Abstract
Previous studies established that transmissible prion diseases could be induced by in vitro-produced recombinant prion protein (PrP) fibrils with structures that are fundamentally different from that of authentic PrP scrapie isoform (PrPSc). To explain evolution of synthetic prions, a new mechanism referred to as deformed templating was introduced. Here, we asked whether an increase in expression level of the cellular form of PrP (PrPC) speeds up the evolution of synthetic strains in vivo. We found that in transgenic mice that overexpress hamster PrPC, PrPC overexpression accelerated recombinant PrP fibril-induced conversion of PrPC to the abnormal proteinase K-resistant state, referred to as atypical PrPres, which was the first product of PrPC misfolding in vivo. However, overexpression of PrPC did not facilitate the second step of synthetic strain evolution-transition from atypical PrPres to PrPSc, which is attributed to the stochastic nature of rare deformed templating events. In addition, the potential of atypical PrPres to interfere with replication of a short-incubation time prion strain was investigated. Atypical PrPres was found to interfere strongly with replication of 263K in vitro; however, it did not delay prion disease in animals. The rate of deformed templating does not depend on the concentration of substrate and is hence more likely to be controlled by the intrinsic rate of conformational errors in templating alternative self-propagating states.
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are fatal neurodegenerative disorders that can arise spontaneously, be inherited, or be acquired through transmission.1 The diversity in the cause of prion diseases can be explained by three mechanisms. Spontaneous TSE arises from the misfolding and aggregation of the normal, cellular isoform of the prion protein, PrPC, into the disease-associated infectious scrapie isoform, PrPSc, and thus, underlies the sporadic forms of prion diseases.2 Second, single point mutations or truncations in the PRNP gene cause inherited prion diseases via facilitating misfolding of PrPC.3, 4, 5 Third, in prion diseases acquired through the transmission, PrPSc seeds initiate prion replication by recruiting host PrPC. According to the conventional view of template-assisted replication mechanisms, prions replicate with high fidelity; ie, the folding pattern of a newly formed PrPSc accurately reproduces that of the PrPSc template.2
Recent studies introduced a new mechanism, referred to as deformed templating, according to which transmissible prion disease can be induced by PrP amyloid fibrils with structures that are fundamentally different from that of PrPSc.6, 7, 8, 9 The mechanism of deformed templating was used to explain transformation of self-replicating states during evolution of prion strains of synthetic origin.8, 10, 11, 12 Amyloid fibrils produced from recombinant Syrian hamster PrP (rPrP) in vitro lacked any detectable PrPSc particles, yet they were able to trigger transmissible TSEs in animals on serial passaging.8, 10 In another illustration of deformed templating, noninfectious fibrils with a stacked β-sheet architecture, prepared from recombinant fungal prion protein HET-s, nucleated the infectious state of HET protein with a β-solenoid structure.13 Further illustrations of the deformed templating mechanism are offered by the so-called quaking or amyloid seeding assays, in which infectious PrPSc nucleates noninfectious self-replicating fibrillar states when exposed to rPrP in vitro.14, 15 In direct support of the deformed templating mechanism, molecular imaging revealed that switching between alternative folding patterns can occur within individual amyloid fibrils.16
Previous studies established a mechanistic model for genesis of authentic PrPSc from noninfectious fibrils that involves two main steps (Figure 1).8, 10 In the first step, rPrP fibrils trigger PrPC misfolding, a process that results in accumulation of an alternative self-replicating state, referred to as atypical PrPres. rPrP and atypical PrPres appear to be similar in structure, because both have a short, C-terminal proteinase K (PK)-resistant core.8, 10, 17 In the second step, formation of authentic PrPSc is triggered via a relatively rare deformed templating event. After the first PrPSc particles are produced through deformed templating, PrPSc can replicate independently of atypical PrPres (Figure 1). This mechanism proposes that two barriers on a pathway from rPrP fibrils to PrPSc exist. The first barrier is attributed to a change in substrate from rPrP to PrPC. In contrast to rPrP, PrPC has a glycophosphatidylinositol-anchor and N-linked glycans, two types of post-translational modifications that might constrain conformational space acquired by self-replicating states.18 The second barrier is attributable to a low rate of deformed templating, the events by which structures different from that of atypical PrPres are generated (Figure 1).
Figure 1.
Schematic presentation of the mechanism, illustrating genesis of PrPSc triggered by rPrP fibrils. In a first step, rPrP fibrils seeded atypical PrPres, a transmissible form of PrP that replicates silently without causing clinical disease. Replication of atypical PrPres occasionally produces PrPSc in seeding events that appears to be rare and stochastic as described for a deformed templating mechanism. PrPSc replicates faster than atypical PrPres and eventually replaces it during serial passages. The two forms atypical PrPres and PrPSc can be distinguished after PK treatment via staining Western blot analyses with 3F4 and SAF-84 antibody. Because PK-resistant regions of atypical PrPres is shorter than that of PrPSc, it binds only SAF-84 antibody (epitope 160 to 170), whereas PrPSc binds both 3F4 (epitope 109 to 112) and SAF-84 antibodies. PK, proteinase K; atypical PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform; rPrP, recombinant prion protein.
In the current study we tested whether substrate plays a role in evolution of authentic PrPSc. Would evolution of infectious PrPSc structures be faster or more efficient in animals overexpressing PrPC? We found that, in transgenic (tg7) mice that overexpress hamster PrPC on an ablated background,19 high concentration of a substrate helped to overcome the first barrier, which is the transition from rPrP to PrPC as a substrate and formation of atypical PrPres. However, overexpression of PrPC did not facilitate crossing the second barrier, which is the transition from atypical PrPres to PrPSc. This result suggests that the rate of deformed templating is not controlled by PrPC expression levels. Moreover, although PrPC overexpression did not speed up the overall rate of evolution of synthetic prions, it expanded the range of self-replicating fibrillar states generated in vitro that were capable of inducing TSEs in animals.
