 9.4 (4.1−29.6) | 5.7 (1.3−15.2) | |
| ART initiated during VL treatment | 40 (27.8) | 8 (18.2) | 32 (32.0) | |
| ART initiated after VL treatment | 41 (28.5) | 18 (40.9) | 23 (23.0) | |
| ART never initiated | 6 (4.2) | 5 (11.4) | 1 (1.0) | |
| Initial VL treatment regimen, n (%) | ||||
| AmBisome and Miltefosine | 59 (40.4) | 13 (29.6) | 46 (45.1) | .08c |
| Other (AmBisome or sodium stibogluconate) | 87 (59.6) | 31 (70.4) | 56 (54.9) | |
| Outcome of first VL episode treated, n (%); n = 139 | ||||
| Clinical cure | 82 (59.0) | 25 (59.5) | 57 (58.8) | .93c |
| Parasitological cure | 57 (41.0) | 17 (40.5) | 40 (41.2) | |
| Characteristics at discharge | ||||
| Spleen size (cm), median (IQR); n = 139 | 0 (0−3) | 0 (0−2) | 0 (0−3) | .21b |
| Body mass index (kg/m 2 ), median (IQR); n = 145 | 17 (16−18) | 17 (16−19) | 17 (16−18) | .37b |
| Hemoglobin (g/dL), median (IQR); n = 137 | 9.1 (8.2−10.5) | 8.9 (8.2−10.3) | 9.1 (8.2−10.5) | .96b |
| Duration of follow-up (months), median (IQR) | 11.4 (4.0−30.0) | 7.8 (3.9−15.3) | 14.4 (4.5−38.7) | .009b |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; VL, visceral leishmaniasis;WHO, World Health Organization.
aFisher exact test.
b2-sample Wilcoxon rank-sum (Mann-Whitney) test.
cχ2 test.
d97 (66.4%) spleen aspirates, 1 bone marrow aspirate (0.7%), 8 lymph node aspirates (5.5%), and 40 (27.4%)—37 primary VL cases and 3 relapse cases—had no parasitological test done.
eCD4 count result is <6 months from VL treatment initiation.
fWHO stage IV or CD4 <50 cells/µL.
gStavudine, lamivudine, and nevirapine; zidovudine, lamivudine, and efavirenz; tenofovir, lamivudine, and efavirenz; zidovudine, lamivudine, and nevirapine; stavudine, lamivudine, and efavirenz.
Risk of Relapse
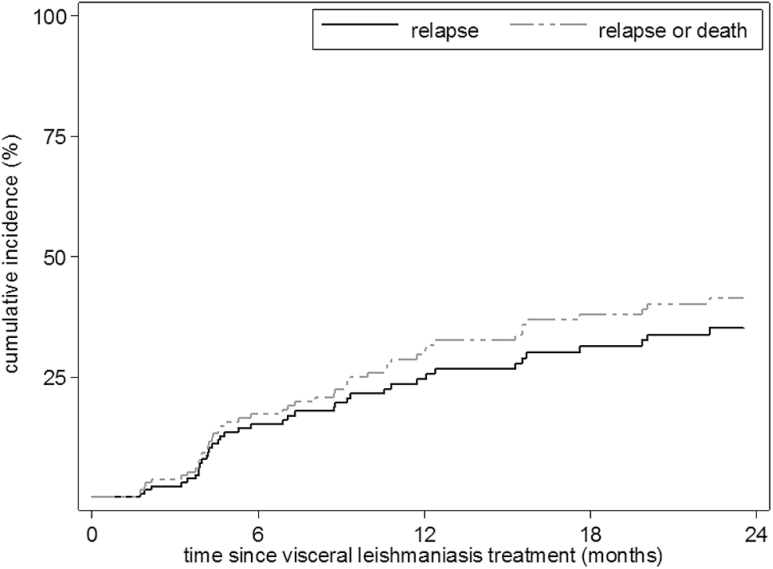
The Kaplan-Meier estimated cumulative incidence was 15% at 6 months, 26% at 12 months, and 35% at 24 months. Estimates of relapse accounting for the competing risk of death were fairly similar (Table 2).
Table 2.
