Our findings demonstrate that molecular epidemiologic monitoring of human immunodeficiency virus (HIV) epidemics using HIV sequence data can identify growing clusters of transmission. Once identified, in-depth analyses may discover structural factors contributing to the change in transmission dynamics addressable through public health interventions.
Keywords: Tijuana, HIV transmission, molecular epidemiology, public policy, injection drug use
Abstract
Background
North Tijuana, Mexico is home to many individuals at high risk for transmitting and acquiring human immunodeficiency virus (HIV). Recently, policy shifts by local government impacted how these individuals were handled by authorities. Here we examined how this affected regional HIV transmission dynamics.
Methods
HIV pol sequences and associated demographic information were collected from 8 research studies enrolling persons in Tijuana and were used to infer viral transmission patterns. To evaluate the impact of recent policy changes on HIV transmission dynamics, qualitative interviews were performed on a subset of recently infected individuals.
Results
Between 2004 and 2016, 288 unique HIV pol sequences were obtained from individuals in Tijuana, including 46.4% from men who have sex with men, 42.1% from individuals reporting transactional sex, and 27.8% from persons who inject drugs (some individuals had >1 risk factor). Forty-two percent of sequences linked to at least 1 other sequence, forming 37 transmission clusters. Thirty-two individuals seroconverted during the observation period, including 8 between April and July 2016. Three of these individuals were putatively linked together. Qualitative interviews suggested changes in policing led individuals to shift locations of residence and injection drug use, leading to increased risk taking (eg, sharing needles).
Conclusions
Near real-time molecular epidemiologic analyses identified a cluster of linked transmissions temporally associated with policy shifts. Interviews suggested these shifts may have led to increased risk taking among individuals at high risk for HIV acquisition. With all public policy shifts, downstream impacts need to be carefully considered, as even well-intentioned policies can have major public health consequences.
Tijuana is a city of 1.6 million people located in the Mexican state of Baja California along the United States–Mexico border [1], with a large transient population, thriving “red light” district, and dynamic illicit drug market [2–4]. Adjacent to Tijuana’s red-light district is the Tijuana River and canal basin, locally called “El Bordo.” Many homeless individuals, up to 4000 at any one time, have historically lived in this region, including deportees from the United States and migrants from Central America [5–7]. Adjacent to this border district is Tijuana’s Zona Centro, a neighborhood with numerous nightclubs that cater to tourists, locals, and gay men. This mix of drugs, sex, and homelessness has placed already vulnerable populations at high risk for acquiring and transmitting human immunodeficiency virus (HIV) [8–10].
In 2015, the Mexican government began to expand the San Ysidro border crossing between the United States and Mexico, and as part of these efforts began to “clean” the homeless population from El Bordo to improve “public safety” [5, 11]. This was accomplished by orchestrated police raids and patrols, and bulldozing of erected dwellings in the area. Many residents were sent to rehabilitation facilities, often involuntarily [12]. Those who wished to avoid placement in rehabilitation facilities were forced to move, and the canal was made inaccessible to pedestrians.
We hypothesized that this dispersal of high-risk individuals and disruption of prevention efforts would change the dynamics of HIV transmission in the region. To evaluate this hypothesis, we used data from cohorts of high-risk individuals (eg, persons who inject drugs [PWID], female sex workers [FSWs], and men who have sex with men [MSM]) [13, 14] collected since 2004 in Tijuana, coupled with near real-time molecular epidemiologic monitoring, to examine the impact of recent public safety measures on the local dynamics of HIV transmission. Herein we describe an outbreak of incident HIV infections among high-risk individuals in Tijuana, which has important implications with respect to unintended consequences of public safety policies.
METHODS
Study Design
To examine the effect of a public policy shift instituted in March 2015 on the regional HIV epidemic, we examined data from 8 research studies in Tijuana screening individuals (n = 2759) for HIV infection between 2004 and 2016. These studies included individuals from multiple risk groups including male clients of FSWs (Amigos and Hombre Seguro), FSWs (Mujer Segura), injection drug–using FSWs (Mujer Mas Segura), FSWs and their noncommercial partners (Proyecto Parejas), a historical study of HIV-infected individuals in Tijuana (Study of HIV Drug Resistance), MSM (Enlaces), prison inmates (La Mesa Health Study), and PWID (Proyecto El Cuete IV [EC]). Participants in Hombre Seguro and Mujer Mas Segura were followed for 1 year with quarterly screening for HIV. Participants in Parejas were followed for 2 years with HIV screening every 6 months. Participants in EC were screened biannually for HIV infection over the last 6 years. All other participants were only screened at a baseline study visit. All individuals identified with HIV infection were included in this analysis (Tables 1 and 2; Figure 1).
