Summary
We report one of the first population-based, age-specific and lineage-specific studies of pediatric hospitalization for influenza B. We found that changes in lineage were associated with higher hospitalization rates.
Keywords: influenza B, hospitalization, children.
Abstract
Background.
Influenza B virus has been perceived to cause less disease burden and milder disease compared with influenza A, but recent studies suggest that influenza B does have a significant impact. We aimed to estimate the burden of influenza B virus infections on hospitalizations in Hong Kong, in the context of virus lineage changes over time.
Methods.
The pediatric age-specific rates of influenza B hospitalization in Hong Kong for 2004–2014 were estimated based on admissions to 2 hospitals that together catered for 72.5% of all pediatric admissions on Hong Kong Island. Influenza B virus was detected by immunofluorescence and culture on nasopharyngeal aspirates. Lineage typing was performed by real-time reverse-transcription polymerase chain reaction.
Results.
A total of 5085 children were recruited on 1 designated day each week, year-round during the 11 years, and 221 (4.3%) tested positive for influenza B. Hospitalization rates were highest in children aged 2 to <5 years with year-to-year variation. Victoria-lineage viruses appeared to be associated with a greater fraction of influenza B hospitalizations in children than of influenza B infections in the general community. Influenza B did not cause significant hospitalization in infants <1 year of age.
Conclusions.
We report one of the first population-based, age- and lineage-specific studies of pediatric hospitalization for influenza B. We found that changes in lineage were associated with higher hospitalization rates and documented that Victoria lineage viruses were associated with greater pediatric hospitalization burden compared with Yamagata lineage viruses.
Influenza leads to a significant burden of hospitalization in children annually. Influenza B was first identified in 1940, and 2 antigenically distinct virus lineages emerged in the mid-1980s, designated as the B/Yamagata and B/Victoria lineages [1]. Influenza B has been perceived to be associated with a lower disease burden and milder disease compared with influenza A, but some recent studies have suggested that influenza B also causes significant mortality and morbidity [2, 3]. Most studies on influenza B burden have not been able to estimate population-based incidence but are reports on frequencies of hospital admissions without defined population denominators. We previously published a study encompassing the influenza seasons of 2003–2004 to 2005–2006 that documented year-to-year variations in influenza B hospitalization [4]. In the current study, we report the age-specific hospitalization disease burden of influenza B in children in Hong Kong over an 11-year period.
METHODS
Study Design
The Hong Kong Special Administrative Region (SAR) of China comprises Hong Kong Island, Kowloon peninsular, the New Territories, and some sparsely populated outlying islands. In 2006, there were 195922 persons <18 years of age residing on Hong Kong Island [4]. Pamela Youde Nethersole Eastern Hospital and Queen Mary Hospital were the only 2 acute care public hospitals on Hong Kong Island serving this population. These 2 hospitals, with a total of 153 pediatric beds, catered for 72.5% of all pediatric admissions from the population of Hong Kong Island in 2006 [4] (the remainder being admitted to private hospitals). While Hong Kong Island is not an entirely closed community, parents from other parts of Hong Kong SAR, namely Kowloon and the New Territories, rarely bring their children across the harbor for acute general pediatric problems as there are 10 public hospitals with pediatric inpatient service serving those areas. Thus, admissions can be related to the pediatric population resident on Hong Kong Island, allowing us to derive population-based estimates of influenza-associated hospitalization burden [4, 5].
Acute respiratory infection was defined as fever ≥38°C (by history or documentation on admission) with any respiratory symptom including cough, runny nose, or sore throat. A systematic sample of all hospital admissions was derived by recruiting all patients aged <18 years of age with a Hong Kong Island home address admitted to either study hospital with acute respiratory infection during 1 designated day (24 consecutive hours) of the week. All recruited patients had a nasopharyngeal aspirate specimen collected for virological testing.
The study protocol was approved by the joint Institutional Review Board of the University of Hong Kong and the Hospital Authority Hong Kong West Cluster, and the Hospital Authority Hong Kong East Cluster Research Ethics Committee, which waived the need for written consent as the investigation was a routine diagnostic test carried out as part of routine care and patient information was delinked from individual patient identification to maintain patient confidentiality.