Materials and Methods
Expression and Purification of rPrP and Formation of rPrP Fibrils
Syrian hamster full-length recombinant PrP that encompass residues 23 to 231 was expressed and purified as previously described,20 with minor modifications.9 Lyophilized rPrP was dissolved in 5 mmol/L HEPES, pH 7.0, immediately before use. To form fibrils for inoculations, a mixture of 0.5 mg/mL rPrP with 50 mmol/L 2-(N-morpholino)ethanesulfonic acid buffer, pH 6.0, and 2.0 mol/L guanidine hydrochloride (GdnHCl) was incubated at 37°C under continuous agitation. Amyloid formation was confirmed by thioflavin T fluorescence assay, epifluorescent microscopy, and electron microscopy as described previously.20 For tg7 mouse inoculations, fibrils were diluted to 0.23 mg/mL rPrP and dialyzed into phosphate-buffered saline (PBS), pH 7.4. To prepare α-rPrP for inoculating the control group, rPrP stock solution was diluted in PBS to a final protein concentration of 0.23 mg/mL. Conformation of α-rPrP was confirmed by circular dichroism. Syrian hamsters were inoculated with fibrils at the concentration of 0.5 mg/mL rPrP dialyzed into PBS, pH 7.4.
Bioassay
All inoculations were performed intracerebrally, under 2% O2/4 minimum alveolar concentration isoflurane anesthesia. Each mouse or hamster received 30 or 50 μL of inoculum, respectively, into the left hemisphere, approximately 3 mm to the left of the midline and approximately 3 mm anterior to a line drawn between the ears. After inoculation, animals were observed daily for disease with the use of a blind scoring protocol. For the first passage, weanling tg7 mice or Syrian hamsters were inoculated with rPrP amyloid fibrils or α-rPrP prepared as described above. Mice and hamsters were euthanized at 510 to 524 and 659 to 664 days after inoculation, respectively, without any sign of clinical disease, and their brains were removed aseptically and saved for analysis and second passage. For the second and third passages, 10% brain homogenates (BHs) prepared by homogenization in PBS, pH 7.4,11 were dispersed by 30 seconds of sonication immediately before inoculation. For the experiment on interference, protein misfolding cyclic amplification with partially deglycosylated substrate (dgPMCA)-derived atypical PrPres produced as described below was diluted 10-fold with PBS supplemented with 1% bovine serum albumin and spiked with 10−4 brain material from a terminally ill animal infected with 263K. As controls, 10-fold diluted dgPMCA-derived atypical PrPres or 10−4 263K in PBS with 1% bovine serum albumin was inoculated separately. This study was performed in strict accordance with the recommendations in the NIH's Guide for the Care and Use of Laboratory Animals.21 The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore (assurance no. A32000-01; permit no. 0215002).
PK Digestion, Western Blot Analysis, and Data Analysis
For the PK digestion in sarcosyl, an aliquot of 10% BH prepared as described previously11 was mixed with an equal volume of 4% sarcosyl in PBS, supplemented with 50 mmol/L Tris, pH 7.5, and digested with 20 μg/mL PK (New England BioLabs, Ipswich, MA) for 30 minutes at 37°C with 1000 rpm shaking with the use of a DELFIA plate shaker (Perkin Elmer, Waltham, MA) placed in an incubator at 37°C. PK digestion was stopped by adding SDS sample buffer and heating the samples for 10 minutes in a boiling water bath. Samples were loaded onto NuPAGE 12% Bis-Tris gels, transferred to polyvinylidene difluoride membrane, and probed with 3F4 or SAF-84 antibodies.
To ensure accurate calculation of PrPSc amount in brains of animals from second and third passage, 10% BHs were dispersed by 30 seconds of sonication of 100-μL aliquots in the microplate horn of Qsonica sonicator (Newton, CT), the total protein concentration was measured by Bradford Assay (Bio-Rad, Hercules, CA), and normalized before performing PK digestion. For statistical analysis, samples from the second and third passages were loaded onto the same gel and were also probed with anti–β-actin antibody (Sigma-Aldrich, St. Louis, MO) to verify equal gel loading. Images were captured with the use of FluorChem imager (ProteinSimple, San Jose, CA). The signal intensity was digitized for densitometry analysis with the use of AlphaView software version 4.1.3 (ProteinSimple), then normalized by β-actin signal, and statistical significance was calculated with the use of t-test.
PMCAb
Normal BH (10%) from healthy hamsters was prepared as described previously8 and was used as a substrate for PMCA with beads (PMCAb).22 For the first round, 10 μL of BH from inoculated animals was added to 90 μL of normal BH. The standard sonication program consisted of 20 seconds of sonication pulses at approximately 150 W applied every 20 minutes during a 24-hour period. For each subsequent round, 10 μL of the reaction from the previous round was added to 90 μL of fresh substrate. Each PMCAb reaction was performed in the presence of two 3/32-inch Teflon beads (McMaster-Carr, Los Angeles, CA). To analyze production of PK-resistant PrP material in PMCAb, 10 μL of sample was supplemented with 5 μL of SDS and 5 μL of PK to a final concentration of SDS and PK of 0.25% and 50 μg/mL, respectively, followed by incubation at 37°C for 1 hour. The digestion was terminated by adding SDS-sample buffer and heating the samples for 10 minutes in a boiling water bath.
dgPMCAb and Generation of Atypical PrPres
To produce substrate for dgPMCAb, 10% normal BH from healthy hamsters prepared for PMCAb (see section above) was treated with peptide N-glycosidase F (New England BioLabs; glycerol-free) as follows. After preclearance of normal BH at 500 × g for 2 minutes, 1500 U/mL peptide N-glycosidase F was added to the supernatant fluid, and the reaction was incubated on a rotator at 37°C for 5 hours. The resulting substrate was used in dgPMCAb with sonication conditions described for PMCAb.