Table 1.
Descriptions of Participating Cohorts in Tijuana, Mexico
| Cohort | Description | Dates |
|---|---|---|
| Amigos | Cross-sectional study of male clients of FSW in partnership with the Hombre Seguro study who were ≥18 years old; reported heroin, methamphetamine, or cocaine use; and had paid or traded something of value for sex with a FSW in Tijuana in the past 4 months. | June 2011–August 2012 |
| El Cuete IV | A longitudinal cohort of PWID that used convenience sampling. | 2010–present |
| Hombre Seguro | A study of male clients of FSW that used convenience sampling. | September 2010–October 2012 |
| Mujer Segura and Mujer Más Segura | A research project studying FSW in Tijuana, some of who used injection drugs. The 2 studies used time location, convenience, and targeted sampling approaches. | 2004–2012 |
| Proyecto Parejas | A research project studying FSW and their noncommercial partners in the Tijuana, Mexico, that used convenience sampling. | 2010–2013 |
| Drug Resistance in Tijuana | A cross-sectional study of drug resistance in chronically HIV-infected individuals in Tijuana. | 2013 |
| Enlaces | A research project comparing partner contact tracing vs venue-based recruitment for identifying undiagnosed men who have sex with men in Tijuana. | 2014–present |
| La Mesa Study | A research project to assess education and perception about HIV in prisoners in Tijuana. | 2016 |
Abbreviations: FSW, female sex worker; HIV, human immunodeficiency virus; PWID, persons who inject drugs.
Table 2.
Breakdown of Data Used in Analysis
| Cohort | Total Participants | Participants With HIV | Incident HIV Cases | Participants With HIV Sequence Data | Gender | HIV Risk Factor | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | TG | MSM | Bisexual | Heterosexual | PWID | |||||
| Amigos | 224 | 9 | NAa | 3 | 3 | 0 | 3 | 1 | |||
| El Cuete | 734 | 50b | 24b | 37b | 20 | 17 | 1 | 2 | 34 | 37 | |
| Hombre Seguro | 215 | 13 | 1 | 12 | 12 | 0 | 4 | 8 | 2 | ||
| Mujer Segura and Mujer Más Segura | 776 | 56 | 2 | 31 | 0 | 31 | 31 | 6 | |||
| Proyecto Parejas | 212 | 13b | 6b | 9b | 4 | 5 | 1 | 8 | 1 | ||
| Drug Resistance in Tijuana | 81 | 81 | NAc | 81d | 41 | 21 | 20 | 6 | 36 | 9 | |
| Enlaces | 114 | 114 | NAc | 109e | 77 | 0 | 13 | 58 | 21 | 11 | 13 |
| La Mesa | 403 | 7 | NAa | 7 | 2 | 5 | 7 | 1 | |||
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; NA, not applicable; PWID, persons who inject drugs; TG, transgender.
aNot applicable as these were only cross-sectional studies.
bOne participant present in both El Cuete and Parejas cohorts.
cNot applicable, as the entry criteria included a new diagnosis of HIV.
dOnly 62 of the participants with HIV sequence data had available demographic data.
eOnly 100 of the participants with HIV sequence data had available demographic data.
Figure 1.
