Summary
This study represents a novel observational endeavor utilizing a population-based patient registry linked with geographically mapped viral surveillance data to assess the impact of influenza and respiratory syncytial virus on severe infective pulmonary exacerbations in children and adults with cystic fibrosis.
Keywords: cystic fibrosis, influenza virus, respiratory syncytial virus, pulmonary exacerbations, surveillance
Abstract
Background.
Characterization of the role of respiratory viral pathogens on cystic fibrosis (CF) pulmonary disease is needed. We aimed to determine the association of influenza and respiratory syncytial virus (RSV) activity with risk of pulmonary exacerbation (PEx) in persons with CF in the United States.
Methods.
We conducted a cohort study from January 2003 to March 2009 using the CF Foundation Patient Registry merged with Centers for Disease Control and Prevention respiratory virus surveillance data. The primary goal was to determine the association between regional influenza or RSV detections with risk of PEx requiring intravenous antibiotics or hospitalization. We analyzed outcomes by geographic region and week of event using multivariable regression models adjusted for demographic and clinical predictors of PEx stratified for children (<18 years) and adults (≥18 years) to calculate relative risks (RRs) of PEx.
Results.
There were 21022 individuals (52% male) in the CF patient cohort in 2003 comprised of 12702 children and 8320 adults. The overall incidence rate of PEx was 521.9 per 10000 person-months. In children, a 10% increase in the proportion of surveillance tests positive for influenza or RSV was significantly associated with increased PEx risk (RR, 1.02; 95% confidence interval [CI], 1.01–1.03) and (RR, 1.05; 95% CI, 1.02–1.07), respectively. In adults, surveillance tests positive for influenza (RR, 1.02; 95% CI, 1.01–1.02), but not RSV (RR, 0.99; 95% CI, .98–1.01), had a significant association with PEx risk.
Conclusions.
Our large CF population–based cohort demonstrated a significant association between PEx risk and influenza activity in children and adults and with RSV activity in children.
Cystic fibrosis (CF) remains the most common genetic fatal condition among white individuals and affects approximately 34000 persons in the United States [1]. CF is characterized by chronic pulmonary disease that ultimately progresses to respiratory failure, which accounts for much of the morbidity and mortality of the disease [2]. CF pulmonary disease is punctuated by acute exacerbation episodes wherein patients may develop increased respiratory or systemic symptoms and decreased forced expiratory volume in 1 second (FEV1) [3]. Pulmonary exacerbations in CF may arise as a consequence of reduced host immunity, resulting in altered airway microbiology and impaired airway clearance [3]. Pulmonary exacerbations are common and are associated with progression to end-stage lung disease and increased mortality. Therefore, efforts to improve interventions, prevention, early identification, and management of pulmonary exacerbations of pulmonary exacerbations in CF are critical [4, 5].
Bacterial pathogens including Pseudomonas aeruginosa and Burkholderia cepacia complex have been implicated in pulmonary exacerbations and disease progression [6, 7]. However, respiratory viruses have been increasingly observed in association with exacerbation events and disease progression [8–11], though their etiologic role and impact on CF lung disease remains to be further delineated [12–14]. Of a number of viral pathogens, influenza and respiratory syncytial virus (RSV) are common, and have been demonstrated to have an association with CF morbidity [10, 15, 16]. Influenza viruses have been identified in sputum samples collected during CF exacerbation episodes [17], and the incidence of exacerbations increases during the influenza season [9]. RSV has been identified in infants and young children with lower respiratory infections, including among persons with CF [15, 18] and, more recently, in high-risk adults and the elderly [19].
Prior studies of association between influenza virus with CF pulmonary exacerbations did not necessarily prove causation. Most evidence comes from small surveillance studies insufficiently powered to demonstrate a clinically meaningful association between respiratory viruses and CF morbidity [10–12], or from ecological studies that fail to adjust for individual patient risks [20]. Our objective was to determine the population-level effect that seasonal influenza and RSV independently have on risk of serious pulmonary exacerbations in children and adults with CF in the United States. We hypothesized that regional seasonal influenza and RSV activity would be independently associated with increased risk of pulmonary exacerbations.
