In Seattle, Washington, between 2014 and 2016, 5% of Neisseria gonorrhoeae cases among men who have sex with men had reduced azithromycin (AZM) susceptibility. The emergence of gonorrhea with reduced susceptibility to AZM should prompt reconsideration of gonorrhea treatment guidelines.
Keywords: antimicrobial resistance, Neisseria gonorrhoeae, men who have sex with men, azithromycin
Abstract
Background
Antimicrobial-resistant Neisseria gonorrhoeae is a major public health threat. The Centers for Disease Control and Prevention (CDC) recommends ceftriaxone 250 mg plus azithromycin (AZM) 1 g for gonorrhea treatment. Resistance to AZM could affect gonorrhea control efforts.
Methods
Using gonococcal isolates collected at the Public Health–Seattle & King County (PHSKC) Sexually Transmitted Disease (STD) Clinic from 2012 to 2016, focusing on 2014–2016, we compared cases with the CDC AZM alert value minimum inhibitory concentration (MIC) (≥2 µg/mL) to those with AZM MIC ≤1 µg/mL, antimicrobial susceptibility profiles and clinical outcomes.
Results
In 2012 and 2013, none of the 263 patients from whom we isolated N. gonorrhoeae from the urethra were infected with organisms with an AZM MIC ≥2 µg/mL. Between 2014 and 2016, 4.4% of 926 gonorrhea cases demonstrated reduced susceptibility to AZM; 93% of these cases occurred among men who have sex with men (MSM). Among MSM, 5.0% of 2014–2016 cases demonstrated reduced susceptibility to AZM. No AZM alert value isolates had concomitant cephalosporin resistance. There were 2 potential treatment failures: 1 pharyngeal infection treated with AZM 2 g alone, and 1 pharyngeal infection that persisted after study drug.
Conclusions
Among MSM with gonorrhea in Seattle, 5% have gonorrhea with reduced susceptibility to AZM. The World Health Organization recommends changing treatment guidelines when >5% of isolates are resistant to a recommended drug. The emergence of resistant AZM gonorrhea should prompt reconsideration of current treatment recommendations, and highlights the need for new therapies for gonorrhea.
The Centers for Disease Control and Prevention (CDC) named antimicrobial-resistant (AMR) gonorrhea as the third most important AMR threat in the United States [1]. Since the 1930s, the gonococcus has developed resistance to sulfonamides, penicillin, tetracyclines, and quinolones, requiring serial changes in recommended therapies [2]. The most recent change occurred in 2012 when CDC removed cefixime as first-line gonorrhea treatment [3] due to an increasing proportion of isolates with reduced susceptibility to the drug. In the United States, the only currently recommended therapy for gonorrhea is ceftriaxone 250 mg and azithromycin (AZM) 1 g [4].
AZM is the most commonly prescribed antibiotic in the United States [5], and case reports indicate that AZM monotherapy can induce “resistance” in Neisseria gonorrhoeae [6]. The Clinical and Laboratory Standards Institute (CLSI) has not defined breakpoints for N. gonorrhoeae AZM resistance due to a lack of clinical data. However, in 2017, CLSI created an epidemiologic cutoff value at a minimum inhibitory concentration (MIC) ≥2 µg/mL based on data indicating that most isolates with AZM MIC ≥2 µg/mL had at least 2 of 4 C2611T mutations of 23s RNA ribosomal subunit [7, 8]. This MIC value also corresponds to the CDC’s definition of gonococcal “alert value” isolates. (In this article we use “alert value” or “reduced susceptibility” to indicate isolates with MIC ≥2 µg/mL.) Gonorrhea with reduced susceptibility to AZM is increasingly recognized globally. In China, 9% of isolates show AZM MIC ≥2 µg/mL [9], and reports have documented sporadic outbreaks of reduced-susceptibility gonorrhea in the United States and the United Kingdom [10–12]. Data from the CDC Gonococcal Isolate Surveillance Project (GISP) found that AZM alert values were stable between 2005 and 2013 [13], but increased 4-fold (0.6% to 2.5%) from 2013 to 2014 [14].
In 2014, we began identifying gonococcal infections at the Public Health–Seattle & King County (PHSKC) Sexually Transmitted Disease (STD) Clinic with elevated MIC to AZM. This paper describes the epidemiology of N. gonorrhoeae with AZM reduced susceptibility in Seattle and its clinical impact between 1 January 2014 and 31 December 2016.