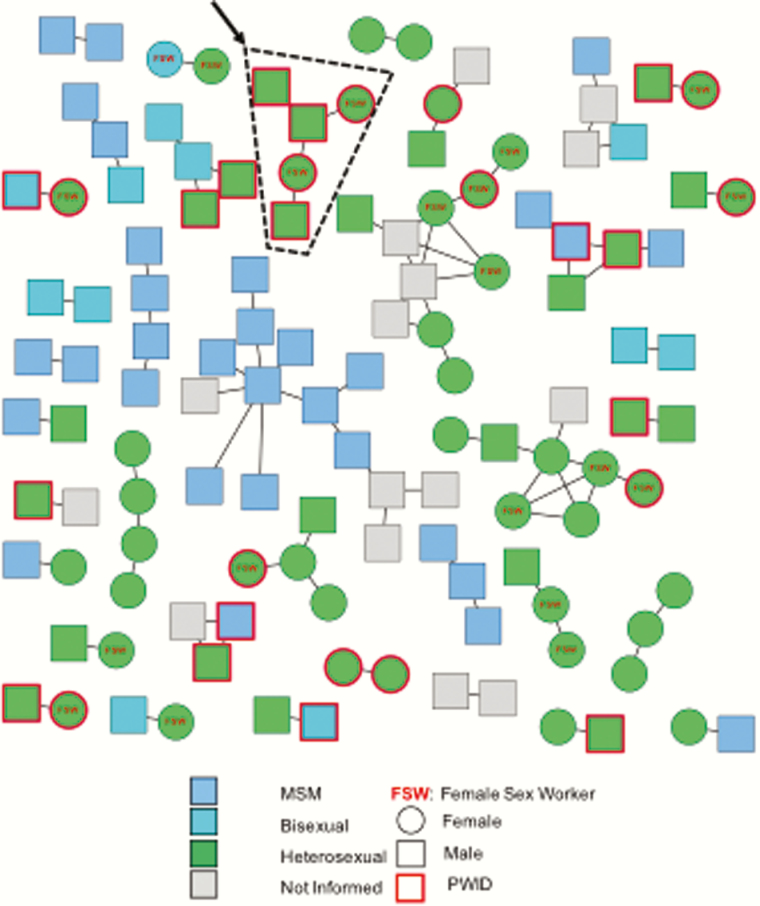
Human immunodeficiency virus transmission network identified in Tijuana, Mexico, between 2004 and 2016. Polygons represent individuals, with the actual shape representing sex, and color representing sexuality. People who inject drugs (PWID) are signified with a red border. Lines connecting polygons represent inferred putative transmission links. Black arrow and dashed line indicate the identified cluster of 5 PWID seroconverters diagnosed in 2012 (n = 2) and between April and May 2016 (n = 3). Abbreviations: FSW, female sex worker; MSM, men who have sex with men; PWID, persons who inject drugs.
Partial HIV type 1 (HIV-1) pol sequence data was generated from blood samples collected from HIV-infected participants as previously described [14], followed by quality filtering and subtyping [15–17]. For each participant, only the HIV sequence obtained from the earliest time point was included. When available, demographic, clinical, and geographic (ie, locations of residence, injection drug use [IDU], and interactions with law enforcement) data were extracted from the de-identified study databases of the participating programs as previously described [13].
Network Analysis
An HIV transmission network was inferred by measuring the genetic distance between all sequences and establishing a putative transmission linkage when the TN93 genetic distance between sequences was <1.5% [18–20]. While these inferred connections may not represent true transmission links, they strongly suggest that the individuals were part of the same transmission chain. Sociodemographic correlates of clustering were analyzed by comparing the characteristics of clustering and nonclustering individuals. Categorical variables were compared using Fisher exact test; continuous variables were evaluated using nonparametric tests.
Subanalysis of Seroconverting PWID
A qualitative analysis was conducted to better understand the impact of recent policy changes on HIV-related behaviors among recent HIV seroconverters in the Parejas and EC studies. HIV seroconverters were defined as those for whom a last HIV-seronegative and first HIV-seropositive test was documented during study follow-up. The subanalysis was limited to these studies because all the recent HIV seroconversions were from these cohorts. Seroconverters who provided consent to be contacted for participation in future studies were approached to participate in this substudy. Semistructured qualitative interviews, ranging from 60 to 90 minutes, were conducted in Spanish with individuals by 3 interviewers who had extensive experience working with vulnerable populations. In-depth interview guides were designed to elicit information on engagement in risk behaviors prior to seroconversion, HIV care and treatment experiences, and social and structural barriers to care. Through biweekly discussions and ongoing analysis of the translated transcripts, investigators determined when conceptual saturation was reached, whereby additional interviews would not elicit new information on the topics of interest [21]. Qualitative analysis involved team members reading through selected transcript excerpts independently and generating a list of codes based on the interview guide (ie, deductive) and emergent themes (ie, inductive). Analysts coded each transcript using MAXQDA 12 (VERBI, Marburg, Germany) and met regularly to discuss and refine codes as needed.
EC participants also underwent interviewer-administered surveys during biannual study visits, where they reported the neighborhood where they primarily had been injecting drugs during the prior 6 months. This information was recorded on paper-based maps and digitized in ArcGIS 10.3.1 (ESRI, Redlands, California) [22]. At each interview EC participants were also asked about any police interactions including incidents of abuse. These encounters were dichotomized into extrajudicial (ie, being sexually or physically assaulted) and judicial encounters with police (ie, being detained or arrested). The physical locations were recorded using Google Street View [22], then aggregated to neighborhood level. Using all waves of follow-up data, we examined the geographic distribution of injection locations and police encounters across neighborhoods using ArcGIS. However, our sample size did not provide sufficient power for statistical comparison in the subset analysis (n = 24) and therefore descriptive statistics were generated to examine shifts in IDU locations and police encounters relative to a participant’s date of HIV seroconversion.