METHODS
Study Population
The CF Foundation Patient Registry (CFFPR) is a population-based registry of all consenting CF patients in the United States. It contains longitudinal encounter-based data relating to demographic, clinical, and socioeconomic parameters [1, 2]. Since 2003, encounter-based data are collected at each outpatient clinical assessment, during outpatient antibiotic therapy administration, during hospitalizations, and at annual reviews of patients followed at accredited CF centers (>115). In a 2012 CFFPR audit, patient data were highly accurate, and >90% of clinical visits and hospitalizations were documented [1]. Of an estimated 33292–34327 individuals with CF in the United States in 2012, the most recent year with available birth and mortality data, 81%–84% of these patients were captured by the registry [2]. Our study included individuals with CF in the CFFPR from 2003 to 2009 merged on a temporal and geographic basis with the US Centers for Disease Control and Prevention (CDC) virologic surveillance data on influenza and RSV tests performed and positive detections [21, 22]. As the CFFPR represents a dynamic cohort, baseline characteristics for the study cohort were from the 2003 annual review. The study was approved by the research ethics board at the University of Washington (institutional review board 18243) and the CF Foundation Patient Registry Committee.
Exposure Assessment
We accessed influenza surveillance data from the United States–World Health Organization (WHO) Collaborating Laboratories and National Respiratory and Enteric Virus Surveillance System (NREVSS) Laboratories from the CDC website (www.cdc.gov/surveillance/nrevss) [23]. We acquired RSV data from NREVSS by a data use request [22]. For influenza, all state public health laboratories, in addition to some county public health laboratories and some large tertiary-care medical centers, participate as WHO collaborating laboratories (http://www.cdc.gov/flu/weekly/fluactivitysurv.htm). The participating laboratories directly report the total numbers of tested and positive specimens by reverse-transcription polymerase chain reaction (RT-PCR) for influenza types A and B on a weekly basis to the CDC [24]. Most WHO collaborating laboratories report influenza A subtype information but do not distinguish influenza B lineage. Data linkage to individuals including clinical or epidemiologic data is not available. The NREVSS is a laboratory-based surveillance system operating on a voluntary basis to collect weekly numbers of tested and positive specimens for RSV and other respiratory or enteric viruses [22]. During the study period from January 2003 through March 2009, laboratories reporting RSV results by antigen detection for ≥30 weeks and averaging ≥10 weekly specimens were included in the analysis [22]. The majority of NREVSS reporting laboratories (85%–90%) are hospital-based laboratories, with the remainder comprised of commercial and public health laboratories.
Influenza and RSV surveillance data were collected on a weekly basis, and were aggregated by 9 census districts and 4 census regions, respectively [22, 23]. Previously, we have shown that CDC influenza surveillance data are highly correlated with other influenza surveillance systems [25]. We calculated the relative proportions of positive specimens by week for influenza (by type and subtype) and RSV. We linked CF-associated pulmonary exacerbation data collected at a subject level on the basis of calendar week and geographic influenza (US Census District) and RSV (US Census Region) surveillance data. As we aimed to determine the association between seasonal influenza activity and pulmonary exacerbations, we ended our study period in March 2009 to avoid the 2009 influenza A(H1N1) pandemic.
Primary Outcome
All persons in the CFFPR during the study period were included for analysis and were considered present for an entire calendar year if they underwent annual review. The primary outcome was a documented CF pulmonary exacerbation requiring home intravenous antibiotic therapy or hospitalization. If an individual had duplicate pulmonary exacerbation events in the same week, or if 2 or more events were spaced within 1 month apart, these were counted as 1 event.
Statistical Analysis
We summarized continuous data using mean and standard deviation (SD) and categorical data using percentages. We categorized patients based on age (time-varying) at year end: <18 years and ≥18 years of age. We calculated incidence rates by dividing the number of exacerbation events by the total person-time of follow-up in the study period. Additionally, we calculated the proportion of patients with the number of annual exacerbation events by categorizing exacerbations (0/1, 2/3, 4/≥5) in a 12-month period.
We estimated the effect of influenza and RSV activity on the risk of CF pulmonary exacerbations by multivariate Poisson regression models adjusted for demographic and clinical covariates. We determined covariates a priori based on literature review of risk factors associated with CF pulmonary exacerbations [9, 26]. Covariates included temporal adjustment (quartic polynomial of year as well as sin and cos of 2π*year), age (as continuous), sex, race (white, African American, other or unknown), CF genotype (heterozygous, homozygous, and other/unknown), insurance status (private, government, none) and pancreatic insufficiency. Sensitivity analysis models adjusted for median household income (based on the average of the 2000 and 2010 zip code of patient residence, per $10000 of income), body mass index (BMI) (as a percentile for children and value for adults) and FEV1% predicted (based on Wang and Hankinson) [27, 28], in addition to the covariates in the primary model. For weeks with no BMI, FEV1% predicted or pancreatic insufficiency values recorded, we used the values from the last week with available data (ie, the last observation carried forward method). FEV1% predicted values from weeks with a pulmonary exacerbation were ignored. The influenza and RSV predictors were used with a 2-week lag (ie, exposure 2 weeks prior to the outcome week). The Poisson models were fitted using the R package biglm for the estimation of generalized linear models in large data sets [29]. We calculated the standard errors for the model coefficients using the nonparametric bootstrap by patient (50 resamples) to account for the repeated measures by patient and for potential overdispersion. All P values were 2-sided and P < .05 was considered statistically significant. Analyses were performed with Stata 13.1 (StataCorp, College Station, Texas), SAS 9.3 (SAS Institute, Cary, North Carolina), and R 3.2.5 [30] software.