Estimated Cumulative Incidence (1 - Kaplan-Meier) of Relapse, Relapse Accounting for Death as a Competing Risk (CR), and Relapse or Death for Patients With Visceral Leishmaniasis and HIV Coinfection Treated by Médecins Sans Frontières in Ethiopia from February 2008 to December 2013
| Total at Beginning (N) | Fail (N) | Cumulative Incidence (95% CI) | Cumulative Incidence (CR) (95% CI) | |
|---|---|---|---|---|
| Relapse | ||||
| Month 3 | 124 | 4 | 0.03 (0.01−0.08) | 0.02 (0.01−0.06) |
| Month 6 | 100 | 15 | 0.15 (0.10−0.23) | 0.14 (0.09−0.21) |
| Month 12 | 72 | 11 | 0.26 (0.19−0.35) | 0.23 (0.16−0.31) |
| Month 24 | 46 | 8 | 0.35 (0.27−0.45) | 0.33 (0.25−0.42) |
| Relapse or death | ||||
| Month 3 | 124 | 6 | 0.04 (0.02−0.09) | − |
| Month 6 | 100 | 16 | 0.17 (0.12−0.25) | − |
| Month 12 | 72 | 15 | 0.31 (0.23−0.39) | − |
| Month 24 | 46 | 10 | 0.41 (0.33−0.51) | − |
Abbreviations: CI, confidence interval; CR, competing risk.
The risk of failure (relapse or death) was 17% at 6 months, 31% at 12 months, and 41% at 24 months (Table 2 and Figure 2).
Figure 2.
Kaplan-Meier estimates of cumulative incidence of relapse and relapse or death at different time points after completion of visceral leishmaniasis treatment.
Predictors of Visceral Leishmaniasis Relapse
The risk of relapse was lower in those on ART at VL diagnosis (adjusted Hazard ratio (aHR), 0.22; 95% confidence interval [CI], 0.10–0.52) or starting ART during VL treatment (aHR, 0.39; 95% CI, 0.17–0.86) and higher in those with a high tissue parasite load (parasite grade 6+) at VL diagnosis (aHR, 6.63; 95% CI, 2.64−16.63) (Table 3). There was a statistically nonsignificant association between larger spleen size and relapse (Table 3).
Table 3.
Predictors and Hazard Ratios for Relapse Among Visceral Leishmaniasis and HIV Coinfected Patients Treated by Médecins Sans Frontières in Ethiopia From February 2008 to December 2013 (N = 146)
| Predictors | n/N (%) | Crude HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| >40 | 9/23 (39.1) | 1.00 | |||
| 30–40 | 18/64 (28.1) | 1.45 (0.65–3.26) | .37 | ||
| <30 | 17/59 (28.8) | 1.00 (0.52–1.95) | .99 | ||
| Sex | |||||
| Male | 43/140 (30.7) | 1.00 | |||
| Female | 1/6 (16.7) | 0.48 (0.07–3.52) | .47 | ||
| Previous VL episode | |||||
| No | 35/110 (31.8) | 1.00 | |||
| Yes | 9/36 (25.0) | 0.80 (0.39–1.67) | .56 | ||
| Advanced HIV stage a | |||||
| No | 7/23 (30.4) | 1.00 | |||
| Yes | 36/110 (32.7) | 0.91 (0.40−2.06) | .83 | ||
| Spleen size at admission (cm) | |||||
| 0 | 2/18 (11.1) | 1.00 | |||