To produce atypical PrPres for in vitro and in vivo interference study, dgPMCA substrate was treated with RNase A (Sigma-Aldrich; catalog no. R4875) for 1 hour at 37°C, as described previously,23 and seeded with 10−9 brain material of an animal from the second passage of synthetic strain S05, which contained predominantly atypical PrPres.10 dgPMCAb was performed for 18 serial rounds with 10-fold dilution between rounds. The absence of PrPSc in the final products was confirmed by failure to amplify in six rounds of PMCAb (data not shown).
Histopathologic Study
Formalin-fixed brain halves divided at the midline were processed for immunohistochemistry staining with the use of the mouse monoclonal anti-PrP antibody 3F4 (dilution 1:1000; BioLegend, San Diego, CA). Blocks were treated in formic acid (96%) before being embedded in paraffin. Epitope recovery was performed by 15 minutes of hydrated autoclaving at 121°C in trisodium citrate buffer, pH 6.0, with 0.05% Tween 20, followed by 5 minutes in 88% formic acid.
Results
2M GdnHCl rPrP Fibrils Induce Formation of Atypical PrPres in tg7 Mice
In previous studies, fibrils produced in vitro with the use of full-length Syrian hamster rPrP in different solvent conditions had different capacities for seeding misfolding of PrPC in vivo, arguing that the efficiency in triggering authentic PrPSc is controlled by the conformation of rPrP fibrils.10 On inoculation of Syrian hamsters, fibrils formed in 0.5 mol/L GdnHCl (referred to as 0.5 M fibrils) induced atypical PrPres in six of seven animals in the first passage and caused transmissible prion disease in the second passage.10 In contrast, no signs of PK-resistant products were detected by Western blot analysis or serial PMCAb assay in animals (0 of 7 animals) inoculated by the fibrils formed in 2 mol/L GdnHCl (2 M fibrils10) (Figure 2A).
Figure 2.
Bioassay of 2 M rPrP fibrils in tg7 mice. A: Western blot analyses of brain homogenates from either tg7 mice inoculated with 2 M rPrP fibrils or α-rPrP or Syrian hamsters inoculated with 2 M rPrP fibrils and stained with 3F4 or SAF-84 antibody. The inoculation of fibril material is referred to as first passage. B: Western blot analyses of brain homogenates from the second passage of 2 M rPrP fibrils or α-rPrP in tg7 mice stained with 3F4 or SAF-84 antibody. PK-resistant bands at 23, 16, and 13 kDa represent di-, mono-, and unglycosylated atypical PrPres, respectively. PK, proteinase K; atypical PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform; rPrP, recombinant prion protein; tg, transgenic.
The current work asked the question whether an increase in expression level of PrPC helps to trigger TSEs by 2 M fibrils that would otherwise fail to induce disease in wild-type animals. Tg7 mice that express PrPC at a level approximately 3.5-fold higher than that in hamster19 were inoculated with 2 M fibrils or rPrP folded into a normal α-helical conformation (α-rPrP). Two of seven tg7 mice inoculated with 2 M fibrils produced atypical PrPres (Figure 2A and Table 1). Discrimination between atypical PrPres and PrPSc is possible because PK-resistant fragments of atypical PrPres encompass residues approximately 150 to 231 and is immunoreactive with SAF-84 (epitope 160 to 170) but not 3F4 antibody (epitope 109 to 112).8, 24 PK-resistant core of PrPSc encompasses residues approximately 90 to 231 and can be detected by both 3F4 and SAF-84. As in previous studies,8, 10, 12 atypical PrPres consisted of three bands of 23, 16, and 13 kDa, corresponding to di, mono-, and unglycosylated C-terminal fragments, respectively, which are immunoreactive with SAF-84 but not 3F4 antibody (Figure 2A). No PK-resistant PrP material was found in the control tg7 group inoculation with α-rPrP (0 of 8) or the hamster group inoculated with 2 M fibrils (0 of 8) (Figure 2A and Table 1). PrPSc was not detected in any of these three animal groups as probed by 3F4 antibody (Figure 2A). In summary, although 2 M fibrils were unable to trigger PrPC misfolding in wild-type animals, they induced formation of atypical PrPres in two of seven animals overexpressing hamster PrPC. This result suggests that an increase in PrPC expression level facilitates crossing of the first barrier, ie, a transition from rPrP fibrils to atypical PrPres.
Table 1.
Bioassay of rPrP Amyloid Fibrils
| Inoculum | Host | ns/nt∗ | nPKres/nT† | Euthanized, days after inoculation |
|---|---|---|---|---|
| 2 M rPrP fibrils (first passage) | tg7 mice | 0/7 | 2‡/7 | 510–524 |
| 2 M rPrP fibrils (first passage) | Hamsters | 0/7 | 0/7 | 659–664 |
| 0.5 M rPrP fibrils (first passage)§ | Hamsters | 0/7 | 6/7 | 723 |
| α-rPrP-monomer (first passage) | tg7 mice | 0/8 | 0/8 | 524 |
| Second passage of 2 M rPrP fibrils from tg7 | tg7 mice | 0/8 | 8/8 | 447 |
| Second passage of α-rPrP from tg7 | tg7 mice | 0/3 | 0/3 | 447 |
| Third passage of 2 M rPrP fibrils from tg7 | Syrian hamsters | 5/5 | 5/5 | 603 |
| Third passage of α-rPrP from tg7 | Syrian hamsters | 0/4 | 0/4 | 602 |
BH, brain homogenate; PK, proteinase K; atypical PrPres, alternative self-replicating state of prion protein; rPrP, recombinant prion protein; tg, transgenic.
Number of animals with clinical signs over the total number of animals survived to the end of the experiment.
Number of animals with PK-resistant PrP in BHs over the total number of animals survived to the end of the experiment.