METHODS
The PHSKC STD Clinic is the only categorical STD clinic in Washington State, with approximately 10000 visits per year. Clinicians obtain specimens for gonorrhea culture from men with signs or symptoms of urethritis and all persons with a positive gonorrhea screening test who return to clinic for treatment. This latter group primarily includes men who have sex with men (MSM) with a positive pharyngeal and/or rectal gonorrhea nucleic acid amplification test (NAAT) and women with a positive endocervical or vaginal NAAT. Clinic policy is to screen MSM using NAAT at all exposed anatomic sites including the pharynx, rectum, and urethra.
Specimens are sent to the PHSKC Laboratory for culture and identification, and N. gonorrhoeae isolates are subsequently sent to the University of Washington’s Neisseria Reference Laboratory (NRL) for antimicrobial susceptibility testing (AST). The STD Clinic and the NRL participate in CDC’s GISP. Urethral isolates undergo AST according to CLSI-recommended agar dilution method [13]. We screen isolates from extragenital and endocervical sites for cefixime and AZM resistance using the Kirby-Bauer disk diffusion method. Isolates with AZM zone diameters ≤30 mm (equivalent to agar dilution AZM MIC ≥0.5–1 µg/mL) are tested by agar dilution [15]. AMR testing includes penicillin, tetracycline, cefixime, ceftriaxone, ciprofloxacin, AZM, and either spectinomycin (until 2015) or gentamicin (starting in 2015). We used CLSI [8] breakpoints to interpret MIC for penicillin (≥2.0 μg/mL), tetracycline (≥2.0 μg/mL), spectinomycin (≥128.0 μg/mL), and ciprofloxacin (≥1.0 μg/mL), and used GISP alert values for AZM (≥2.0 μg/mL), cefixime (≥0.25 μg/mL), and ceftriaxone (≥0.125 μg/mL) as CLSI has not established breakpoints for these antimicrobials.
In early 2014, concurrent with the first isolates with increased MIC to AZM, we began recommending test of cure (TOC) 4 weeks after treatment for patients with N. gonorrhoeae with AZM MIC ≥2 µg/mL. Public health staff contacted patients infected with gonococcal isolates with cefixime, ceftriaxone, or AZM alert values to encourage them to return for TOC.
We used clinical and behavioral data collected as part of routine clinic evaluations to identify factors associated with AZM reduced susceptibility, using χ2 test to compare proportions and t tests to compare means/medians. We considered P < .05 significant; all analyses were conducted with Stata 12.0 software (StataCorp, College Station, Texas). For most patients, standardized behavioral data were collected using a computer self-interview; patients who did not complete the self-interview were asked standardized questions by clinicians [16]. Because virtually all AZM reduced-susceptibility infections occurred in MSM, we compared MSM gonorrhea cases with and without AZM alert values. Because we do not conduct full AST on extragenital and endocervical specimens, our analyses comparing antimicrobial susceptibility profiles compare all isolates with AZM MIC ≥2.0 μg/mL to all urethral isolates with AZM MIC ≤1.0 μg/mL. Throughout the manuscript, we define cases (as opposed to isolates) as temporally separate clinical events; some patients had >1 isolate from different anatomic sites as part of a single case, and some patients had >1 case during the observation period. As a public health activity, our analysis did not require institutional review board approval.