RESULTS
Baseline Cohort Characteristics
In 2003, there were a total of 21022 patients (48.2% female) of mean age 17.1 (SD, 11.9) years in the CFFPR meeting our inclusion criteria (Table 1). Of these, 12702 were children (48.9% female), and 8320 were adults aged ≥18 years (47.0% female). The majority of the cohort was of white race (90.1%), had medical insurance (private or health maintenance organization) (64.1%), and had a median income by residence location of $42 659. Both adults and children had a median of 4 clinical encounters and a median of zero hospitalizations for a pulmonary exacerbation. Adults had a lower mean lung function measured as %FEV1 predicted (60% vs 86%), and increased prevalence of P. aeruginosa (82% vs 48%) and B. cepacia complex (4% vs 2%) infection but lower prevalence of Staphylococcus aureus infection (42% vs 61%) than children in the patient cohort. Overall, 45% of the patients were delta-F508 homozygous, 34% were delta-F508 heterozygous, and the remaining 21% either unknown/other with no difference in genotypic distribution comparing children and adults.
Table 1.
Baseline Characteristics of Study Cohort From the Cystic Fibrosis Foundation Patient Registry (2003)
| Characteristic | <18 y (n = 12702) |
≥18 y (n = 8320) |
All Subjects (N = 21022) |
|---|---|---|---|
| Female sex | 6217 (49) | 3908 (47) | 10125 (48) |
| Age at CF diagnosis, y, mean (SD) | 1.4 (2.6) | 5.9 (10.4) | 3.2 (7.2) |
| Age group at CF diagnosis | |||
| 0–3 y | 10853 (85) | 5451 (66) | 16304 (78) |
| >3–5 y | 802 (6) | 577 (7) | 1379 (7) |
| >5 y | 1047 (8) | 2292 (28) | 3339 (16) |
| Age at year end (2003), y, mean (SD) | 9.5 (5.0) | 28.7 (9.4) | 17.1 (11.8) |
| Age group at year end (2003) | |||
| <6 y | 3652 (29) | 0 | 3652 (17) |
| ≥6–12 y | 4506 (35) | 0 | 4506 (21) |
| ≥12–18 y | 4544 (36) | 0 | 4544 (22) |
| ≥18–36 y | 0 | 6614 (79) | 6614 (31) |
| ≥36 y | 0 | 1706 (21) | 1706 (8) |
| Genotype | |||
| Other/unknown | 2248 (18) | 1933 (23) | 4181 (20) |
| Delta F508 heterozygous | 4296 (34) | 2934 (35) | 7230 (34) |
| Delta F508 homozygous | 6158 (48) | 3453 (42) | 9611 (45) |
| Race | |||
| White, non-Hispanic | 11164 (88) | 7775 (93) | 18939 (90) |
| Black, non-Hispanic | 559 (4) | 211 (3) | 770 (4) |
| Other/unknown | 979 (8) | 334 (4) | 1313 (6) |
| Infection status | |||
| Pseudomonas aeruginosa present | 6143 (48) | 6806 (82) | 12949 (62) |
| Burkholderia complex present | 254 (2) | 333 (4) | 587 (3) |
| Staphylococcus aureus present | 7748 (61) | 3494 (42) | 11242 (53) |
| FEV1% predicted | |||
| No. | 8520 | 7392 | 15912 |
| Mean (SD) | 86 (21) | 60 (24) | 74 (26) |
| FEV1, L | |||
| No. | 8520 | 7394 | 15914 |
| Mean (SD) | 1.9 (0.8) | 2.3 (1.0) | 2.1 (0.9) |
| Insurance status | |||
| Private/HMO | 7966 (63) | 5507 (66) | 13473 (64) |
| Public | 4379 (34) | 2406 (29) | 6785 (32) |
| Other/unknown | 40 (0) | 59 (1) | 99 (0) |
| No insurance | 317 (2) | 348 (4) | 665 (3) |
| Median zip code income (IQR) | $42083 | $43077 | $42659 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CF, cystic fibrosis; FEV1, forced expiratory volume in 1 second; HMO, health maintenance organization; IQR, interquartile range; SD, standard deviation.