In two BHs atypical PrPres were found by PK digestion assay.
Data are from Makarava et al.10
In previous experiments, wild-type animals had already produced subclinical levels of PrPSc by the first passage on inoculation of 0.5 M fibrils.10 In contrast, no PrPSc was found in the first passage of 2 M fibrils in tg7 mice, either by Western blot analysis (Figure 2A) or serial PMCAb (data not shown). Lack of detectible PrPSc in tg7 mice suggests that overexpression of PrPC does not help to speed up the transition from atypical PrPres to PrPSc, ie, crossing of the second barrier. Alternatively, unlike atypical PrPres produced by 0.5 M fibrils, atypical PrPres generated by 2 M fibrils might be unable to produce authentic PrPSc. To address this question, a second passage was conducted in tg7 mice.
Overexpression of PrPC Does Not Facilitate Transition from Atypical PrPres to PrPSc
Brain materials from one tg7 mouse positive for atypical PrPres and one control tg7 mouse inoculated with α-rPrP were used to produce second passage in tg7 mice. All eight animals from the second passage of 2 M fibrils showed substantial amounts of atypical PrPres in their brains, but they did not develop TSE signs for up to 447 days after inoculation (Figure 2B and Table 1). In agreement with the model presented in Figure 1, small amounts of PrPSc were detected in all brains by Western blot analysis, in addition to atypical PrPres (Figure 2B). No atypical PrPres or PrPSc was found in the second passage of α-rPrP (Figure 2B). In good agreement with previous studies,8, 10, 12 atypical PrPres was found to be transmissible, and its serial passaging resulted in formation of PrPSc. However, because PrPSc appeared only in the second passage, overexpression of PrPC did not seem to affect the rate of deformed templating events that led to PrPSc.
Further evidence that the high level of PrPC expression does not speed up deformed templating events was found in a comparison of the amounts of atypical PrPres and PrPSc in the following animal groups: Tg7 mice from second passage of 2 M fibrils and asymptomatic hamsters from the second passage of 0.5 M fibrils.10 The amounts of atypical PrPres were much higher in tg7 mice than in hamsters (Figure 3), which is consistent with higher expression levels of PrPC in tg7 mice. However, the amounts of PrPSc in the second passage of 2 M fibrils were approximately the same as in the asymptomatic animals from the second passage of 0.5 M fibrils (Figure 3). The size of the PK-resistant bands and the glycoform ratio were similar in atypical PrPres generated from 0.5 M fibrils in hamsters and from 2 M fibrils in tg7 mice. In summary, these results demonstrate that overexpression of PrPC accelerates accumulation of atypical PrPres but does not help crossing the second barrier, ie, the transition from atypical PrPres to PrPSc.
Figure 3.
Comparison of PrPSc and atypical PrPres amounts in animals from the second passage. Western blot analyses of brain homogenates from the second passage of 0.5 M rPrP fibrils in Syrian hamsters or the second passage of 2 M rPrP fibrils or α-rPrP in tg7 mice. Brain materials were treated with increasing concentrations of PK as indicated and stained with 3F4 or SAF-84 antibody. PK, proteinase K; atypical PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform; rPrP, recombinant prion protein; tg, transgenic.
Third Passage of 2 M Fibrils Leads to Accumulation of PrPSc and Clinical Disease
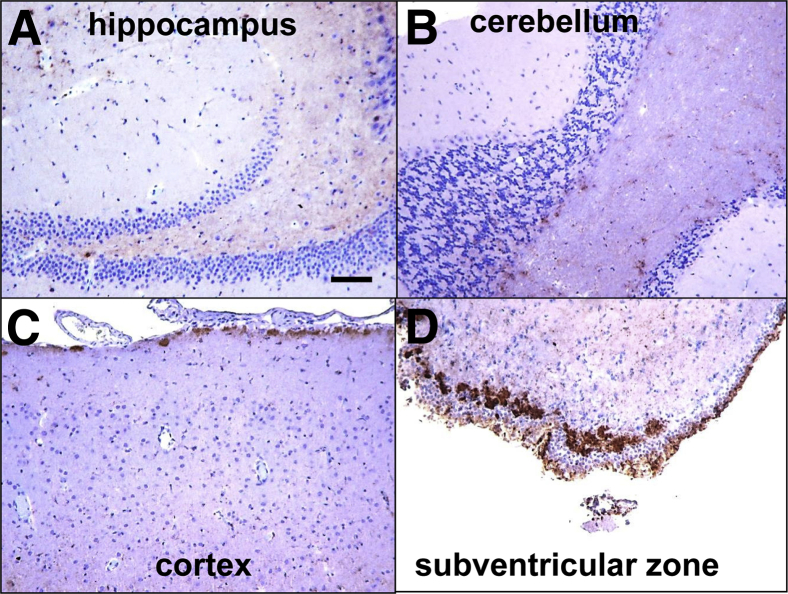
According to the model in Figure 1, atypical PrPres and PrPSc are two alternative self-propagating forms that compete for the same substrate. In previous studies, PrPSc outcompeted and replaced atypical PrPres during serial passaging.8, 10 To test whether this is the case for transmissible materials produced from 2 M fibrils and to allow more time for this process to take place, the third passage of 2 M fibrils was performed in Syrian hamsters instead of tg7 mice. Atypical PrPres continued to propagate in the third passage, although its amount declined compared with the amounts in the second passage (Figure 4A). Nevertheless, this highlights the highly transmissible nature of this state. Remarkably, the amounts of PrPSc increased significantly in the third passage compared with the group from the second passage (Figure 4, A and B, and Supplemental Figure S1). Moreover, clinical disease was observed at approximately 500 days after inoculation (Table 1). Consistent with slow disease progression in previous studies on hamster synthetic strains,8, 10 the clinical disease here progressed slowly, and animals approached terminal stages approximately 100 days after the first signs. Staining of brain slices with 3F4 revealed large plaques in the subpial and subependymal region (Figure 5), which is a hallmark of hamster synthetic strains (Figure 5).8, 9, 10 Diffuse, synaptic PrP immunoreactivity was observed in multiple brain areas, including hippocampus and cerebellum (Figure 5). In summary, similar to the previous studies on synthetic strains S05 and LOTSS,8, 10 serial passaging led to accumulation of PrPSc, a decline in the amounts of atypical PrPres, and a development of clinical prion disease.