RESULTS
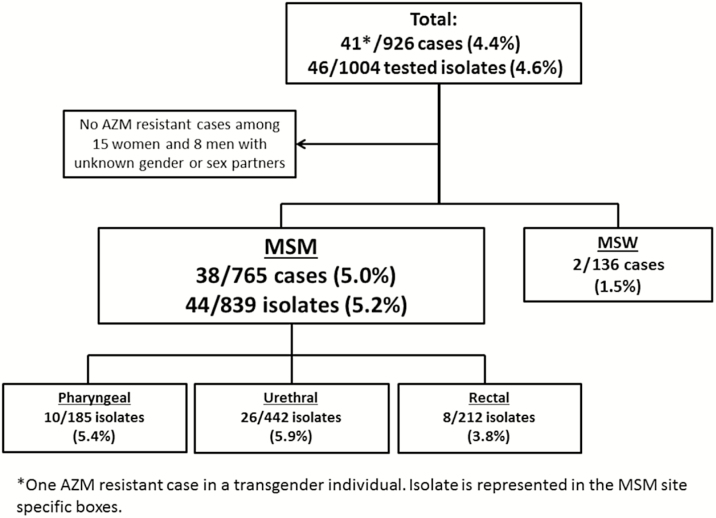
None of the 292 tested urethral isolates obtained from 263 patients at the PHSKC STD Clinic 2012–2013 demonstrated reduced susceptibility to AZM. However, 4.7%, 3.9%, and 4.7% of gonococcal cases diagnosed at the PHSKC STD Clinic in 2014, 2015, and 2016, respectively, had AZM alert values. Between 1 January 2014 and 31 December 2016, the NRL conducted AST on 1004 isolates from 825 individual patients who had 926 cases of gonorrhea. (Eighty-four persons had >1 separate case of gonorrhea.) This included 577 urethral, 198 pharyngeal, 216 rectal, 11 endocervical, and 2 unknown site isolates. The 926 cases included 765 cases in MSM, 136 in men who have sex with women only (MSW), 8 cases in men with unknown gender of sex partners, 15 cases in women, and 2 cases in transgender individuals. Forty-one of the 926 (4.4%) cases were caused by N. gonorrhoeae with reduced susceptibility to AZM. Thirty-eight of the 41 (93%) AZM-reduced susceptibility cases occurred in MSM; 2 occurred in MSW (both in 2016). A total of 38 of 765 (5.0%) MSM gonococcal cases were AZM alert values, compared with 2 of 136 (1.5%) cases in MSW (P = .068). Annually, the proportion of MSM cases with AZM alert values was 5.4%, 4.8%, and 4.6% in 2014, 2015, and 2016, respectively. Among MSM isolates, 10 of 185 (5.4%) pharyngeal, 8 of 212 (3.8%) rectal, and 26 of 442 (5.9%) urethral isolates were resistant to AZM (P = .523) (Figure 1).
Figure 1.
Flowchart of the number and proportion of azithromycin alert value gonococcal isolates and cases identified at the Public Health–Seattle & King County Sexually Transmitted Disease Clinic, 2014–2016. Abbreviations: AZM, azithromycin; MSM, men who have sex with men; MSW, men who have sex with women.
The AZM MIC required to inhibit growth of 50% of organisms (MIC50), MIC required to inhibit growth of 90% of organisms (MIC90), and MIC geometric means did not vary during 2014–2016 (Table 1). Because European criterion [17] for AZM resistance is MIC ≥1 µg/mL, we examined urethral isolates with MIC 1 µg/mL by year. Overall, 20 isolates had AZM MIC 1 µg/mL: 7 (1.9%) in 2014, 8 (2.6%) in 2015, and 5 (1.5%) in 2016. All but 1 of these were from MSM. Using this lower breakpoint to define AZM resistance, the percentage of isolates with reduced susceptibility to AZM was 7.2%, 6.5%, and 5.9% in 2014, 2015, and 2016. Comparatively, in 2012 and 2013, 5 (3.4%) and 4 (2.8%) of tested urethral isolates had AZM MIC ≥1 µg/mL.
Table 1.
Azithromycin Minimum Inhibitory Concentrations (MICs), Geometric Means, Counts, and Percentages of MIC Cut Points for All Tested Isolates at the Public Health–Seattle & King County Sexually Transmitted Disease Clinic by Year: 2012–2016
| Parameter | 2012 (n = 149)a | 2013 (n = 143)a | 2014 (n = 359) | 2015 (n = 306) | 2016 (n = 339) |
|---|---|---|---|---|---|
| AZM MIC50 | 0.25 μg/mL | 0.25 μg/mL | 0.25 μg/mL | 0.25 μg/mL | 0.25 μg/mL |
| AZM MIC90 | 0.5 μg/mL | 0.5 μg/mL | 1.0 μg/mL | 1.0 μg/mL | 1.0 μg/mL |
| Geometric mean | 0.21 μg/mL | 0.23 μg/mL | 0.30 μg/mL | 0.29 μg/mL | 0.27 μg/mL |
| MIC ≥2 μg/mL | 0 | 0 | 19 (5.3%) | 12 (3.9%) | 15 (4.4%) |
| MIC ≥1 μg/mL | 5 (3.4%) | 4 (2.8%) | 26 (7.2%) | 20 (6.5%) | 20 (5.9%) |
Abbreviations: AZM, azithromycin; MIC, minimum inhibitory concentration; MIC50, the MIC required to inhibit growth of 50% of organisms; MIC90, the MIC required to inhibit growth of 90% of organisms.
a2012 and 2013 isolates are urethral only.