| 1–5 | 16/40 (40.0) | 4.12 (0.95–17.99) | .06 | ||
| ≥5 | 25/86 (29.1) | 3.75 (0.89–15.88) | .07 | ||
| Body mass index at admission (kg/m 2) | |||||
| <16 | 18/58 (31.0) | 1.00 | |||
| ≥16 | 20/76 (26.3) | 0.73 (0.39–1.38) | .33 | ||
| Hemoglobin level at admission (g/dL) | |||||
| <7 | 7/33 (21.2) | 1.00 | |||
| 7−10 | 23/74 (31.1) | 1.34 (0.58–3.14) | .49 | ||
| >10 | 13/37 (35.1) | 1.55 (0.62–3.88) | .35 | ||
| Parasite grade at VL diagnosis | |||||
| <6+ | 26/84 (30.9) | 1.0 | 1.00 | ||
| 6+ | 10/22 (45.4) | 3.22 (1.53−6.78) | .002 | 6.63 (2.64−16.63) | <.001 |
| Not done: serological/clinical diagnosisb | 8/40 (20.0) | 0.49 (0.22−1.09) | .08 | 0.49 (0.22−1.09) | .08 |
| Tuberculosis | |||||
| Yes | 10/32 (31.2) | 1.00 | |||
| No | 33/113 (29.2) | 1.00 (0.49–2.03) | .99 | ||
| ART regimen c | |||||
| Non–tenofovir-based | 24/76 (31.6) | 1.00 | |||
| Tenofovir-based | 15/64 (23.4) | 1.01 (0.53–1.94) | .98 | ||
| Timing of ART initiation | |||||
| After VL treatment | 18/41 (43.9) | 1.00 | |||
| During VL treatment | 8/40 (20.0) | 0.47 (0.20−1.07) | .07 | 0.39 (0.17−0.86) | .02 |
| Before VL treatment | 13/57 (22.8) | 0.49 (0.24−1.01) | .05 | 0.22 (0.10−0.52) | <.001 |
| Never initiated | 5/6 (83.3) | 2.04 (0.75−5.55) | .16 | ||
| Initial VL regimen | |||||
| Other (AmBisome or sodium stibogluconate) | 31/87 (35.6) | 1.00 | |||
| AmBisome and Miltefosine | 13/59 (22.0) | 0.85 (0.44–1.63) | .62 | ||
| Spleen size at discharge (cm) | |||||
| 0 | 26/81 (32.1) | 1.00 | |||
| 1–5 | 13/44 (29.6) | 1.06 (0.54–2.06) | .87 | ||
| ≥5 | 2/14 (14.3) | 0.55 (0.13–2.30) | .41 | ||
| Body mass index at discharge (kg/m 2) | |||||
| <16 | 38/118 (32.2) | 1.00 | |||
| ≥16 | 5/27 (18.5) | 0.67 (0.27–1.71) | .41 | ||
| Hemoglobin level at discharge (g/dL) | |||||
| 0 | 1/13 (7.7) | 1.00 | |||
| 7−10 | 27/76 (35.5) | 4.45 (0.60–32.8) | .14 | ||
| >10 | 15/48 (31.2) | 3.49 (0.46–26.5) | .22 | ||
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; VL, visceral leishmaniasis.
aWHO stage IV or CD4 <50 cells/µL.
b37 are primary VL cases and 3 are relapse VL cases.
cStavudine, lamivudine and nevirapine; zidovudine, lamivudine and efavirenz; tenofovir, lamivudine and efavirenz; zidovudine, lamivudine and nevirapine; stavudine, lamivudine and efavirenz.
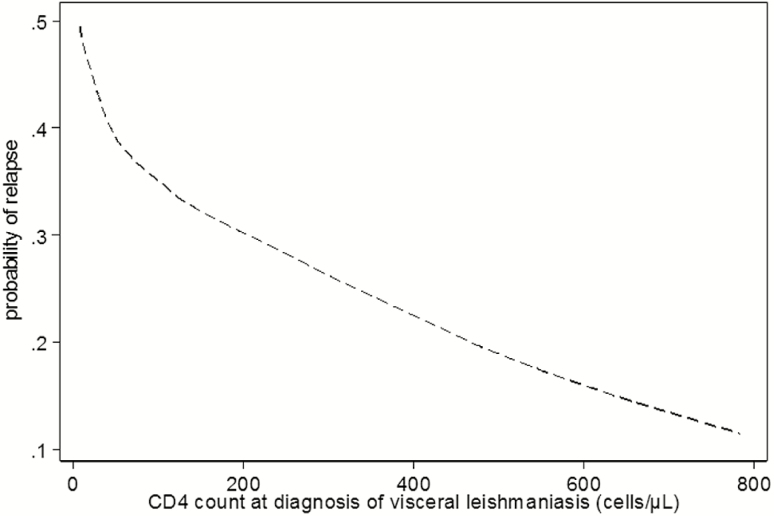
Given the high number of missing CD4 counts, this variable was not included in the main predictor analysis, but the available data demonstrated an increased risk of relapse with lower CD4 counts (Figure 3).