Laboratory Methods
Nasopharyngeal aspirate specimens from all recruited patients were tested for influenza B virus by direct antigen detection by direct immunofluorescence test, and by virus culture, at the Virology Laboratory at the University of Hong Kong and the Public Health Laboratory Services Branch, Centre for Health Protection, Department of Health, respectively. The direct immunofluorescence antigen test was carried out as previously described using IMAGEN respiratory screen and typing reagents (Oxoid Ely Ltd, United Kingdom) [6]. All the specimens found positive in the respiratory screen with a pooled immunofluorescence reagent were further identified using antibody reagents to influenza B using the IMAGEN typing kit.
Culture for respiratory viruses was done by inoculation 150 µL of the nasopharyngeal aspirate-virus transport medium suspension onto monolayers of continuous cell lines, including Martin-Darby canine kidney (MDCK), African green monkey kidney, human epidermoid laryngeal carcinoma, and human embryonal rhabdomyosarcoma, in culture tubes at the virology laboratory of the Department of Health as previously described [7]. This laboratory is the designated National Influenza Centre for Hong Kong within the World Health Organization (WHO) influenza laboratory network. MDCK was the cell line used for influenza virus isolation, and inoculated cells were maintained in serum-free medium with trypsin (2 µg/mL) and incubated at 33°C for 7 days. The cultures were examined daily for cytopathic effect, and immunofluorescence and hemagglutination inhibition tests were used for identification and antigenic characterization of influenza viruses, respectively.
Influenza B lineages were differentiated by real-time reverse-transcription polymerase chain reaction as described previously with modification [8]. In brief, reaction conditions were established for the LightCycler 96 system in a total reaction mixture volume of 25 L containing 1X reaction mix (Invitrogen), 0.5 U SuperScriptIII Platinum RT Taq polymerase (Invitrogen), 900 nM forward primer (5-ACCCTACAR AMTTGGAACYTCAGG-3), 600 nM reverse primer (5-ACAGCCCAAGCCATTGTTG-3), 150 nM Yamagata probe MGB437 (5-FAM–AATCCGMTYTTACTGGTAG–MGB-3), 100 nM Victoria probe MGB470 (5-VIC–ATCCGTTTCCATTGGTAA–MGB-3), and 5 L of RNA. Cycling conditions were 30 minutes at 50°C, then 2 minutes at 95°C, followed by 50 cycles of 15 seconds at 95°C and 30 seconds at 55°C.
For comparison, we also obtained data on the community circulation of influenza B virus lineages in Hong Kong based on laboratory surveillance done by the virology laboratory of the Department of Health. In these surveillance data, approximately 95% of the positive results are detected from specimens from adult and pediatric patients hospitalized in public and private hospitals; the remainder is from outpatient clinics.
Statistical Analysis
Hospitalization rates and 95% confidence intervals for each age group were estimated using Poisson regression models. The numerator in each incidence rate was the number of laboratory-confirmed influenza admissions in that age group, while the denominator was the estimated person-years at risk each year and was included in regression models as an offset term. Person-years at risk was estimated by the population of Hong Kong Island in that age group obtained from the Census and Statistics Department of the Hong Kong government based on regular population censuses, divided by 7 to allow for the study design of sampling 1 day per week, and further divided by 0.725 to arrive at the proportion of children served by the 2 study hospitals. Census data for infants aged <6 months were lacking. Therefore, we used half the population <1 year of age as the denominator, assuming a constant birth rate over the year. Statistical analyses were conducted using SAS version 9.02 (SAS Institute, Cary, North Carolina) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 5085 children were included in the entire study spanning 11 years. Two hundred twenty-one children (4.3%) tested positive for influenza B. Among all the hospitalized patients infected with influenza B, only 11 were <12 months of age. Of 436 infants <6 months of age, only 4 (0.9%) had influenza B virus infection compared to 28 (6.4%) with influenza A virus infection and 98 (22.5%) with respiratory syncytial virus (RSV) infection. Likewise, of the 629 infants 6–12 months of age, 7 (1.1%) had influenza B virus infection while 36 (5.7%) had influenza A virus infection and 108 (17.2%) had RSV infection. Of the 4020 children >12 months of age, 210 (5.2%) had influenza B infection compared to 440 (10.9%) with influenza A and 326 (8.1%) with RSV infection.