Description of Respiratory Virus Activity During 2003–2009
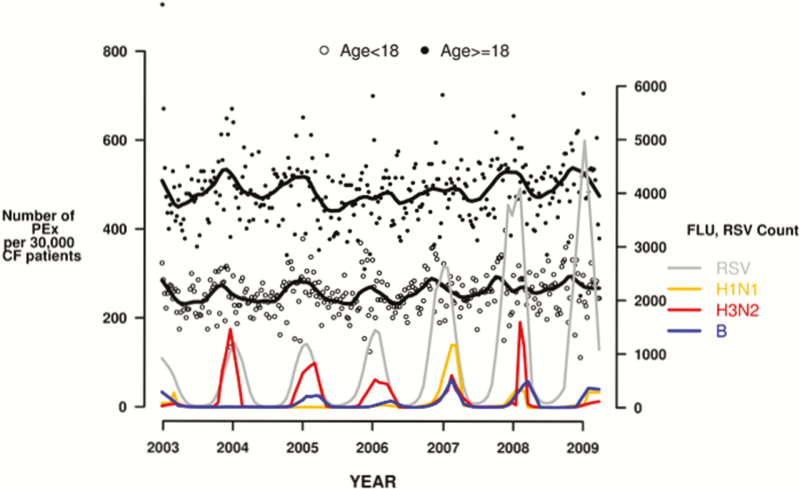
Surveillance revealed that different influenza virus types and subtypes were dominant during annual epidemics and RSV detections increased in the latter years (Figure 1). Specifically with regards to influenza, influenza A(H3N2) was the predominant circulating strain over 4 years (2004–2006 and 2008), and seasonal influenza A(H1N1) was the predominant strain in 2007. In 2003–2004 and 2008–2009 (inclusive of March 2009), influenza A(H1N1) and influenza B were relatively codominant (Figure 1). The study period ended before the emergence of 2009 pandemic influenza A(H1N1), and thus activity of that strain was not accounted for in the analyses. Further, regional and temporal variations occurred in respiratory virus activity over the study period (data not shown).
Figure 1.
Respiratory viruses in cystic fibrosis. Abbreviations: CF, cystic fibrosis; FLU, influenza; PEx, pulmonary exacerbation; RSV, respiratory syncytial virus.
Pulmonary Exacerbation Seasonality
A total of 12336 pulmonary exacerbations occurred in the 2003 calendar year comprising 5396 events in children and 6940 events in adults. In the study period, there was a total of 91291 exacerbation episodes in 31568 individuals (Table 2). Of these, 37169 occurred in children and 54122 occurred in adults. The proportion of patients with at least 1 exacerbation episode was 34%–36% annually and this was consistent over the study period. The crude incidence rate of pulmonary exacerbations was 375.2 per 10000 person-months in children and 713.5 per 10000 person-months in adults. Assessment of exacerbation frequency demonstrated that at least 58% of patients had ≥1 annual event, and 22% had ≥5 events (Table 2). Approximately 86.5% of total exacerbation episodes were managed in hospitals, and a greater proportion of children were hospitalized than adults (93.4% vs 81.9%). An annual increase in pulmonary exacerbations occurred during the winter months, and pulmonary exacerbations were temporally associated with increases in influenza and RSV activity (Figure 1).
Table 2.
Number of Cystic Fibrosis Pulmonary Exacerbations Among Patients in the Cystic Fibrosis Registry From January 2003 Through March 2009 (n = 31 568)
| Parameter | Total PEx | Hospitalization | Home IV Antibiotics | Hospitalization and Home IV Antibiotics |
|---|---|---|---|---|
| PEx | ||||
| No. (%) of subjects with | ||||
| 0 events | 13130 (42) | 14089 (45) | 19449 (62) | 20761 (66) |
| ≥1 event | 18438 (58) | 17479 (55) | 12119 (38) | 10807 (34) |
| 1–2 events | 7818 (25) | 8090 (26) | 6362 (20) | 6507 (21) |
| 3–4 events | 3589 (11) | 3476 (11) | 2510 (8) | 2283 (7) |
| ≥5 events | 7031 (22) | 5913 (19) | 3247 (10) | 2017 (6) |
| No. (%) of events | ||||
| Overall | 91291 | 79054 | 43719 | 31692 |
| Age <18 y | 37169 (41) | 34731 (44) | 14504 (33) | 12214 (39) |
| Age ≥18 y | 54122 (59) | 44323 (56) | 29215 (67) | 19478 (61) |
Abbreviations: IV, intravenous; PEx, pulmonary exacerbation.