RESULTS
Two hundred eighty-eight sequences were obtained from unique individuals sampled between 2004 and 2016; 262 (91.0%) were HIV-1 subtype B, and 26 (9.0%) were non-B (including 11 CRF29, 8 CRF3, 4 CRF28, and 5 others). These represent 5%–16% of the estimated cases in Tijuana [23]. Among individuals with reported demographic data, 46.4% (116/250) were MSM, 30.5% (80/262) were women, 42.1% (99/235) reported transactional sex, and 27.8% (65/234) were PWID. A total of 42.4% (122/288) of sequences linked to at least 1 other sequence, forming 37 transmission clusters (range, 2–14 individuals) (Figure 1). This included a transmission cluster made up of 5 PWID, including 3 individuals with recent seroconversion. Clustering status was not associated with age, IDU, history of syphilis, transactional sex, or deportation; only female sex was associated with clustering (P = .04) (Table 3). A total of 13.5% (5/37) of inferred clusters included only women. There was some segregation of clustering by risk group, with PWID comprising 100% of 5 clusters and MSM comprising 100% of another 5 clusters; however, there was mixing of risk factors in other clusters, with 12 other clusters including at least 1 PWID.
Table 3.
Comparison of Clustering and Nonclustering Study Participants
| Characteristic | Total | Not Clustering | Clustering | P Value |
|---|---|---|---|---|
| No. | 288 | 166 (57.6) | 122 (42.7) | |
| Age, y, median (IQR) | 33 (28–40) | 33.5 (28–42) | 33 (26–39) | ns |
| Gender | ||||
| Male | 166/262 (63.4) | 103/147 (70.1) | 63/115 (54.8) | .01 |
| Female | 80/262 (30.5) | 37/147 (25.8) | 43/115 (37.4) | .04 |
| Transgender | 16/262 (6.1) | 7/147 (4.8) | 9/115 (7.8) | ns |
| Sexual behavior | ||||
| MSM | 126/261 (48.3) | 76/147 (51.7) | 50 (43.9) | ns |
| MSW and WSM | 135/261 (51.7) | 71/147 (48.3) | 64 (56.1) | |
| Injection drug use | 69/235 (29.4) | 41 /138 (29.7) | 28/97 (28.9) | ns |
| History of syphilis | 22/60 (36.7) | 11/34 (32.4) | 11/26 (42.3) | ns |
| Transactional sex | 99/236 (41.9) | 57/138 (41.3) | 42/98 (42.9) | ns |
| Deportation | 58/232 (24.6) | 35/136 (25.7) | 23/96 (24.0) | ns |
| Study | ||||
| Amigos/Hombre Seguro | 15/288 (5.2) | 8/166 (4.8) | 7/122 (5.7) | |
| Alicia | 7/288 (2.4) | 2/166 (1.2) | 5/122 (4.1) | |
| El Cuete | 37/288 (12.8) | 24/166 (14.5) | 13/122 (10.7) | |
| Drug resistance in Tijuana | 81/288 (28.1) | 57/166 (34.3) | 24/122(19.7) | <.01 |
| Enlaces | 108/288 (37.5) | 60/166 (36.1) | 48/122 (39.3) | |
| Mujer Segura/Mas Mujer Segura | 31/288 (10.8) | 12/166 (7.2) | 19/122 (15.6) | .03 |
| Proyecto Parejas | 9/288 (3.1) | 3/166 (1.8) | 6/122 (4.9) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; MSM, men who have sex with men; MSW, men who have sex with women; ns, not significant; WSM, women who have sex with men.
HIV Seroconversions
Incident infections were identified during the follow-up phase of the studies (ie, observed HIV seroconversion) (Supplementary Materials). Six participants of the Parejas study seroconverted, including 5 of whom reported IDU within 6 months of seroconversion, and 4 with HIV sequences that clustered with at least 1 other participant. During the 2-year follow-up of the Parejas study, only 3 of 6 seroconverters initiated and were adherent to their antiretroviral therapy (ART) regimen. Twenty-four EC participants seroconverted during follow-up, including 1 participant who was enrolled in both the Parejas and EC studies. Twelve of 24 (50%) of seroconversions occurred prior to 2015. Sequence data were available for 15 of 24 (62.5%) of the participants who seroconverted. Among individuals who seroconverted prior to 2015 and for whom sequences were available (n = 8/12), 4 (50.0%) were genetically linked to another infection. From 2015 into 2016, we identified 12 additional seroconversions in the EC cohort, defined here as recent seroconversions. From these 12 individuals, only 7 (58.3%) sequences were available. Three of these 7 participants (42.9%), diagnosed with HIV in April and May of 2016, linked together and to a pair of other PWID who seroconverted in 2012. The other 4 recent seroconverters with available sequence data did not share inferred linkages with other cohort members. Three additional seroconverters were identified from 2 of the other studies (Hombre Seguro and Mujer Mas Segura), but only 1 HIV sequence was available. Overall, seroconverters were not more likely to cluster than the rest of the sampled population (11/21 [52.4%] vs 111/267 [41.7%]; P = .37).