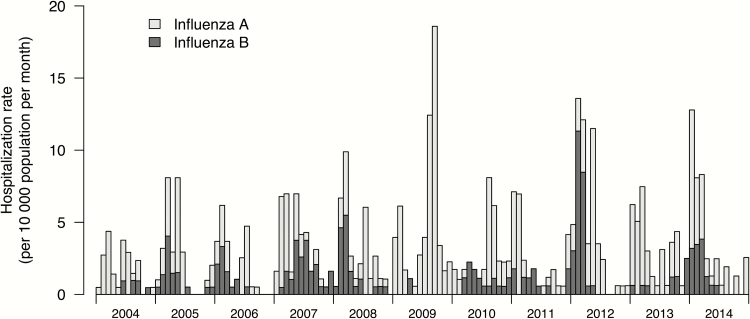
Of the 221 samples positive for influenza B, 175 were available for lineage analysis. Both lineages co-circulated in most years, with the exception when a Yamagata lineage virus (in 2004 and 2007) or a Victoria lineage virus (in 2006) accounted for ≥95% of influenza B viruses identified (Table 1). There was almost no influenza B circulation in Hong Kong in 2009, the year when influenza A(H1N1pdm09) emerged. There was also very little influenza B circulation in 2013. In 2007, 2008, 2010, 2012, and 2014, influenza B circulation accounted for >3% of all respiratory samples from inpatients and outpatients tested by the government virology laboratory. There was no predictable seasonality with frequent overlap with that of influenza A. Influenza B caused hospitalization in almost all months in a few years (2007 and 2010) (Figure 1).
Table 1.
Age-Specific Rates of Hospitalizations (95% Confidence Interval) Associated With Confirmed Influenza B Yamagata or Victoria Lineage per 10000 Residents, 2004–2014
| Year | Predominant Lineage in Hong Kong, % | <6 mo | 6–12 mo | 1 to <2 y | 2 to <5 y | 5 to <10 y | 10 to <15 y | 15 to <18 y | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B/Yamagata | B/Victoria | B/Yamagata | B/Victoria | B/Yamagata | B/Victoria | B/Yamagata | B/Victoria | B/Yamagata | B/Victoria | B/Yamagata | B/Victoria | B/Yamagata | B/Victoria | ||
| 2004 | 97% (Yam)-like | 0 (0–86.7) | 0 (0–86.7) | 0 (0–86.7) | 0 (0–86.7) | 0 (0–44.4) | 0 (0–44.4) | 7.1 (.9–25.7) | 0 (0–13.1) | 6.5 (1.8–16.6) | 1.6 (0–9.0) | 0 (0–5.2) | 0 (0–5.2) | 0 (0–8.8) | 0 (0–8.8) |
| 2005 | 72% (Yam)-like | 0 (0–91.1) | 0 (0–91.1) | 0 (0–91.1) | 0 (0–91.1) | 12.8 (.3–71.4) | 12.8 (.3–71.4) | 19.4 (6.3–45.2) | 7.7 (.9–28.0) | 3.4 (.4–12.4) | 0 (0–6.3) | 0 (0–5.3) | 4.3 (.9–12.6) | 0 (0–8.9) | 2.4 (.1–13.4) |
| 2006 | 96% (Vic)-like | 0 (0–95.7) | 0 (0–95.7) | 0 (0–95.7) | 0 (0–95.7) | 0 (0–50.3) | 0 (0–50.3) | 0 (0–15.6) | 25.4 (9.3–55.3) | 0 (0–6.7) | 14.5 (6.3–28.7) | 0 (0–5.4) | 2.9 (0.4–10.6) | 0 (0–9.0) | 2.4 (0.1–13.6) |