Multivariate Model of Pulmonary Exacerbation
In a multivariate Poisson regression model, influenza virus activity (expressed per 10% change in test positivity) was significantly associated with pulmonary exacerbation risk in children (relative risk [RR], 1.02; 95% CI, 1.01–1.03; P < .001) and adults (RR, 1.02; 95% CI, 1.01–1.02; P < .001) after adjusting for demographic, clinical, and socioeconomic factors (Table 3). RSV activity (percentage test positivity, per 10%) was associated with increased exacerbation risk in children (RR, 1.05; 95% CI, 1.02–1.07; P < .001) but did not achieve statistical significance in the adult cohort (RR, 1.01; 95% CI, 1.00–1.03; P = .07) (Table 3).
Table 3.
Relative Risk of Pulmonary Exacerbations in Children and Adults With Cystic Fibrosis
| Parameter | Children (<18 y) RR (95% CI) |
P Value | Adults (≥18 y) RR (95% CI) |
P Value |
|---|---|---|---|---|
| Male sex | 0.77 (.74–.81) | <.001 | 0.78 (.75–.82) | <.001 |
| Race | ||||
| White (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||
| Other/unknown | 1.03 (.88–1.20) | .7 | 0.96 (.84–1.08) | .5 |
| African-American | 0.92 (.84–1.01) | .08 | 0.87 (.80–.95) | .002 |
| Age, per 5 y | 1.55 (1.52–1.58) | <.001 | 0.95 (.95–.96) | <.001 |
| Genotype | ||||
| Heterozygous (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||
| Homozygous | 1.09 (1.04–1.15) | .001 | 1.08 (1.04–1.13) | <.001 |
| Other/unknown | 0.99 (.93–1.07) | .9 | 0.98 (.93–1.04) | .6 |
| Insurance | ||||
| None (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||
| Government | 2.91 (2.55–3.32) | <.001 | 3.00 (2.79–3.23) | <.001 |
| Private only | 1.62 (1.41–1.85) | <.001 | 1.64 (1.52–1.77) | <.001 |
| Pancreatic sufficiency | 1.37 (1.29–1.45) | <.001 | 1.44 (1.37–1.52) | <.001 |
| % influenza positive, per 10% | 1.02 (1.01–1.03) | <.001 | 1.02 (1.01–1.02) | <.001 |
| % RSV positive, per 10% | 1.05 (1.02–1.07) | <.001 | 1.01 (1.00–1.03) | .07 |
A multivariate Poisson regression model incorporating confounders was utilized for children and adults. Effects of temporal adjustments that were present in the multivariate model (quartic polynomial of year as well as sin and cos of 2π*year) are not shown.
Abbreviations: CI, confidence interval; RR, relative risk; RSV, respiratory syncytial virus.
Sensitivity Analyses
We conducted sensitivity analyses in relation to covariates in our multivariate models that had a substantial number of observations (person-weeks) with missing data. Specifically, models additionally adjusted for lung function (FEV1 predicted), median income, and BMI resulted in loss of 55% of person-weeks for the child data and 43% of the person-weeks for the adult data. The estimated effects of influenza virus activity on the pulmonary exacerbation risk from the sensitivity analysis models were similar to the results from the primary analysis models (Table 4).
Table 4.
Relative Risk of Pulmonary Exacerbations in Children and Adults With Cystic Fibrosis (Sensitivity Analysis)
| Parameter | Children (<18 y) RR (95% CI) |
P Value | Adults (≥18 y) RR (95% CI) |
P Value |
|---|---|---|---|---|
| Male sex | 0.82 (.79–.86) | <.001 | 0.75 (.72–.79) | <.001 |
| Race | ||||
| White (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||
| Other/unknown | 0.89 (.75–1.06) | .2 | 0.91 (.78–1.06) | .2 |
| Black | 0.93 (.84–1.02) | .11 | 0.97 (.89–1.06) | .5 |
| Age, per 5 y | 1.22 (1.18–1.25) | <.001 | 0.91 (.90–.92) | <.001 |
| Genotype | ||||
| Heterozygous (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||
| Homozygous | 1.02 (.98–1.07) | .3 | 1.07 (1.02–1.11) | .007 |
| Other/unknown | 0.91 (.86–.97) | .004 | 0.93 (.88–.99) | .03 |
| Insurance | ||||
| None (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||
| Government | 2.45 (2.04–2.93) | <.001 | 2.61 (2.36–2.88) | <.001 |
| Private only | 1.81 (1.51–2.17) | <.001 | 1.80 (1.62–2.00) | <.001 |
| Median income, per $10000 | 0.99 (.97–1.00) | .02 | 1.00 (.99–1.01) | .5 |
| Pancreatic sufficiency | 1.56 (1.38–1.76) | <.001 | 1.20 (1.11–1.29) | <.001 |
| BMI, percentile, per 10% | 0.99 (.98–1.00) | .01 | 0.99 (.99–1.00) | .06 |
| FEV1% predicted, per 20% | 0.55 (.54–.56) | <.001 | 0.62 (.61–.63) | <.001 |
| % influenza positive, per 10% | 1.03 (1.02–1.04) | <.001 | 1.02 (1.01–1.03) | <.001 |
| % RSV positive, per 10% | 1.01 (.98–1.03) | .5 | 1.00 (.98–1.01) | .7 |
The above represents a multivariate Poisson regression model done as a sensitivity analysis for children and adults. Represents approximately 50% of total data due to missing data in additional covariates in the model (refer to Supplementary Methods). Effects of temporal adjustments that were present in the multivariate model (quartic polynomial of year as well as sin and cos of 2π*year) are not shown.