Figure 3.
Association between CD4 cell counts at diagnosis of visceral leishmaniasis and the estimated risk of relapse (LOWESS graph).
The predictors remained similar in competing risk and sensitivity analyses (data not shown).
DISCUSSION
In this study, we determined the risk and predictors of VL relapse in HIV coinfected patients in Ethiopia. The cumulative risk of relapse was 15% at 6 months, 26% at 12 months, and 35% at 24 months. Being on ART at VL diagnosis or starting ART during VL treatment was associated with a lower risk of relapse. Those with a high tissue parasite load (parasite grade 6+) at VL diagnosis were at increased risk.
Despite the variations across studies regarding population characteristics and setting, the 1-year risk of relapse was relatively consistent [7, 20]. In Ethiopia, ter Horst et al. [7] reported that among patients on ART, with a median follow-up duration of 7 months, the risk of relapse was 22% (definitive timeline not indicated). For patients with CD4 counts between 100 and 200 cells/µL—as was the case for most patients in our study—the 1-year risk was estimated at 15% for primary VL and 22−30% for relapse VL [7]. In India, Burza et al. [20] reported a 1-year risk of 18.5%. In our study, during the first year of follow-up, the number of relapses was highest in the first 6 months (63.3%), which is consistent with other East African studies [7, 8].
In an Ethiopian clinical trial comparing antimonials with miltefosine, the relapse rate at 6 months was 11% with antimonial therapy and 25% with miltefosine therapy [21]. Our estimate of 15% lies between these 2 extremes. In a recent single-arm trial on PSP, the risk of VL was 23% at 12 months [8] and 31% at 24 months (unpublished data). However, as this study enrolled patients deemed at the highest risk of VL relapse, the findings cannot directly be compared with our study. For instance, baseline CD4 counts were lower, more patients with (multiple) relapses were enrolled, and all cases were cured with parasitological confirmation before enrolment. A recent systematic review suggested a risk reduction of approximately 50% with secondary prophylaxis [6].
Studies on predictors of VL relapse in people living with HIV/AIDS have yielded conflicting findings [6]. In Ethiopia, ter horst et al. [7] reported that patients with a history of multiple VL episodes were at increased risk of relapse. A systematic review on predictors of relapse reported a previous history of VL relapse as one of the predictors [6]. In our study, there was no association; however, we enrolled comparatively few patients with history of VL relapse. Similarly, in 2 Indian studies [20, 22], history of VL relapse was not predictive of VL relapse.
We observed a moderate effect of ART, as was documented in another Ethiopian study [7] and comparable to an Indian study [20]. ART scaling-up is likely to reduce relapse rates. As ART also reduces mortality, timely ART initiation in all coinfected patients remains vital [23]. A high parasite grade was associated with treatment failure and mortality in Ethiopia [24] and was additionally associated with relapse in our study. Possibly, the high parasite load on admission might be associated with a higher residual (possibly submicroscopic) parasite load at discharge, although many other factors might contribute as well. For instance, the high load at diagnosis might be caused by patient factors indicating poor immunological control or by more advanced/severe VL, with more extensive damage to the immune system. Our study indicates the importance of baseline parasite load assessment for all coinfected patients, as this allows identifying those at highest risk of adverse outcomes including relapse. As coinfected patients are prone to treatment failure, parasitological testing at VL diagnosis and end of treatment could improve the evaluation of treatment response [5].
Our predictors of relapse are relatively simple indicators that can be easily identified by clinicians in most VL treatment settings, making them clinically useful. They can improve patient management by identifying those requiring closer medical follow-up or additional interventions like secondary prophylaxis. The strengths of this study include the relatively long patient follow-up and relatively low rate of lost to follow-up. The study represents the best evidence-base on the risk and predictors of VL relapse in coinfected patients in East Africa.