| 2007 | 95% (Yam)-like | 24.6 (.6–136.8) | 0 (0–90.6) | 49.1 (5.9–177.4) | 0 (0–90.6) | 26.1 (3.2–94.4) | 13.1 (0.3–72.8) | 32.8 (14.2–64.6) | 0 (0–15.1) | 11.5 (4.2–25.0) | 0 (0–7.1) | 1.5 (0–8.5) | 0 (0–5.6) | 0 (0–9.1) | 0 (0–9.1) |
| 2008 | 72% (Yam)-like | 0 (0–86.2) | 0 (0–86.2) | 23.4 (.6–130.2) | 0 (0–86.2) | 12.6 (0.3–70.0) | 0 (0–46.4) | 12.0 (2.5–34.9) | 8.0 (1.0–28.8) | 6.1 (1.3–17.7) | 6.1 (1.3–17.7) | 0 (0–5.9) | 1.6 (0–8.9) | 2.5 (.1–14.1) | 0 (0–9.3) |
| 2009 | 59% (Vic)-like | 0 (0–82.5) | 0 (0–82.5) | 0 (0–82.5) | 0 (0–82.5) | 0 (0–44.8) | 12.2 (.3–67.7) | 0 (0–14.4) | 0 (0–14.4) | 0 (0–8.0) | 2.2 (.1–12.0) | 0 (0–6.2) | 0 (0–6.2) | 0 (0–9.5) | 0 (0–9.5) |
| 2010 | 69% (Vic)-like | 0 (0–78.8) | 0 (0–78.8) | 0 (0–78.8) | 0 (0–78.8) | 0 (0–43.2) | 11.7 (.3–65.2) | 3.8 (.1–21.1) | 7.6 (.9–27.3) | 2.3 (.1–12.8) | 16.1 (6.5–33.1) | 0 (0–6.6) | 5.3 (1.1–15.6) | 0 (0–9.7) | 0 (0–9.7) |
| 2011 | 57% (Vic)-like | 0 (0–75.4) | 0 (0–75.4) | 0 (0–75.4) | 0 (0–75.4) | 0 (0–41.7) | 0 (0–41.7) | 0 (0–13.6) | 22.1 (8.1–48.1) | 2.5 (.1–13.7) | 14.7 (5.4–32.1) | 0 (0–6.9) | 1.9 (0–10.5) | 0 (0–9.9) | 0 (0–9.9) |
| 2012 | 59% (Yam)-like | 0 (0–72.0) | 19.5 (.5–108.7) | 39.0 (4.7–140.9) | 19.5 (.5–108.7) | 10.9 (.3–60.5) | 21.7 (2.6–78.5) | 32.1 (14.7–61.0) | 32.1 (14.7–61.0) | 21.0 (9.1–41.4) | 10.5 (2.9–26.9) | 2.0 (.1–11.0) | 2.0 (.1–11.0) | 0 (0–10.0) | 0 (0–10.0) |
| 2013 | 68% (Yam)-like | 18.8 (.5–104.6) | 0 (0–69.3) | 0 (0–69.3) | 0 (0–69.3) | 0 (0–38.8) | 0 (0–38.8) | 10.5 (2.2–30.6) | 0 (0–12.9) | 5.7 (.7–20.6) | 5.7 (.7–20.6) | 2.1 (.1–11.8) | 2.1 (.1–11.8) | 0 (0–10.3) | 0 (0–10.3) |
| 2014 | 78% (Yam)-like | 0 (0–66.6) | 0 (0–66.6) | 0 (0–66.6) | 0 (0–66.6) | 10.2 (.3–56.7) | 10.2 (.3–56.7) | 10.2 (2.1–29.8) | 6.8 (.8–24.6) | 18.6 (6.8–40.4) | 15.5 (5.0–36.1) | 2.3 (.1–12.6) | 2.3 (.1–12.6) | 0 (0–10.5) | 0 (0–10.5) |
Figure 1.
Rates of hospitalization associated with influenza B in children <18 years of age by calendar month, 2004–2014. Hospitalization rates for influenza A over the same period are presented for comparison.