Among MSM, there was no association of AZM reduced-susceptibility gonorrhea with human immunodeficiency virus (HIV) status, age, race/ethnicity, number of sexual partners, drug use, or previous gonorrhea or chlamydial infection (Table 2). Comparing antimicrobial susceptibility profiles from urethral isolates to AZM alert value isolates, isolates with AZM ≥2 µg/mL were more susceptible to penicillin (P = .005) and ciprofloxacin (P < .001) (Table 3). None of the AZM alert value isolates had concomitant reduced susceptibility to third-generation cephalosporins. Between 2014 and 2016, the NRL identified 17 (3%) isolates with reduced susceptibility to cefixime (MIC ≥0.25 μg/mL) and 3 (0.5%) isolates with a ceftriaxone alert value (MIC ≥0.125 μg/mL). The majority of cefixime/ceftriaxone alert value isolates (95%) occurred among MSM. There were no differences in mean MIC to any of the tested drugs between urethral and extragenital isolates with AZM MIC ≥2 µg/mL.
Table 2.
Comparison of Clinical and Demographic Characteristics of Men Who Have Sex With Men (MSM) Gonorrhea Cases With Azithromycin Alert Value Versus All Other MSM Gonorrhea Cases at the Public Health–Seattle & King County Sexually Transmitted Disease Clinic, 2014–2016
| Characteristic | MSM AZM Alert Value Cases (n = 38) | All Other MSM Cases (n = 727) |
P Valuea |
|---|---|---|---|
| HIV infected | 3 (7.9) | 139 (19.1) | .107 |
| Age, y, median (IQR) | 29 (25–34) | 29 (25–37) | .497 |
| Race/ethnicity | .732 | ||
| Black/African American | 2 (5.3) | 71 (9.8) | |
| White | 27 (71.1) | 455 (62.6) | |
| Asian | 2 (5.3) | 35 (4.8) | |
| Hispanic/Latino | 3 (7.9) | 97 (13.3) | |
| Native American/Alaska Native | 0 (0) | 10 (1.4) | |
| Unknown | 4 (10.5) | 59 (8.1) | |
| No. of sex partners <2 mo, median (IQR) | 3 (2–4) | 3 (2–5) | .476 |
| Methamphetamine use <12 mo | 4 (10.5) | 88 (12.1) | .771 |
| Popper use <12 mo | 9 (23.7) | 193 (26.6) | .696 |
| Crack or cocaine <12 mo | 1 (2.6) | 56 (7.7) | .246 |
| Any illicit drug useb <12 mo | 11 (29.0) | 237 (32.6) | .639 |
| Lifetime history of gonorrhea | 18 (47.4) | 264 (36.3) | .169 |
| Gonorrhea diagnosis <12 mo | 7 (18.4) | 183 (25.2) | .348 |
| Chlamydia or NGU < 12 mo | 6 (15.8) | 133 (18.3) | .820 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AZM, azithromycin; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; NGU, nongonococcal urethritis.
aχ2 test for comparison of proportions and t tests for comparison of means.
bAny illicit drug use includes methamphetamines, poppers, crack, cocaine, or any report of injection drug use. It does not include drugs for erectile dysfunction.
Table 3.