Seroconversions in PWID
From 2015 through July 2016, we noticed an increase in the frequency of HIV seroconversions in the EC cohort (n = 12 recent seroconversions, including 8 from April to July of 2016). Qualitative interviews (n = 19 [13 women and 6 men]) of past and recent seroconverters provided a common theme, which was the need to relocate both residence and location of IDU after the public safety intervention in the El Bordo region. In the interviews, individuals who remained after the cleaning of El Bordo reported being forced to find new venues for their IDU. Some described moving east in Tijuana to another shooting gallery (gallery 1, Figure 2). These participants noted that even in this location, local law enforcement officials continued to perform daily raids to keep the canal basin clear of PWID and homeless individuals, leading to further dispersal of these individuals. Several interviewees also stated that shooting galleries in Tijuana’s Zona Norte continued to be under Federal Police and Army surveillance, also causing mobilization of street-based PWID living in Zona Norte. Per the interviewees, a significant number of these individuals ended up moving to inject nearby in galleries 2 and 3 (Figure 2). More recently, the interviewees stated that gallery 1 was now completely shut down after the installation of a 24/7 checkpoint controlled by the Federal Police. This has led most new arrivals to the El Bordo region, including those released or who escaped from rehabilitation centers, to find their way to gallery 2. The interviewees also noted that, after the second stage of the canal raids in May–June 2016 and initiation of the construction of a new international pedestrian crossing, gallery 2 has become one of the main locations for drug distribution in Tijuana.
Figure 2.
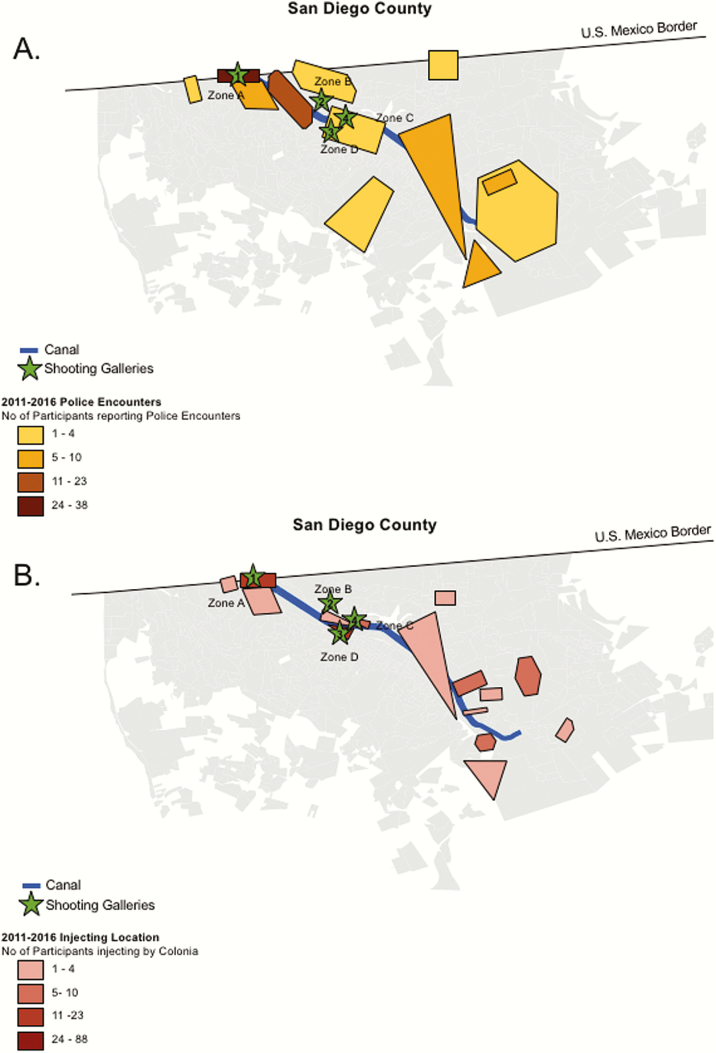
Schematic map of the frequency of police encounters (A) and injection locations (B) by colonia (neighborhood) in Tijuana among incident human immunodeficiency virus cases in the Proyecto El Cuete IV study during 2011–2016. Data were collected from interviews performed at study visits every 6 months.