Abbreviations: BMI, body mass index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; RR, relative risk; RSV, respiratory syncytial virus.
DISCUSSION
Respiratory viruses are often found in samples collected during CF-associated pulmonary exacerbations, but their clinical importance remains understated and poorly understood [12]. Diagnostic test limitations, study designs, and emergence or presence of novel or established bacterial pathogens, respectively, in CF patient airways have contributed to the challenges in determining the specific role of viral pathogens in CF pulmonary disease progression [8, 11, 31]. Thus, we utilized a population-based approach with individual and community-level data to identify the association of influenza and RSV with risk of pulmonary exacerbations in individuals with CF during a 63-month period in the United States. Herein we demonstrated that regional influenza activity had a significant association with risk of pulmonary exacerbations in children and adults with CF, and RSV had a similar association in children with CF. These associations were significant even after accounting for known clinical and socioeconomic confounders supporting the role of respiratory viruses in pulmonary exacerbation events.
To our knowledge, our study is the first of its kind to assess respiratory virus disease burden within a patient registry, facilitating individual-level analyses and the adjustment for clinical predictors of severe respiratory disease. Our results regarding the impact of influenza and RSV on CF pulmonary exacerbations adds to our understanding of the potential causative roles of these respiratory viral pathogens have on lung disease progression. Our dataset was massive (>150000 observations describing approximately 34000 persons followed weekly for over 6 years), and we had extremely high precision to measure influenza and RSV-associated events while adjusting for potential confounding variables including presence and severity of risk factors for CF pulmonary exacerbation. As studies of this size are not feasible in many chronic lung disease populations (ie, chronic obstructive pulmonary disease) wherein increased risk of respiratory virus illness exists [32–34], our study may serve as a risk model to guide future preventive strategies for persons with severe chronic lung disease in general.
Influenza-associated illnesses are a significant threat on a global scale, and influenza has been associated with an average of 28000 severe illness hospitalizations and 36000 deaths annually in the United States alone [26, 35]. Increased risk of influenza illness and death has been demonstrated in persons with chronic lung disease including CF [9]. While influenza pneumonia may directly cause CF lung exacerbations, it is relatively rare in the general population [36]. Furthermore, influenza virus infection is associated with secondary bacterial infections and may alter lung flora in CF initiating pulmonary exacerbations [8, 11, 13]. Either process may be averted by vaccination.
When influenza vaccination rates were assessed in the CF population, they were lower than recommended goals, suggesting that a portion of influenza illnesses may be prevented by further efforts to improve influenza vaccine coverage [37, 38]. However, influenza vaccine effectiveness is typically only moderate in magnitude, and during the study period it ranged from 33% to 67% in the United States [39]. With these effectiveness estimates, vaccine failures during the study period would not have been uncommon. We have previously demonstrated that persons with CF who have ≥4 pulmonary exacerbations annually have nearly an order of magnitude greater risk for influenza-associated pulmonary exacerbations compared with the overall cohort [9]. Given the elevated risk for this subgroup of persons with CF, chemoprophylaxis with neuraminidase inhibitors against influenza virus infection should be considered during the influenza season [40].