Comparison of Antimicrobial Susceptibility Profiles of All Non-azithromycin Alert Value Urethral Isolates Versus Azithromycin Alert Value Isolates From Public Health–Seattle & King County Sexually Transmitted Disease Clinic, 2014–2016
| Antimicrobial (Breakpoint) | All Non-AZM Alert Value Urethral Isolates (n = 541) | AZM Alert Value Isolates (n = 46) | P Value | |||
|---|---|---|---|---|---|---|
| No. Resistant (%) | Median MIC (Range) | No. Resistant (%) | Median MIC (Range) | For % | For Mean MIC | |
| Azithromycin ECV/alert value (≥2 µg/mL) |
0 | 0.25 (0.03–1) | 46 (100) | 4 (2 to >256) | <.001 | <.001 |
| Cefixime alert value (≥0.25 µg/mL) |
17 (3.1) | 0.015 (0.015–0.25) | 0 | 0.015 (0.015–0.06) | .386 | .036 |
| Ceftriaxone alert value (≥0.125 µg/mL) |
3 (0.6) | 0.008 (0.008–0.25) | 0 | 0.008 (0.008–0.03) | 1.00 | .061 |
| Tetracycline (≥2 µg/mL) | 194 (35.9) | 1 (0.25–16) | 19 (41.0) | 1 (0.25–4) | .523 | .027 |
| Spectinomycin (2014) (≥128 µg/mL) |
0 | NA | 0 | NA | NA | NA |
| Gentamicin (2015–2016) (≥32 µg/mL) |
0 | 8 (4–16) | 0 | 8 (4–8) | NA | .014 |
| Penicillin (≥2 µg/mL) |
102 (18.9) | 1 (0.25–16) | 1 (2.2) | 1 (0.25–2) | .005 | .018 |
| Ciprofloxacin (>0.5 µg/mL) | 178 (32.9) | 0.015 (0.015–16) | 3 (6.5) | 0.015 (0.015–16) | <.001 | .006 |
Abbreviations: AZM, azithromycin; ECV, epidemiologic cutoff value; MIC, minimum inhibitory concentration; NA, not applicable.
The majority (71%) of the 41 cases of reduced-susceptibility AZM gonorrhea were treated with ceftriaxone and AZM. Five (12%) were treated with a study drug, 2 (5%) were treated with ceftriaxone and doxycycline, and 1 (2%) was treated with gentamicin and AZM. Another 3 men (7%) were treated with 2 g of AZM-monotherapy, the recommended alternative treatment for patients with a β-lactam allergy until May 2015. (We do not have treatment records for 1 man.) Twenty-one men (51%) returned for a TOC at a median of 27 days (range, 3–142) following treatment. Among men with TOC, 3 tested positive. One reported condomless sex with his untreated partner and was classified as reinfected. We classified the other 2 men as potential treatment failures. The first was treated with study drug, and was initially infected at the urethra and pharynx; only his pharyngeal TOC by culture was positive at 7 days. We do not have documentation of his re-treatment. The second patient, a 30-year-old HIV-infected MSM, appeared to fail AZM monotherapy. He presented with symptomatic urethritis and received AZM 2 g alone due to a needle phobia. After treatment, his pharyngeal gonococcal culture returned positive; rectal testing was negative. The MIC of both urethral and pharyngeal isolates was 4.0 µg/mL. He underwent TOC 28 days following treatment, and denied any symptoms or sexual activity since treatment. Pharyngeal TOC by NAAT was positive; culture was not done. He was re-treated with ceftriaxone 250 mg intramuscularly and doxycycline 100 mg twice daily for 7 days.
DISCUSSION
In 2014, the PHSKC STD Clinic witnessed the beginning of a sustained increase in N. gonorrhoeae infections with reduced susceptibility to AZM among MSM, among whom 5% of gonococcal infections now have AZM MIC ≥2 µg/mL. While we did not observe a concurrent increase in AZM alert value gonorrhea in heterosexuals, we had very few non-MSM N. gonorrhoeae isolates, and identified 2 AZM alert value gonococcal infections in MSW in 2016, raising concern that the potentially resistant infections seen in MSM may have spread into other populations. The World Health Organization and CDC recommend removing an antimicrobial from recommended treatment regimens when >5% of circulating isolates are resistant or the therapy is <95% effective [18]. If we consider alert value isolates resistant, we are now at that threshold among MSM in King County, Washington. This occurrence raises the issue of whether AZM should continue to be part of standard gonorrhea treatment. Our findings also highlight the need to expand antimicrobial susceptibility surveillance for gonorrhea, particularly among heterosexuals.