Figure 4.
Third passage of 2 M rPrP fibrils or α-rPrP. A: Western blot analyses of brain homogenates from the third passage of 2 M rPrP fibrils or α-rPrP conducted in Syrian hamsters. Analysis of brain materials from the second passage of 2 M rPrP fibrils is provided for comparison. Western blot analyses were stained with 3F4 or SAF-84 antibody, as indicated. 3F4 reacts only with PrPSc, whereas SAF-84 stains both PrPSc and atypical PrPres. B: Quantification and statistical analysis of PrPSc in animal brains from the second and third passages of 2 M fibrils. For calculating means ± SD, the Western blot analysis presented in Supplemental Figure S1 (eight animals form the second and five animals from the third passages) stained with 3F4 antibody was used. PrPSc signal intensity was normalized by β-actin signal intensity. ∗∗∗P < 0.0005. PK, proteinase K; atypical PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform; rPrP, recombinant prion protein.
Figure 5.
PrP depositions in hamsters from the third passage. A–D: PrP immunoreactivity stained with 3F4 antibody in hippocampus (A), cerebellum (B), cortex (C), and subventricular zone (D) in animals from the third passage of 2 M fibrils. Scale bar = 200 μm. PrP, prion protein.
Interference between Atypical PrPres and PrPSc
Considering the competing nature of atypical PrPres and PrPSc and because replication of atypical PrPres alone does not cause the disease,8, 10, 12 we were interested in testing whether atypical PrPres material could be used to suppress replication of PrPSc and to delay or prevent prion disease. To prepare atypical PrPres, brain-derived material from an asymptomatic S05 animal of the second passage with high concentration of atypical PrPres10 was used to seed a dgPMCAb reaction (the PMCAb format that uses partially deglycosylated PrPC as a substrate17). To make sure that the inoculum lacks even miniscule amounts of PrPSc, 18 serial dgPMCAb rounds were conducted with RNase-treated substrate (see Materials and Methods).
In support of our hypothesis, in vitro experiments revealed that the presence of atypical PrPres interfered with the amplification of PrPSc of hamster-adapted scrapie strain 263K in PMCAb (Figure 6). Notably, in dgPMCAb that uses partially deglycosylated PrPC as a substrate,17 atypical PrPres was amplifiable regardless of whether 263K PrPSc was present in the reaction mixture (Figure 6).
Figure 6.
Competition between atypical and PrPSc in PMCAb or dgPMCAb. Serial PMCAb or dgPMCAb reactions were seeded with 10−4 diluted 263K brain material (lanes 2 to 4), 10 μL of dgPMCAb reaction that contained atypical PrPres (lanes 5 to 7) or a mixture of 263K and atypical PrPres (lanes 8 and 9). Three serial rounds were conducted with 10-fold dilution between rounds. Western blot analyses were stained with 3F4 or SAF-84 antibody as noted. dgPMCAb, protein misfolding cyclic amplification with beads and partially deglycosylated substrate; PK, proteinase K; PMCAb, protein misfolding cyclic amplification with beads; atypical PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform.
Next, we tested the interference effect with the use of animal bioassay. Hamsters were inoculated with 10−4 diluted 263K brain-derived material alone, 10−4 diluted 263K brain-derived material mixed with dgPMCAb-derived atypical PrPres, or dgPMCAb-derived atypical PrPres. As expected, animals inoculated with 263K brain material developed first clinical sings of TSE at 84 ± 3 days after inoculation (16 of 16) and showed a strong signal on Western blot analysis (Figure 7). Animals inoculated with a mixture of 263K and atypical PrPres developed clinical diseases at 84 ± 8 days after inoculation (6 of 6) and showed an equally strong signal on Western blot analysis. Unfortunately, mixing atypical PrPres with 263K did not delay the incubation time to disease nor slow the progression of the disease. Animals inoculated with atypical PrPres alone were asymptomatic, and no PrPSc or atypical PrPres were detected in their brains at 116 days after inoculation (Figure 7). However, the animals inoculated with atypical PrPres did show substantial amounts of atypical PrPres in their brain, although without any clinical signs when euthanized at 606 days after inoculation (Figure 7). This result establishes that, although atypical PrPres is transmissible, it takes a long time to accumulate in vivo, and atypical PrPres does not effectively interfere with a short-incubation time strain.
Figure 7.
Bioassay for interference between atypical and PrPSc. Hamsters were inoculated with 10−4 263K brain-derived material alone, 10−4 263K brain-derived material mixed with dgPMCAb-derived atypical PrPres, or dgPMCAb-derived atypical PrPres alone. Animals inoculated with atypical PrPres were euthanized at 116 or 606 dpi. Western blot analyses were stained with SAF-84 antibody. dgPMCAb, protein misfolding cyclic amplification with beads and partially deglycosylated substrate; dpi, days after inoculation; PK, proteinase K; atypical PrPres, alternative self-replicating state of prion protein; PrPSc, prion protein scrapie isoform.