Qualitative findings were supported by quantitative surveys conducted biannually, as 63% (15/24) of EC seroconverters reported shifting injection locations during the follow-up period and, of these, 47% (7/15) moved to nonadjacent neighborhoods. Of the recent seroconverters, one-third (4/12) moved injection locations during the period of seroconversion, traveling an average of 6.81 km (standard deviation, 8.88 km) (Supplementary Table 2). In addition, all EC seroconverters reported a police encounter during the follow-up period, with the majority reporting an occurrence within 1 year of HIV diagnosis (83% [20/24]). A majority of these encounters involved an arrest or detention (75% [18/24]), but a quarter (6/24) reported being physically or sexually assaulted during their interaction with police. Furthermore, such extrajudicial encounters were more likely to occur within 1 month of seroconversion (67% [4/6]) than judicial encounters (28% [5/18]), but this was not statistically significant due to the small sample size.
We next examined spatial overlap between location of drug use and police encounters among the 24 EC seroconverters, covering the period 2011–2016 (Figure 2). To protect the identity and geolocation of study participants, the physical location of events were masked by transforming and rotating the original locations. Most of these encounters, judicial and extrajudicial, occurred along the Tijuana river canal. The highest frequency occurred in the Zona Norte neighborhood, which is located adjacent to the Mexico–US border and includes the “El Bordo” region. Similarly, most seroconverters reported IDU in neighborhoods near the Tijuana river canal, most commonly in neighborhoods demarcated by zones B, C, and D. Overall, the neighborhoods where PWID interacted with police closely overlapped with the neighborhoods where PWID primarily use drugs (Pearson correlation coefficient, r = 0.85).
DISCUSSION
While migration and deportation continue to play an important role in the HIV epidemic in Tijuana [13, 14], structural factors may also be impacting this local epidemic. The concentration of individuals engaging in high-risk behaviors, as in the red-light and border districts of Tijuana, continues to propel ongoing HIV transmission. These risks have been partly addressed by the work of a few nongovernmental organizations (NGOs) working to prevent further transmission through screening and harm reduction services. Here, we examined the impact of public safety measures designed to shift the homeless population along the border on the dynamics of HIV transmission in this local epidemic using near real-time molecular epidemiologic methods.
Our molecular epidemiologic analysis demonstrated a high proportion of clustered infections among the sampled population (>40%), similar to rates of clustering in other densely sampled populations [19, 24]. This proportion was higher (~50%) but not significantly different in the subgroup of incident infections, suggesting that less than half of new transmissions were likely from unsampled infected individuals [25]. Our analyses of these incident infections found an active transmission cluster in early to mid-2016, comprised of several linked infections in individuals with primary HIV infection, a group known to be more infectious and at higher risk for transmission, due to high viral loads [26–28]. This cluster was comprised entirely of PWID, with sequences from 3 recently infected individuals linked to 2 heroin-injecting PWID who seroconverted in 2012. This is not surprising, as <5% of HIV-infected persons sampled in some of these studies were receiving ART [29]. Based on this molecular epidemiologic surveillance information, federal and state public health authorities in Tijuana, Mexico, were notified. To better understand this outbreak, we used both qualitative interviews and geospatial analysis to identify and explore structural factors that could be associated with these transmissions.
According to our interviews, after being displaced from “El Bordo” by city urban development projects and the corresponding public safety policy shift toward efforts to “clean” the Tijuana river and canal basin along the US–Mexico border in March 2015, PWID and homeless people were forced to relocate [6, 11]. Some were forced into drug rehabilitation in the outskirts of Tijuana and further south in Baja California [6, 11]. Participants described police encounters where individuals found with drug paraphernalia would be sent to city jail and belongings confiscated. Some participants described extrajudicial encounters where police would request money or sexual favors as a condition to not be detained. Fear of these encounters has led PWID to avoid needle exchange programs and shift their IDU to new “underground” shooting galleries away from the canal region. Displacement and relocation, as described by many PWID, were identified as structural factors possibly contributing to onward HIV transmission. Research in other populations of PWID has shown that disruption of social networks, particularly the introduction of new contacts as may result from relocation to new shooting galleries, is associated with increased probability of engaging in high-risk IDU [30].