The rapid rise from zero AZM alert value isolates in 2013 to 5% of cultured gonococcal isolates in 2014 is similar, but larger in magnitude, to a trend observed in GISP data [13]. Nationally, there was a 4-fold increase in AZM alert values, from 0.6% of isolates in 2013 to 2.5% in 2014. Similarly, Canadian surveillance data showed an 8-fold increase in AZM with MIC ≥2 µg/mL between 2011 (0.4%) and 2014 (3.3%) [19]. In Guangzhou, China, the proportion of tested isolates with AZM MIC ≥2 µg/mL more than doubled between 2009 and 2013, when 10% had an AZM MIC ≥2 µg/mL (though only 100 isolates were tested) [9]. Ten years ago, ciprofloxacin resistance first appeared in the United States among MSM on the West Coast, presumably due to resistant organisms spread from Asia. The epidemiologic pattern we observed, with AZM alert value gonorrhea seemingly emerging first among MSM in Seattle, Washington, is reminiscent of the US experience with ciprofloxacin resistance, which is now widespread nationally [2]. Curiously, other West Coast jurisdictions did not see as large of an increase in AZM alert values in 2014 as Seattle and, per GISP, the area with the largest proportion of AZM alert value isolates was in the Midwest [14]. It remains unclear whether organisms with AZM reduced susceptibility are a consequence of transmission of organisms from Asia, de novo emergence of resistance, or a combination of both.
Our observations, and other developments in the field of STDs, should prompt some reconsideration of current STD treatment guidelines. AZM is the most commonly prescribed antibiotic in the United States [5], being used mostly for upper respiratory tract infections. In the realm of STDs, AZM monotherapy is recommended for the treatment of chlamydial infection and the empiric treatment of sexually transmitted infection syndromes (ie, nongonococcal urethritis [NGU] and cervicitis). However, AZM is problematic for several reasons. First, observational data suggest that the drug is 20% less effective than doxycycline for rectal chlamydial infection [20], which is very common in MSM [21] and may be common, and undiagnosed, in women [22]. Second, AZM monotherapy induces resistance in Mycoplasma genitalium [23], a common but infrequently diagnosed pathogen causing NGU. Third, use of AZM for chlamydia and NGU in persons harboring asymptomatic, undiagnosed N. gonorrhoeae has the potential to expose the gonococcus to AZM monotherapy. Case reports and retrospective studies have found that AZM monotherapy can result in elevated N. gonorrhoeae MIC [6, 11, 24], and in vitro data support this idea [25]. Thus, AZM appears to be inferior to doxycycline for at least some chlamydial infections, and may promote both M. genitalium and gonococcal resistance. Because of these concerns, European guidelines have recently changed to recommend the use of doxycycline for NGU [26].
AZM may also be problematic as part of gonorrhea treatment regimens. The addition of AZM to ceftriaxone or cefixime for the treatment of gonorrhea was originally justified as a means to treat concurrent, undiagnosed chlamydial infection. The 2-drug regimen for gonorrhea was subsequently endorsed, even in persons without chlamydia, as a means to thwart the development of gonorrhea AMR. Though seemingly reasonable, very few clinical data exist to support this recommendation, and it is possible that combination therapy is actually promoting resistance. AZM has a very long half-life (approximately 68 hours) [27], whereas ceftriaxone usually clears the body between 30 and 45 hours (half-life 8 hours) [28]. Thus, gonococci not eradicated in the first 30 hours following treatment may be exposed to AZM monotherapy for up to 14 days. Given the many concerns related to AZM’s use for STDs, it may be time to reconsider the drug’s role in STD treatment recommendations.
Fortunately, over the surveillance period, 2014–2016, the PHSKC STD Clinic did not experience any treatment failures among persons treated with ceftriaxone-containing regimens. Ceftriaxone resistance remains very rare, and the recommended 250-mg dose would be expected to cure virtually all isolates currently circulating in the United States [29]. However, most other developed nations, including the United Kingdom [30], Europe [31], and Australia [32] recommend 500 mg of ceftriaxone in combination with 1 g or 2 g of AZM. Japanese authorities recommend 1 g ceftriaxone alone [33]. No clinical data exist to suggest that these higher doses are superior for the majority of circulating gonorrhea isolates, but insofar as treatment recommendations are designed to retard the selection of resistant organism and their transmission, it may be worthwhile to consider increasing the ceftriaxone dose in the United States. Ideally, prior to such a recommendation we would possess laboratory and/or clinical evidence that using a higher dose of ceftriaxone prevents the development of AMR.