Discussion
Current work provides new insight into the evolution of transmissible prion states and helps to refine the model developed from previous studies.8, 10 First, we showed that although 2 M rPrP fibrils failed to induce PrPSc and TSE in wild-type animals, they triggered prion replication de novo in tg7 mice. Because serial transmission of α-rPrP failed to induce TSE in tg7 animals, this effect appears to be fibril specific. Taken together these results illustrate that the range of self-replicating conformations generated in vitro that are capable of inducing TSEs can be expanded in animal models that overexpress PrPC. Second, the current work illustrates that overexpression of PrPC helps to overcome the first barrier, ie, the transition from rPrP fibrils to atypical PrPres. Because atypical PrPres is the first product of PrPC misfolding seeded by rPrP fibrils in vivo, the first barrier is attributable to a change in a substrate from rPrP to PrPC. Third, overexpression of PrPC did not facilitate passing the second barrier, which is the transition from atypical PrPres to PrPSc. PrPSc is produced in seeding events that appear to be rare and stochastic in nature and are described by a deformed templating mechanism.8, 10 Current results argue that the rate of deformed templating does not depend on the expression level of a substrate but is likely to be controlled by the nature of the template, intrinsic rate of conformational errors in templating, and, possibly, the biochemical environment. We do not know whether the rate of deformed templating events depends on cofactors and/or their species specificity. If it does, cofactors might not only shape the strain-specific structure, as was shown in a previous study,25 but also regulate evolutionary processes. In summary, although the transition from rPrP to atypical PrPres happened more efficiently in tg7 mice, overexpression of PrPC did not speed up disease development.
Previous studies on mouse synthetic prions showed that a range of PrPSc conformations with different incubation times to disease and disease phenotypes can be generated in mice overexpressing mouse PrPC with the use of rPrP fibrils produced in vitro under different solvent conditions.26, 27, 28, 29, 30 It is not clear whether any atypical PrP states are involved in the evolution of synthetic mouse prions, because no mouse atypical PK-resistant states were described. Nevertheless, because the structures of mouse rPrP fibrils generated in vitro and PrPSc were found to be fundamentally different,31, 32 a transition from fibril-specific to PrPSc-specific structures should involve deformed templating, whether it occurs directly or via as yet unknown intermediate steps. In a manner similar to evolution of synthetic hamster strains,11 physical and biological properties of mouse strains changed gradually on serial passages,27, 29, 30 highlighting the common trends in evolution of strains of synthetic origin.
As judged from the size and position of the PK-resistant core, atypical PrPres of synthetic origin is similar to abnormal C-terminal PK-resistant fragments found in most individuals with sporadic Creutzfeldt-Jakob disease, iatrogenic Creutzfeldt-Jakob disease, or mice infected with mouse-passaged hamster scrapie.33, 34, 35 Similar C-terminal PK-resistant fragments were found in atypical bovine spongiform encephalopathy and in certain types of ovine scrapie.36, 37 In a report, co-accumulation of atypical PrPres and PrPSc was reported in a patient who succumbed to sporadic Creutzfeldt-Jakob disease with a prolonged clinical course.38 Nevertheless, the relation between atypical PrPres of synthetic and natural origins has not yet been established.24
Previously infecting a host with two prion strains, one with a short and another with a long incubation time, was shown to lead to an extension of the incubation period to disease compared with the period for the short incubation time strain, a phenomenon known as prion strain interference.39, 40, 41, 42, 43 In the current study, we tested whether atypical PrPres can replace a long incubation time strain and interfere with replication of a short incubation time strain. Although transmissible, atypical PrPres does not produce prion disease8, 10, 12 and thereby might offer advantages as an interfering agent. In vitro, atypical PrPres and PrPSc could be selectively amplified from their mixtures in two PMCAb formats via modification of the biochemical environment and the PrPC glycosylation status.17 Unfortunately, mixing atypical PrPres with the short incubation time strain 263K did not delay the incubation time to the disease. There are several possible explanations for the lack of interference. Similar to PrPSc, atypical PrPres undergoes clearance after inoculation. Accumulation of atypical PrPres after clearance might occur slower than accumulation of the short incubation time strains, such as 263K, leading to a development of TSE before atypical PrPres had a chance to accumulate. In addition, atypical PrPres might not be effective as an interfering agent because of differences in neurotropism between atypical PrPres and 263K and/or differences in subcellular replication sites. Deposition of atypical PrPres in different brain regions was found to overlap only partially with that of natural or synthetic prion strains.12 Unlike PrPSc, atypical PrPres did not show perineuronal or perivascular immunoreactivity.12 If the replication rates of PrPSc and atypical PrPres were not controlled exclusively by a substrate but also availability of state-specific cellular cofactors as well, differences in cellular cofactors might contribute too to the apparent lack of an interference effect.17 Failure to delay the disease could be due to differences in the experimental design between the current and previous studies. In previous studies the long incubation time strains were inoculated much earlier than the short incubation time strains, allowing the long incubation time strains more time to spread through cellular replication sites.39, 40, 41, 42, 43 Finally, the most recent studies that used two-dimensional analysis of PrP sialoglycoforms revealed that only a small subfraction of the PrPC that is recruited as a substrate into PrPSc could also serve as a substrate for atypical PrPres.44 This explains why atypical PrPres replicates slower than PrPSc.
Conclusions
In previous studies, a mechanistic model for genesis of authentic PrPSc from noninfectious fibrils that involves two main steps was established.8, 10 The current study tested whether expression levels of substrate play a role in evolution of synthetic prions. We found that overexpression of PrPC facilitated the first step toward PrPSc, ie, the transition from rPrP fibrils to atypical PrPres. However, the second step toward PrPSc, which is controlled by the rate of deformed templating events, did not depend on the expression levels of substrate. These results suggest that the intrinsic rate of conformational errors in templating alternative self-propagating states is independent of substrate concentration. Nevertheless, overexpressing PrPC helped to expand the range of self-replicating states generated in vitro that are capable of inducing TSEs in animals. In addition, the current work explored the potential of atypical PrPres to interfere with replication of a short incubation time prion strain. Atypical PrPres was found to interfere effectively with replication of short incubation time strain 263K in vitro; however it did not delay prion disease in animals inoculated with 263K.