Geospatial analysis and survey data corroborated the information provided to us by our interviewees. Using data collected during the biannual study visits for HIV incident cases in the EC study, we found a high degree of overlap between the neighborhoods where PWID used drugs and the locations where PWID reported police encounters, consistent with the policy shift to clean these areas of homeless and PWID. Moreover, more than half of the 24 EC seroconverters moved injection locations during the study period, and most reported a police encounter in the year prior to HIV diagnosis. Although only an association, we suspect that some of this mobility was reflective of the policing environment and, more specifically, the policy shift toward removing the homeless from El Bordo and known shooting galleries. Consistent with this, 3 of our recently identified clustering individuals described an interaction with police in the year before HIV diagnosis, followed by shifting their IDU to a different shooting gallery where they felt safe from the police. Notably, this shooting gallery was away from NGOs offering harm reduction services. Thus, the dispersion of individuals from locations known and frequented by NGOs hampered efforts to provide these services.
This analysis was limited by small sample size, focused sampling of high-risk individuals, and mix of sampling techniques used by the participating studies [13]. While a more dense and random sampling of the population would have provided more insight into the entire Tijuana HIV epidemic, in our focused population we identified a clustering rate of approximately 40%, similar to other molecular epidemiologic studies of regional epidemics [19, 24, 31]. Thus, while we could not capture changes in transmission dynamics across this complex epidemic, our results provide important insights in this high-risk subpopulation. Further, in 2014, Mexico transitioned from Global Fund support to government support of its HIV prevention programs by virtue of its middle-income status. This has led to widespread reports of shortages in sterile syringes and fewer condoms available to high-risk populations. This change may also be contributing to shifts in the current dynamics of HIV transmission in the region.
While migration and high-risk behavior remain important drivers of the Tijuana HIV epidemic, these results highlight the importance of local structural factors in changing the dynamics of transmission. While well-intentioned, these changes in policy toward high-risk homeless and PWID living in the border region, and efforts to relocate and involuntarily commit individuals to rehabilitation, has driven some high-risk behaviors underground, where neither the government nor NGOs are able to provide adequate harm reduction support (ie, needle exchange services). We believe these shifts led to increased risk-taking behavior (ie, needle sharing), resulting in the observed outbreak. This reduction in access has been compounded by the end of Mexico’s Global Fund support, which sponsored many harm reduction services. The implications of such policy changes need to be carefully considered, as significant downstream effects, such as increased HIV transmission, may occur. Continued serologic and molecular surveillance of high-risk populations will be critical to the early identification of new outbreaks and epidemiologic trends necessitating public health responses.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. S. R. M. coordinated the data collection, analyzed the demographic data, and wrote the report. A. C. analyzed the sequence data and generated figures. T. L. G. performed the geospatial analysis. P. E. G.-Z. collected samples and demographic data, and performed and analyzed the qualitative interviews. J. K. S. assisted with analysis of the qualitative interviews. H. A.-R., J. R. C., A. V., K. D. W., and T. L. P. collected and provided sequence and demographic data. D. M. S. helped design the study and edit the report. S. A. S. collected and provided sequence and demographic data, helped design the study, and edited the report.
Acknowledgments. We thank Joel O. Wertheim and Steven Weaver for assistance with the clustering analysis, and Susan Little and Richard Garfein for providing access to HIV sequence data from participants in San Diego.
Financial support. This work was supported by the National Institutes of Health (grant numbers K23AI093163 to S. R. M., K01DA031031 to K. D. W., K01DA034523 to T. L. G., R37DA019829, R01DA027772, and R01DA023877 to S. A. S., DP1DA034978 to D. M. S., and P30AI036214, R01DA029008, R01MH065849, and R01MH087054 to T. L. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Instituto Nacional de Estadística Geografía e Informática de Mexico. Geografia. 2011. Available at: www.inegi.org.mx. Accessed 1 February 2017.