Our findings may also have implications for how clinicians and public health authorities treat the sex partners of persons with gonorrhea. Cefixime, an oral third-generation cephalosporin that is considerably less potent against N. gonorrhoeae than ceftriaxone, is widely used in combination with AZM for gonorrhea expedited partner therapy (EPT). (EPT is provision of treatment to sex partners of infected patients without the partners’ medical evaluation, usually by having the infected patient give their partners medication or a prescription.) In some states, including Washington, EPT is widely prescribed for heterosexuals and has been shown to significantly reduce gonorrhea reinfection [34, 35]. EPT is not recommended for MSM, due both to the higher risk of AMR gonorrhea and high rates of concurrent syphilis and undiagnosed HIV [4]. However, heterosexual EPT use might need to be curtailed if AZM reduced-susceptibility gonorrhea becomes widespread in MSW and women. Our data on AMR gonorrhea among heterosexuals are extremely limited, and the current GISP system does not collect specimens from women. Our observation of a small number of AZM alert value gonococcal infections in heterosexuals, as well as recent increases in heterosexual syphilis cases in King County and elsewhere [36], suggests that the current epidemic of bacterial sexually transmitted infections in MSM may be spreading to heterosexual populations, and highlights the need to expand gonorrhea surveillance among heterosexuals. CDC has recently launched an expanded gonorrhea surveillance project, SURRG (Strengthening US Response to Resistant Gonorrhea), which should greatly augment the availability of data on AMR gonorrhea.
Despite several strengths, this analysis is subject to many limitations. First, only a subset of persons infected with gonorrhea in King County underwent culture for antimicrobial surveillance. Men infected with gonorrhea who attend the STD Clinic may be different than those diagnosed elsewhere. Second, as indicated above, we have very limited data on non-MSM. Third, our findings come from a single US city, and the extent to which the rise in reduced susceptibility AZM we observed has affected other parts of the United States is not certain. Fourth, we did not conduct full AST on all extragenital and endocervical isolates. We screened these isolates using disk diffusion, possibly underestimating the number of isolates with low-level, elevated AZM MIC (approximately 1–2 µg/mL). Last, we did not perform molecular testing on pre- and posttreatment isolates from our suspected treatment failures, so we cannot verify that they were not reinfections. However, given that the patients with positive TOC were treated with AZM monotherapy and an experimental drug, and denied reexposure, we believe these are true treatment failures.
In summary, the proportion of gonococcal infections in King County, Washington, with reduced susceptibility to AZM increased dramatically between 2013 and 2016. This increase was concentrated among MSM, among whom approximately 5% of gonococcal infections are caused by AZM alert value organisms. At least 1 treatment failure with AZM monotherapy occurred, and this regimen should be avoided. While none of the AZM alert value isolates were also resistant to a cephalosporin, a number of recent findings in the STD field may prompt reconsideration of continuing to recommend AZM for STD treatments. Cefixime plus AZM for EPT should be avoided among MSM, and surveillance efforts should be expanded to determine if EPT is still a reasonable partner management option for heterosexuals. Further research is needed to understand how antibiotic use affects the development of resistance in N. gonorrhoeae at both the individual and population level, and to develop new gonorrhea treatment options.
Notes
Financial support. This work was funded by the National Institutes of Health (NIH) (grant number K23-AI113185 to L. A. B.); a Centers for Disease Control and Prevention program grant (GISP PS001411-03 to K. K. H. and O. O. S.); and the University of Washington Center for AIDS Research, a NIH–funded program (grant number P30 AI027757).
Potential conflicts of interest. L. A. B. has received research support from Hologic. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 2015 International Society for STD Research World Congress, Brisbane, Australia.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta, GA: CDC, 2013. [Google Scholar]
- 2. Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012; 366:485–7. [DOI] [PubMed] [Google Scholar]
- 3. Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 2012; 61:590–4. [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. STD treatment guidelines 2015. Atlanta, GA: CDC, 2015. [Google Scholar]
- 5. Hicks LA, Taylor TH, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 368:1461–2. [DOI] [PubMed] [Google Scholar]
- 6. Soge OO, Harger D, Schafer S et al. . Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011). Sex Transm Dis 2012; 39:877–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grad YH, Harris SR, Kirkcaldy RD et al. . Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 2016; 214:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI Supplement M100. 27th ed. Wayne, PA: CLSI, 2017. [Google Scholar]
- 9. Liang JY, Cao WL, Li XD et al. . Azithromycin-resistant Neisseria gonorrhoeae isolates in Guangzhou, China (2009–2013): coevolution with decreased susceptibilities to ceftriaxone and genetic characteristics. BMC Infect Dis 2016; 16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 2011; 16. [PubMed] [Google Scholar]
- 11. Gose SO, Soge OO, Beebe JL, Nguyen D, Stoltey JE, Bauer HM. Failure of azithromycin 2.0 g in the treatment of gonococcal urethritis caused by high-level resistance in California. Sex Transm Dis 2015; 42:279–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papp JR, Abrams AJ, Nash E et al. . Azithromycin resistance and decreased ceftriaxone susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerg Infect Dis 2017; 23:830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkcaldy RD, Soge O, Papp JR et al. . Analysis of Neisseria gonorrhoeae azithromycin susceptibility in the United States by the Gonococcal Isolate Surveillance Project, 2005 to 2013. Antimicrob Agents Chemother 2015; 59:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirkcaldy RD, Harvey A, Papp JR et al. . Neisseria gonorrhoeae antimicrobial susceptibility surveillance—the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65:1–19. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Program (GISP) protocol Available at: https://www.cdc.gov/std/gisp/gisp-protocol-may-2016.pdf.