Similarly, RSV has been attributed to significant morbidity, especially in infants and children [10, 15]. Our study supports this finding as RSV had a significant association with pulmonary exacerbation risk in the children cohort. Pavilizumab, a monoclonal antibody vaccine, did not demonstrate significant efficacy for RSV infection in children with CF in a single clinical trial despite having benefit in reduction of hospitalization in other high risk populations [41]. Purified fusion protein vaccines have also been studied in CF pediatric cohorts to prevent RSV infection, without demonstrable efficacy [42, 43]. Given the trend we saw with RSV-associated adult pulmonary exacerbations, it is possible that particular subsets of adults with frequent pulmonary exacerbations are at significantly increased risk and should be considered for future vaccines or next-generation RSV monoclonal antibodies.
Although our study was a large CF population-based study spanning several years, it has limitations to consider. We used ecologic linkages between CF outcomes and regional respiratory virus activity. Typically, studies to assess the burden of respiratory virus disease are ecologic comparisons of aggregated events data from administrative databases and surveillance data over time [9, 35, 44]. While ecological in nature, our study incorporated patient-level predictors of pulmonary exacerbations, thus strengthening our measures of association. As our influenza and RSV data were obtained from the CDC surveillance databases, we did not have access to respiratory virus laboratory test results from persons in our cohort or the ability to confirm influenza or RSV infections in relation to exacerbation events. In this regard, as the diagnosis of pulmonary exacerbations is clinician based, we relied on the accuracy of the CFFPR data. Influenza vaccination and RSV prophylaxis data were not incorporated, as these data were not consistently or at all available during the study period. We did not study other respiratory viral pathogens found in CF pulmonary infections such as rhinovirus or human metapneumovirus, as available surveillance data were not as comprehensive. Sputum bacteriology was not incorporated as only data for P. aeruginosa were available, and we sought to confirm our earlier findings regarding influenza in CF [9]. Due to the study design with weekly data for analysis, there was a possibility of duplicate counting of pulmonary exacerbation events, although we attempted to exclude them. However, if there was duplicate counting of exacerbation events, and if they occurred at random, this would have biased results toward the null as influenza and RSV seasons only overlapped by approximately one-third of each year.
In conclusion, our population-based study demonstrated that both influenza and RSV have a significant association with pulmonary exacerbation risk even after accounting for important confounders in individuals with CF. Given the potential deleterious effects of viral pathogens in CF, exploration of novel vaccines or chemoprophylactic strategies in this population is warranted.
Supplementary Material
Notes
Author contributions. M. N. and U. K. were primarily responsible for data analysis. R. S. was responsible for the creation of the manuscript. J. R. O., K. M. N., and C. H. G. were responsible for the project’s inception and supervision. All authors contributed to the final manuscript. J. R. O. serves as guarantor of the work.
Acknowledgments. The authors thank the Cystic Fibrosis (CF) Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of their institutions.
Financial support. This work was supported by the Robert Wood Johnson Harold Amos Medical Faculty Development Program (J. R. O.). C. H. G. receives funding from the Cystic Fibrosis Foundation, the National Institutes of Health (grant numbers R01HL103965, R01HL113382, R01AI101307, UM1HL119073, P30DK089507), and the US Food and Drug Administration (grant number R01FD003704). R. S. is supported by fellowship funding from Cystic Fibrosis Canada and the Canadian Institutes of Health Research.
Potential conflicts of interest. All authors: No potential conflicts of interest. No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Knapp EA, Fink AK, Goss CH, et al. The cystic fibrosis foundation patient registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc 2016; 13:1173–9. [DOI] [PubMed] [Google Scholar]
- 2. Cystic Fibrosis Foundation. Patient registry annual data report 2012. Bethesda, MD: Cystic Fibrosis Foundation; 2012. [Google Scholar]
- 3. Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax 2007; 62:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenbit AE, Flume PA. Pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med 2011; 17:442–7. [DOI] [PubMed] [Google Scholar]
- 5. Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001; 153:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Folescu TW, da Costa CH, Cohen RW, da Conceição Neto OC, Albano RM, Marques EA. Burkholderia cepacia complex: clinical course in cystic fibrosis patients. BMC Pulm Med 2015; 15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34:91–100. [DOI] [PubMed] [Google Scholar]
- 8. Ramsey BW, Gore EJ, Smith AL, Cooney MK, Redding GJ, Foy H. The effect of respiratory viral infections on patients with cystic fibrosis. Am J Dis Child 1989; 143:662–8. [DOI] [PubMed] [Google Scholar]