- 2. Magis-Rodríguez C, Lemp G, Hernandez MT, Sanchez MA, Estrada F, Bravo-García E. Going north: Mexican migrants and their vulnerability to HIV. J Acquir Immune Defic Syndr 2009; 51(Suppl 1):S21–5. [DOI] [PubMed] [Google Scholar]
- 3. O’Connor A-M. Mexico's City of Promise: Immigrants from across the country are pouring into Tijuana. Los Angeles Times, 26 January 1998. [Google Scholar]
- 4. Strathdee SA, Magis-Rodriguez C, Mays VM, Jimenez R, Patterson TL. The emerging HIV epidemic on the Mexico-U.S. border: an international case study characterizing the role of epidemiology in surveillance and response. Ann Epidemiol 2012; 22:426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archiold RC. As Mexican border town tries to move on, some are stuck in limbo. New York Times, 27 November 2014. [Google Scholar]
- 6. Zabludovsky K. The two faces of Tijuana. BuzzFeed News; 2016. Available at: https://www.buzzfeed.com/karlazabludovsky/tijuana-wants-you-to-forget-everything-you-know-about-it?utm_term=.qrDOYM30v#.efpoVaJ7B. Accessed 1 February 2017. [Google Scholar]
- 7. Nieves R. Stuck between two countries. CNN; 22 February 2014. Available at: http://www.cnn.com/2014/02/21/us/u-s-mexico-border-purgatory/index.html. Accessed 1 February 2017. [Google Scholar]
- 8. Zhang X, Martinez-Donate AP, Simon NE et al. . Risk behaviours for HIV infection among travelling Mexican migrants: the Mexico-US border as a contextual risk factor. Glob Public Health 2016; 12:65–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Martinez-Donate AP, Nobles J, Hovell MF, Rangel MG, Rhoads NM. Substance use across different phases of the migration process: a survey of Mexican migrants flows. J Immigr Minor Health 2015; 17:1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rangel MG, Martinez-Donate AP, Hovell MF et al. . A two-way road: rates of HIV infection and behavioral risk factors among deported Mexican labor migrants. AIDS Behav 2012; 16:1630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suarez M. Where have all the squatters gone? San Diego Reader, 14 March 2015. [Google Scholar]
- 12. Guerrero J. Tijuana mandates drug treatment for hundreds of homeless. KPBS, 2015. Available at: http://www.kpbs.org/news/2015/apr/13/tijuana-homeless-get- compulsory-treatment/. Accessed 1 February 2017. [Google Scholar]
- 13. Mehta SR, Wertheim JO, Brouwer KC et al. . HIV transmission networks in the San Diego-Tijuana border region. EBioMedicine 2015; 2:1456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta SR, Delport W, Brouwer KC et al. . The relatedness of HIV epidemics in the United States–Mexico border region. AIDS Res Hum Retroviruses 2010; 26:1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gifford RJ, Liu TF, Rhee SY et al. . The calibrated population resistance tool: standardized genotypic estimation of transmitted HIV-1 drug resistance. Bioinformatics 2009; 25:1197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kosakovsky Pond SL, Posada D, Stawiski E et al. . An evolutionary model-based algorithm for accurate phylogenetic breakpoint mapping and subtype prediction in HIV-1. PLoS Comput Biol 2009; 5:e1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose PP, Korber BT. Detecting hypermutations in viral sequences with an empha- sis on G –> A hypermutation. Bioinformatics 2000; 16:400–1. [DOI] [PubMed] [Google Scholar]
- 18. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993; 10:512–26. [DOI] [PubMed] [Google Scholar]
- 19. Little SJ, Kosakovsky Pond SL, Anderson CM et al. . Using HIV networks to inform real time prevention interventions. PLoS One 2014; 9:e98443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wertheim JO, Leigh Brown AJ, Hepler NL et al. . The global transmission network of HIV-1. J Infect Dis 2014; 209:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods 2005; 18:59–82. [Google Scholar]
- 22. Beletsky L, Arredondo J, Werb D et al. . Utilization of Google enterprise tools to georeference survey data among hard-to-reach groups: strategic application in international settings. Int J Health Geogr 2016; 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brouwer KC, Strathdee SA, Magis-Rodríguez C et al. . Estimated numbers of men and women infected with HIV/AIDS in Tijuana, Mexico. J Urban Health 2006; 83:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chan PA, Hogan JW, Huang A et al. . Phylogenetic investigation of a statewide HIV-1 epidemic reveals ongoing and active transmission networks among men who have sex with men. J Acquir Immune Defic Syndr 2015; 70:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grabowski MK, Lessler J, Redd AD et al. . Rakai Health Sciences Program The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS Med 2014; 11:e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiridou M, Geskus R, de Wit J, Coutinho R, Kretzschmar M. Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS 2004; 18:1311–20. [DOI] [PubMed] [Google Scholar]
- 27. Quinn TC, Wawer MJ, Sewankambo N et al. . Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 28. Koopman JS, Jacquez JA, Welch GW et al. . The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 14:249–58. [DOI] [PubMed] [Google Scholar]
- 29. Smith LR, Patterson TL, Magis-Rodriguez C et al. . Engagement in the HIV care continuum among key populations in Tijuana, Mexico. AIDS Behav 2016; 20:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costenbader EC, Astone NM, Latkin CA. The dynamics of injection drug users’ personal networks and HIV risk behaviors. Addiction 2006; 101:1003–13. [DOI] [PubMed] [Google Scholar]
- 31. Dennis AM, Hué S, Hurt CB et al. . Phylogenetic insights into regional HIV transmission. AIDS 2012; 26:1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.