Acknowledgment
We thank Pamela Wright for editing the manuscript.
Footnotes
See related Commentary on page 761
Supported by NIH grants R01 NS045585 and R01 NS074998 (I.V.B.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.11.013.
Supplemental Data
Western blot analyses of brain homogenates from the second and third passages of 2 M rPrP fibrils. Western blot analyses were stained with 3F4 or SAF-84 antibody. Western blot analysis of β-actin is provided as a loading control; signal intensity of β-actin was used for normalizing signal intensity of 3F4-positive materials for statistical analysis of PrPSc amounts. PK, proteinase K; PrPSc, prion protein scrapie isoform; rPrP, recombinant prior protein.
References
- 1.Prusiner S.B. Prion diseases and the BSE crisis. Science. 1997;278:245–251. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 2.Cohen F.E., Prusiner S.B. Pathologic conformations of prion proteins. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner S.B., Scott M.R. Genetics of prions. Annu Rev Genet. 1997;31:139–175. doi: 10.1146/annurev.genet.31.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Sigurdson C.J., Nilsson K.P., Hornemann S., Heikenwalder M., Manco G., Schwartz P., Ott D., Rulicke T., Liberski T., Julius C., Falsiq J., Stitz L., Wuthrich K., Aguzzi A. De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci U S A. 2009;106:304–309. doi: 10.1073/pnas.0810680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson W.S., Borkowski A.W., Faas H., Steele A.D., King O.D., Watson N., Jasanoff A., Lindquist S. Spontaneous generation of prion infectivity in fatal familial insomnia knockin mice. Neuron. 2009;63:438–450. doi: 10.1016/j.neuron.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makarava N., Baskakov I.V. Genesis of transmissible protein states vie deformed templating. Prion. 2012;6:252–255. doi: 10.4161/pri.19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makarava N., Baskakov I.V. The evolution of transmissible prions: the role of deformed templating. PLoS Pathog. 2013;9:e1003759. doi: 10.1371/journal.ppat.1003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Genesis of mammalian prions: from non-infectious amyloid fibrils to a transmissible prion disease. PLoS Pathog. 2011;7:e1002419. doi: 10.1371/journal.ppat.1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makarava N., Kovacs G.G., Bocharova O.V., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Ostapchenko V.G., Budka H., Rohwer R.G., Baskakov I.V. A new mechanism for transmissible prion diseases. J Neurosci. 2012;32:7345–7355. doi: 10.1523/JNEUROSCI.6351-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarava N., Kovacs G.G., Savtchenko R., Alexeeva I., Budka H., Rohwer R.G., Baskakov I.V. Stabilization of a prion strain of synthetic origin requires multiple serial passages. J Biol Chem. 2012;287:30205–30214. doi: 10.1074/jbc.M112.392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs G.G., Makarava N., Savtchenko R., Baskakov I.V. Atypical and classical forms of the disease-associated state of the prion protein exhibit distinct neuronal tropism, deposition patterns, and lesion profiles. Am J Pathol. 2013;183:1539–1547. doi: 10.1016/j.ajpath.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan W., Bian W., McDonald M., Kijac A., Wemmer D.E., Stubbs G. Heterogeneous seeding of a prion structure by a generic amyloid form of the fungal prion-forming domain HET-s(218–289) J Biol Chem. 2013;288:29604–29612. doi: 10.1074/jbc.M113.505511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atarashi R., Moore R.A., Sim V.L., Hughson A.G., Dorward D.W., Onwubiko H.A., Priola S.A., Caughey B. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4:645–650. doi: 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- 15.Colby D.W., Zhang Q., Wang S., Groth D., Legname G., Riesner D., Prusiner S.B. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci U S A. 2007;104:20914–20919. doi: 10.1073/pnas.0710152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makarava N., Ostapchenko V.G., Savtchenko R., Baskakov I.V. Conformational switching within individual amyloid fibrils. J Biol Chem. 2009;284:14386–14395. doi: 10.1074/jbc.M900533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarava N., Savtchenko R., Baskakov I.V. Selective amplification of classical and atypical prions using modified protein misfolding cyclic amplification. J Biol Chem. 2013;288:33–41. doi: 10.1074/jbc.M112.419531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breydo L., Sun Y., Makarava N., Lee C.I., Novitskaia V., Bocharova O.V., Kao J.P., Baskakov I.V. Nonpolar substitution at the C-terminus of the prion protein, a mimic of the glycosylphosphatidylinositol anchor, partially impairs amyloid fibril formation. Biochemistry. 2007;46:852–861. doi: 10.1021/bi061923v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kercher L., Favara C., Chan C.C., Race R., Chesebro B. Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types. Am J Pathol. 2004;165:2055–2067. doi: 10.1016/S0002-9440(10)63256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bocharova O.V., Breydo L., Parfenov A.S., Salnikov V.V., Baskakov I.V. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical property of PrP(Sc) J Mol Biol. 2005;346:645–659. doi: 10.1016/j.jmb.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 21.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council . ed 8. National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 22.Gonzalez-Montalban N., Makarava N., Ostapchenko V.G., Savtchenko R., Alexeeva I., Rohwer R.G., Baskakov I.V. Highly efficient protein misfolding cyclic amplification. PLoS Pathog. 2011;7:e1001277. doi: 10.1371/journal.ppat.1001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Montalban N., Makarava N., Savtchenko R., Baskakov I.V. Relationship between conformational stability and amplification efficiency of prions. Biochemistry. 2011;50:7933–7940. doi: 10.1021/bi200950v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klimova N., Makarava N., Baskakov I.V. The diversity and relationship of prion protein self-replicating states. Virus Res. 2015;207:113–119. doi: 10.1016/j.virusres.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deleault N.R., Walsh D.J., Piro J.R., Wang F., Wang X., Ma J., Rees J.R., Supattapone S. Cofactor molecules maintain infectious conformation and restrict strain properties in purified prions. Proc Natl Acad Sci U S A. 2012;109:E1938–E1946. doi: 10.1073/pnas.1206999109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legname G., Baskakov I.V., Nguyen H.O., Riesner D., Cohen F.E., DeArmond S.J., Prusiner S.B. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 27.Colby D.W., Giles K., Legname G., Wille H., Baskakov I.V., DeArmond S.J., Prusiner S.B. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci U S A. 2009;106:20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colby D.W., Wain R., Baskakov I.V., Legname G., Palmer C.G., Nguyen H.O., Lemus A., Cohen F.E., DeArmond S.J., Prusiner S.B. Protease-sensitive synthetic prions. PLoS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaemmaghami S., Watts J.C., Nquyen H.O., Hayashi S., DeArmond S.J., Prusiner S.B. Conformational transformation and selection of synthetic prion strains. J Mol Biol. 2011;413:527–542. doi: 10.1016/j.jmb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghaemmaghami S., Colby D.W., Nquyen H.O., Hayashi S., Oehler A., DeArmond S., Prusiner S.B. Convergent replication of mouse synthetic prion strains. Am J Pathol. 2013;182:866–874. doi: 10.1016/j.ajpath.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wille H, McDonald M, Bian W, Kendall A, Borovinskiy A, Cohen F, Prusiner S, Stubbs G: X-ray fiber diffraction reveals major structural differences between brain-derived prions and recombinant prion protein amyloid. Oct 8–10, 2008, Madrid, Spain, International Conference Prion-2008, 2008, Abstract 39.