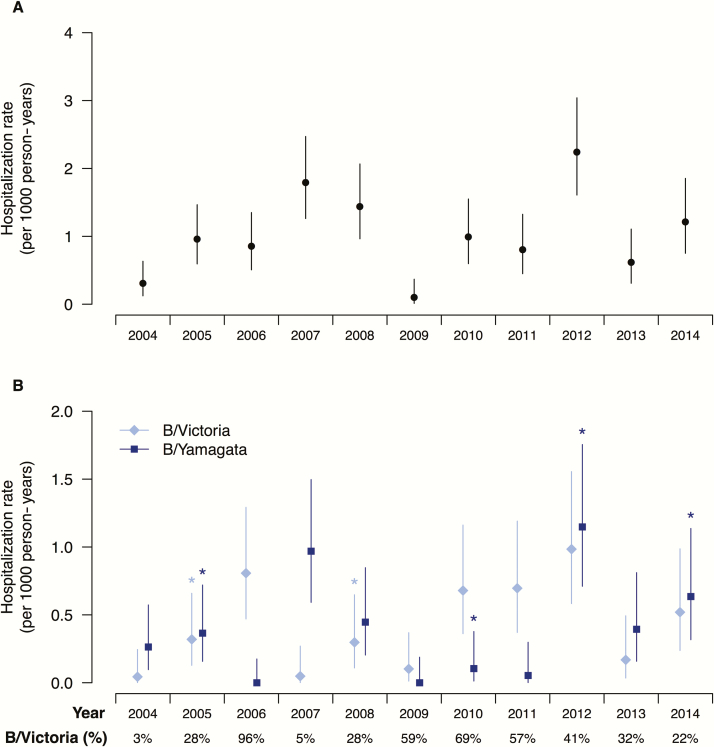
There was significant year-to-year fluctuation in disease burden in terms of pediatric hospitalizations due to influenza B in the 11 study years, largely correlating with influenza B circulation across the whole of Hong Kong (Figure 2A). Lineage-specific hospitalization rates also fluctuated (Figure 2B). The proportion of children with chronic illness at high risk for influenza complications varied from year to year, ranging from 11% to 38%. In general, hospitalization rates were consistently highest in children aged 2 to <5 years and markedly higher in younger children <2 years of age and older children 5 to <10 years of age (Table 1). However, there was year-to-year variation. The rate of 38.7 per 10000 in 2005 was significantly higher than that of 7.1 per 10000 in 2004 (P = .03) in those aged 2 to <5 years. There was also an apparent increase in hospitalizations in 2007 (65.6 per 10000) compared with 25.4 per 10000 in 2006 (P = .06) in this age group when the predominant virus switched from a Victoria lineage virus to a Yamagata lineage virus. Influenza B did not appear to cause significant hospitalization in infants <1 year of age. Hospitalization in this age group was only documented in 4 of the 11 study years, and all 4 years were dominated by circulation of the Yamagata lineage viruses after several years of Victoria lineage virus circulation.
Figure 2.
Annual hospitalization rates of pediatric patients infected with influenza B viruses overall (A), and identified as Victoria or Yamagata lineage (B) in Hong Kong, 2004–2014. An asterisk above particular estimates indicates the year when there was an antigenic change in the corresponding lineage. The bottom row reports the annual proportion of laboratory detections of influenza B cases belonging to Victoria lineage among all influenza B detections with lineage typing done. The proportions of laboratory detections of influenza B/Yamagata lineage virus among all influenza B detections can be calculated as 1 minus the proportion of B/Victoria detections, and are therefore not shown.
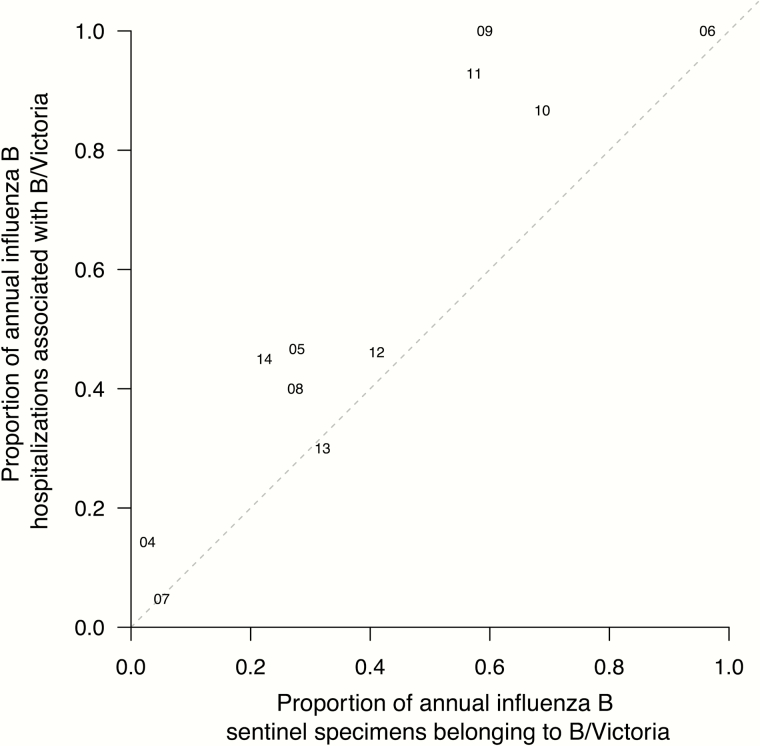
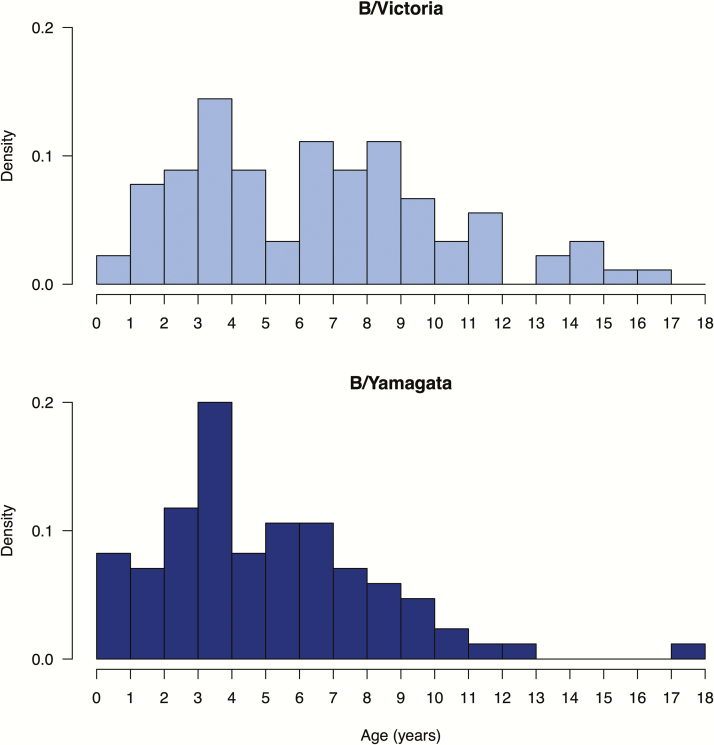