There are several limitations to this study. Although our study compares well to others, the risk of relapse might be underestimated due to loss to follow-up. This is inherent in studies on neglected tropical diseases conducted in remote settings in highly mobile populations. In addition, the follow-up of some patients was censored as PSP was available at the end of the study period. Without this, the relapse rate could have been higher. CD4 counts were missing for a substantial number of patients. VL diagnosis and cure were not systematically parasitological confirmed during the entire study period, reflecting current reality in field settings. As a retrospective study, we could only study predictors from among the collected variables.
In conclusion, in Ethiopia the risk of VL relapse among coinfected patients was high, particularly in those not on ART or presenting with a high tissue parasite load. These patients should be preferentially targeted for secondary prophylaxis and/or regular medical follow-up. Timely ART initiation in all coinfected patients is crucial.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the staff of Abdurafi health center and Leishmania Research and Treatment Center at the University of Gondar. C. A. has received a PhD scholarship granted from the European Union Seventh Framework Program (FP7/2007‐2013) under grant agreement n° 305178 via AfriCoLeish project.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the European Union Seventh Framework Program (FP7/2007‐2013) under grant agreement number 305178 via the AfriCoLeish project, which granted a PhD scholarship to C. A.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet 2005; 366:1561–77. [DOI] [PubMed] [Google Scholar]
- 2. Alvar J, Vélez ID, Bern C et al. ; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012; 7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvar J, Cañavate C, Gutiérrez-Solar B et al. . Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev 1997; 10:298–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alvar J, Aparicio P, Aseffa A et al. . The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 2008; 21:334–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J. Visceral leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis 2014; 8:e2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis 2011; 5:e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ter Horst R, Collin SM, Ritmeijer K, Bogale A, Davidson RN. Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis 2008; 46:1702–9. [DOI] [PubMed] [Google Scholar]
- 8. Diro E, Ritmeijer K, Boelaert M et al. . Use of pentamidine as secondary prophylaxis to prevent visceral leishmaniasis relapse in HIV infected patients, the first twelve months of a prospective cohort study. PLoS Negl Trop Dis 2015; 9:e0004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Control of the leishmaniases. 2010. [Google Scholar]
- 10. ter Horst R, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K. Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. Am J Trop Med Hyg 2009; 80:929–34. [PubMed] [Google Scholar]
- 11. Meredith SE, Kroon NC, Sondorp E et al. . Leish-KIT, a stable direct agglutination test based on freeze-dried antigen for serodiagnosis of visceral leishmaniasis. J Clin Microbiol 1995; 33:1742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Médecins Sans Frontières. Médecins Sans Frontières kala azar manual. 2014. [Google Scholar]
- 13. Ritmeijer K, ter Horst R, Chane S et al. . Limited effectiveness of high-dose liposomal amphotericin B (AmBisome) for treatment of visceral leishmaniasis in an Ethiopian population with high HIV prevalence. Clin Infect Dis 2011; 53:e152–8. [DOI] [PubMed] [Google Scholar]
- 14. Chulay JD, Bryceson AD. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg 1983; 32:475–9. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Treatment of tuberculosis: guidelines. 2010. [PubMed] [Google Scholar]
- 16. Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2008; 22:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royston P. Multiple imputation of missing values. Stata J 2004; 4:227–41. [Google Scholar]
- 18. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009; 170:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med 1997; 16:901–10. [DOI] [PubMed] [Google Scholar]
- 20. Burza S, Mahajan R, Sinha PK et al. . Visceral leishmaniasis and HIV co-infection in Bihar, India: long-term effectiveness and treatment outcomes with liposomal amphotericin B (AmBisome). PLoS Negl Trop Dis 2014; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ritmeijer K, Dejenie A, Assefa Y et al. . A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis 2006; 43:357–64. [DOI] [PubMed] [Google Scholar]
- 22. Sinha PK, van Griensven J, Pandey K et al. . Liposomal amphotericin B for visceral leishmaniasis in human immunodeficiency virus-coinfected patients: 2-year treatment outcomes in Bihar, India. Clin Infect Dis 2011; 53:e91–8. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization. The use of antiretroviral drugs for treating and preventing HIV infection. 2016. [PubMed] [Google Scholar]
- 24. Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis 2014; 8:e2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.