- 9. Ortiz JR, Neuzil KM, Victor JC, Wald A, Aitken ML, Goss CH. Influenza-associated cystic fibrosis pulmonary exacerbations. Chest 2010; 137:852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armstrong D, Grimwood K, Carlin JB, et al. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol 1998; 26:371–9. [DOI] [PubMed] [Google Scholar]
- 11. Wang EE, Prober CG, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med 1984; 311:1653–8. [DOI] [PubMed] [Google Scholar]
- 12. Wat D, Gelder C, Hibbitts S, et al. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros 2008; 7:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hament JM, Kimpen JL, Fleer A, Wolfs TF. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol 1999; 26:189–95. [DOI] [PubMed] [Google Scholar]
- 14. Van Ewijk BE, Wolfs TF, Aerts PC, et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res 2007; 61:398–403. [DOI] [PubMed] [Google Scholar]
- 15. Abman SH, Ogle JW, Butler-Simon N, Rumack CM, Accurso FJ. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr 1988; 113:826–30. [DOI] [PubMed] [Google Scholar]
- 16. Pribble CG, Black PG, Bosso JA, Turner RB. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr 1990; 117:200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns JL, Emerson J, Kuypers J, et al. Respiratory viruses in children with cystic fibrosis: viral detection and clinical findings. Influenza Other Respir Viruses 2012; 6:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 20. Ortiz JR, Neuzil KM, Victor JC, Wald A, Aitken ML, Goss CH. Influenza-associated cystic fibrosis pulmonary exacerbations. Chest 2010; 137:852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Overview of influenza surveillance in the United States. Atlanta, GA: CDC, 2006. [Google Scholar]
- 22. Centers for Disease Control and Prevention. The National Respiratory and Enteric Virus Surveillance System (NREVSS). Atlanta, GA: CDC; Available at: https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html. Accessed on 1 August 2016. [Google Scholar]
- 23. Centers for Disease Control and Prevention. Past weekly surveillance reports Available at: https://www.cdc.gov/flu/weekly/pastreports.htm. Accessed on 1 August 2016.
- 24. Centers for Disease Control and Prevention. Fluview—a weekly influenza surveillance report prepared by the Influenza Division Atlanta, GA: CDC, 2016. [Google Scholar]
- 25. Ortiz JR, Zhou H, Shay DK, Neuzil KM, Fowlkes AL, Goss CH. Monitoring influenza activity in the United States: a comparison of traditional surveillance systems with Google flu trends. PLoS One 2011; 6:e18687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortiz JR, Neuzil KM, Rue TC, et al. Population-based incidence estimates of influenza-associated respiratory failure hospitalizations, 2003 to 2009. Am J Respir Crit Care Med 2013; 188:710–5. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993; 15:75–88. [DOI] [PubMed] [Google Scholar]
- 28. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–87. [DOI] [PubMed] [Google Scholar]
- 29. Chandra P, Wolfenden LL, Ziegler TR, et al. Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: a case series. Photodermatol Photoimmunol Photomed 2007; 23:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria, 2013. [Google Scholar]
- 31. Freymuth F, Vabret A, Galateau-Salle F, et al. Detection of respiratory syncytial virus, parainfluenzavirus 3, adenovirus and rhinovirus sequences in respiratory tract of infants by polymerase chain reaction and hybridization. Clin Diagn Virol 1997; 8:31–40. [DOI] [PubMed] [Google Scholar]
- 32. Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 162:167–73. [DOI] [PubMed] [Google Scholar]
- 33. Smith CB, Kanner RE, Golden CA, Klauber MR, Renzetti AD., Jr Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J Infect Dis 1980; 141:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiselka MJ, Kent J, Cookson JB, Nicholson KG. Impact of respiratory virus infection in patients with chronic chest disease. Epidemiol Infect 1993; 111:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 36. Rello J, Pop-Vicas A. Clinical review: primary influenza viral pneumonia. Crit Care 2009; 13:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ortiz JR, Neuzil KM, Victor JC, Aitken ML, Goss CH. Predictors of influenza vaccination in the cystic fibrosis foundation patient registry, 2006 through 2007. Chest 2010; 138:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wat D, Gelder C, Hibbitts S, et al. Is there a role for influenza vaccination in cystic fibrosis? J Cyst Fibros 2008; 7:85–8. [DOI] [PubMed] [Google Scholar]
- 39. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 40. Fiore A, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep 2011; 60:1–24. [PubMed] [Google Scholar]
- 41. Robinson K, Odelola OA, Saldanha IJ, McKoy NA. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database Syst Rev 2013; 6:CD007743. [DOI] [PubMed] [Google Scholar]
- 42. Piedra PA, Cron SG, Jewell A, et al. ; Purified Fusion Protein Vaccine Study Group Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine 2003; 21:2448–60. [DOI] [PubMed] [Google Scholar]
- 43. Piedra PA, Grace S, Jewell A, et al. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J 1996; 15:23–31. [DOI] [PubMed] [Google Scholar]
- 44. Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses 2009; 3:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.