The Victoria-lineage virus appeared to be associated with excess hospitalization in children when hospitalization is plotted against the proportion of Victoria lineage viruses in circulation each year (Figure 3). In all of the confirmed influenza B hospitalizations included in this study, the mean age of hospitalized children was 6.0 years for B/Victoria vs 4.5 years for B/Yamagata (P = .007) (Figure 4). With the exception of 2012, no infant <12 months of age was hospitalized for Victoria lineage virus infection.
Figure 3.
Association between the proportion of influenza B hospitalizations in children associated with B/Victoria viruses (y-axis) and the proportion of community detections of influenza B associated with B/Victoria viruses (x-axis) each year. Note that the proportion of B/Yamagata can be derived as 1 minus the proportion with B/Victoria. The 2 digits indicate the corresponding year in the 21st century (eg, 09 corresponds to the calendar year of 2009). Points above the diagonal line indicate years in which the B/Victoria viruses were identified more frequently in hospitalized children with influenza B than among influenza B infections in the community. Points below the diagonal line indicate years in which the B/Yamagata viruses were identified more frequently in hospitalized children with influenza B than among influenza B infections in the community.
Figure 4.
Age distribution of pediatric hospitalizations associated with B/Victoria and B/Yamagata lineage viruses.
DISCUSSION
In this study, we documented age-specific rates of hospitalizations due to influenza B lineages in children over a period of 11 years. Disease burden in terms of influenza B hospitalizations varied from year to year and must be interpreted within the context of overall virus activity, the influenza B lineages in circulation in each year, the emergence of antigenically different viruses, and influenza vaccination coverage. Athough we did not have complete vaccination data on all the patients in the present study, in a previously reported vaccine effectiveness study conducted between 2009 and 2014, we reported that influenza vaccination coverage is very low in children in Hong Kong, with 8.8% of those hospitalized for a respiratory illness other than influenza B receiving influenza vaccine [9]. Meanwhile, 2 previous studies reported that 1.7% and 3.9% of pregnant women in Hong Kong were vaccinated during their pregnancy [10, 11]. Therefore, the impact of influenza vaccination on hospitalization disease burden documented in this study is likely to be minimal.