- 16. Dombrowski J, Kerani R, Golden M.. Routine CASI increased sexual history completeness among MSM STD clinic patients. In: Centers for Disease Control and Prevention STD Prevention Conference, Minneapolis, MN, 2012. [Google Scholar]
- 17. European Committee on Antimicrobial Susceptibility Testing Clinical breakpoint tables version 7.1 Available at: http://www.eucast.org/clinical_breakpoints/. Accessed 7 November 2017.
- 18. World Health Organization. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 19. Martin I, Sawatzky P, Liu G et al. . Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada. Emerg Infect Dis 2016; 22:65–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kong FY, Tabrizi SN, Fairley CK et al. . The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis. J Antimicrob Chemother 2015; 70:1290–7. [DOI] [PubMed] [Google Scholar]
- 21. Barbee LA, Dombrowski JC, Kerani R, Golden MR. Effect of nucleic acid amplification testing on detection of extragenital gonorrhea and chlamydial infections in men who have sex with men sexually transmitted disease clinic patients. Sex Transm Dis 2014; 41:168–72. [DOI] [PubMed] [Google Scholar]
- 22. Gratrix J, Singh AE, Bergman J et al. . Evidence for increased chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 2015; 60:398–404. [DOI] [PubMed] [Google Scholar]
- 23. Bissessor M, Tabrizi SN, Twin J et al. . Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 2015; 60:1228–36. [DOI] [PubMed] [Google Scholar]
- 24. Wind CM, de Vries E, Schim van der Loeff MF et al. . Decreased azithromycin susceptibility of Neisseria gonorrhoeae isolates in patients recently treated with azithromycin. Clin Infect Dis 2017; 65:37–45. [DOI] [PubMed] [Google Scholar]
- 25. Chisholm SA, Dave J, Ison CA. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother 2010; 54:3812–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horner PJ, Blee K, Falk L, van der Meijden W, Moi H. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS 2016; 27:928–37. [DOI] [PubMed] [Google Scholar]
- 27. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Inc, 2010. [Google Scholar]
- 28. ROCEPHIN [package insert]. South San Francisco, CA: Genentech USA, Inc, 2013. [Google Scholar]
- 29. Chisholm SA, Mouton JW, Lewis DA, Nichols T, Ison CA, Livermore DM. Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink?J Antimicrob Chemother 2010; 65:2141–8. [DOI] [PubMed] [Google Scholar]
- 30. Bignell C, Fitzgerald M; Guideline Development Group; British Association for Sexual Health and HIV UK UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 2011; 22:541–7. [DOI] [PubMed] [Google Scholar]
- 31. Bignell C, Unemo M, Board STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2013; 24:85–92. [DOI] [PubMed] [Google Scholar]
- 32. Australasian Sexual Health Alliance. Australian STI management guidelines 2014. Available at: http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management. Accessed 9 June 2017.
- 33. Barbee LA. Preparing for an era of untreatable gonorrhea. Curr Opin Infect Dis 2014; 27:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Golden MR, Whittington WL, Handsfield HH et al. . Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005; 352:676–85. [DOI] [PubMed] [Google Scholar]
- 35. Stenger MR, Kerani RP, Bauer HM et al. . Patient-reported expedited partner therapy for gonorrhea in the United States: findings of the STD surveillance network 2010–2012. Sex Transm Dis 2015; 42:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2015. Atlanta, GA: CDC, 2015. [Google Scholar]