- 32.Ostapchenko V.G., Sawaya M.R., Makarava N., Savtchenko R., Nilsson K.P., Eisenberg D., Baskakov I.V. Two amyloid states of the prion protein display significantly different folding patterns. J Mol Biol. 2010;400:908–921. doi: 10.1016/j.jmb.2010.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou W.Q., Capellari S., Parchi P., Sy M.S., Gambetti P., Chen S.G. Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem. 2003;278:40429–40436. doi: 10.1074/jbc.M308550200. [DOI] [PubMed] [Google Scholar]
- 34.Satoh K., Muramoto T., Tanaka T., Kitamoto N., Ironside J.W., Nagashima K., Yamada M., Sato T., Mohri S., Kitamoto T. Association of an 11-12 kDa protease-resistant prion protein fragment with subtypes of dura graft-associated Creutzfeldt-Jakob disease and other prion diseases. J Gen Virol. 2003;84:2885–2893. doi: 10.1099/vir.0.19236-0. [DOI] [PubMed] [Google Scholar]
- 35.Lawson V.A., Priola S.A., Meade-White K., Lawton M., Chesebro B. Flexible N-terminal region of prion protein influences conformation of protease-resistant prion protein isoforms associated with cross-species scrapie infection in vivo and in vitro. J Biol Chem. 2004;279:13689–13695. doi: 10.1074/jbc.M303697200. [DOI] [PubMed] [Google Scholar]
- 36.Biacabe A.G., Jacobs J.G., Bencsik A., Langeveld J.P., Baron T.G. H-type bovine spongiform encephalopathy: complex molecular features and similarities with human prion diseases. Prion. 2007;1:61–68. doi: 10.4161/pri.1.1.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baron T., Bencsik A., Vulin J., Biacabe A.G., Morignat E., Verchere J., Betemps D. A C-terminal protease-resistant prion fragment distinguishes ovine “CH1641-like” scrapie from bovine classical and L-Type BSE in ovine transgenic mice. PLoS Pathog. 2008;4:e1000137. doi: 10.1371/journal.ppat.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues-Martinez A.B., de Munain A.L., Ferrer I., Zarranz J.J., Atares B., Villagra N.T., Arteagoitia J.M., Garrido J.M., Juste R.A. Coexistence of protease sensitive and resistant prion protein in 129VV homozygous sporadic Creutzfeldt-Jakob disease: a case report. J Med Case Rep. 2012;6:348. doi: 10.1186/1752-1947-6-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickinson A.G., Fraser H., Meikle V.M., Outram G.W. Competition between different scrapie agents in mice. Nat New Biol. 1972;237:244–245. doi: 10.1038/newbio237244a0. [DOI] [PubMed] [Google Scholar]
- 40.Kimberlin R.H., Walker C.A. Competition between strains of scrapie depends on the blocking agent being infectious. Intervirology. 1985;23:74–81. doi: 10.1159/000149588. [DOI] [PubMed] [Google Scholar]
- 41.Bartz J.C., Kramer M.L., Sheehan M.H., Hutter J.A., Ayers J.I., Bessen R.A., Kincaid A.E. Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol. 2007;81:689–697. doi: 10.1128/JVI.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schutt C.R., Bartz J.C. Prion interference with multiple prion isolates. Prion. 2008;2:61–63. doi: 10.4161/pri.2.2.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shikiya R.A., Ayers J.I., Schutt C.R., Kincaid A.E., Bartz J.C. Coinfecting prion strains compete for a limiting cellular resource. J Virol. 2010;84:5706–5714. doi: 10.1128/JVI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makarava N., Savtchenko R., Baskakov I.V. Two alternative pathways for generating transmissible prion disease de novo. Acta Neuropathol Commun. 2015;3:69. doi: 10.1186/s40478-015-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analyses of brain homogenates from the second and third passages of 2 M rPrP fibrils. Western blot analyses were stained with 3F4 or SAF-84 antibody. Western blot analysis of β-actin is provided as a loading control; signal intensity of β-actin was used for normalizing signal intensity of 3F4-positive materials for statistical analysis of PrPSc amounts. PK, proteinase K; PrPSc, prion protein scrapie isoform; rPrP, recombinant prior protein.