It is commonly reported that influenza B mainly affects school-aged children [12–14]. In this 11-year study, we also documented significant population-based hospitalization rates in children 5 to <10 years of age, but hospitalization rates were highest in children aged 2 to <5 years (Table 1). In an earlier study of 2004–2005, we also documented the highest hospitalization rates in children aged 2 to <5 years [4]. This may reflect that influenza B virus infections in the younger age group were more likely to result in hospitalization, either due to more severe disease or a lower admission threshold in this younger age group. In a 20-year retrospective study of pediatric inpatients and outpatients from Finland, Peltola et al found that outpatient consultation and hospitalization were highest in children <2 years of age [15]. A population-based study for children aged <6 years in the United States indicated that the age group with the highest influenza hospital admissions was infants aged <6 months while influenza A viruses were predominant during the study period [16].
Interestingly, unlike influenza A, which resulted in the highest hospitalization in the youngest age group [5], we found that influenza B caused much lower hospitalization rates in infants <12 months of age. Specifically, hospitalization of infants <6 months was only seen in 3 of the 11 years (Table 1). This was also documented in the Tecumseh Study, which found no influenza B virus infections in 43 infants <1 year of age and only 1 infection among 135 patients (0.7%) aged 1–2 years [12]. Recently, a birth cohort study in Vietnam also showed that influenza B virus infection in infants <1 year of age was rare [17]. Lineage characterization was not performed in these studies. The epidemiology of influenza B virus infections or hospitalization in this very young age is not well described in the literature because many influenza B studies focused on school-aged and older children. Protection from maternal antibodies may explain the very low incidence of influenza B hospitalization in these infants. Furthermore, the difference in hospitalization rates for influenza A and influenza B in very young infants may be explained by higher prevalence of protective maternal antibody against influenza B when compared to influenza A, as influenza B viruses are believed to evolve more slowly than influenza A, allowing for a higher background immunity against influenza B. In addition, there was hardly any hospitalization of infants <6 months of age due to Victoria lineage viruses even during a time of significant influenza B Victoria lineage circulation (supplementary appendix). Unlike the rest of the world where the Victoria lineages were not found between the 1990s and early 2000, they have persisted in mainland China and Hong Kong since the 1980s [18, 19]. Moreover, Vijaykrishna et al found that Victoria viruses were generally more transmissible than Yamagata viruses [20]. Thus, it is likely that there would be a greater level of immunity to B/Victoria lineage viruses in women of childbearing age in Hong Kong that may explain the low hospitalization rates in these young infants. Further studies to correlate maternal antibodies with infant infection of respective influenza B lineage would help to confirm this hypothesis.
Some previous studies have suggested that B/Victoria lineage viruses result in more infections or hospitalizations in the younger age groups (<15 years) compared to B/Yamagata viruses in studies of all ages [19, 21, 22]. In this study of pediatric age groups, we provided age-specific rates and found that while the Victoria lineage viruses appear to cause higher rates of hospitalization, hospitalized children with B/Yamagata lineage viruses were in fact younger than those hospitalized with B/Victoria lineage viruses, and almost all hospitalizations in children aged <12 months were attributed to B/Yamagata lineage viruses. Vijaykrishna et al showed that Victoria lineage viruses have higher rates of antigenic drift, and expected the lower rates of antigenic drift of B/Yamagata lineage viruses to skew the age distribution of cases toward younger individuals after maternal antibody has waned [20]. Our study findings support their hypothesis. A recent study from Bangkok did not compare hospitalization rates but concluded that the B/Victoria lineage viruses were associated with a longer duration of hospitalization, and a higher number of fatal cases and pneumonia [23]. However, the study included all age groups with a trend toward a higher proportion of patients aged ≥65 years in the B/Victoria lineage group.
An example of the impact of a change in lineage predominance was observed in 2007. In that year, Hong Kong had substantial influenza B activity (Figure 1), and we documented significant pediatric hospitalization in all months except January that year [24]. Yamagata lineage virus dominated with 95% of influenza B in circulation in Hong Kong following 2006, which was a year of Victoria lineage dominance (97%). The abrupt switch of lineage predominance may explain the high virus circulation and disease burden. The impact of emergence of a different clade could also be seen: In 2012, the Yamagata lineage viruses only accounted for 58% of influenza B in Hong Kong but there was an increase in hospitalization due to Yamagata lineage viruses, including in infants 6–12 months of age, that was not seen in the previous 2 years (2010 and 2011), with similar ratio of Victoria to Yamagata lineage virus circulation (Table 2 and Figure 2). Although we did not perform antigenic analysis on the strains from our individual patients, WHO reported the emergence of clade 2 Yamagata lineage viruses that were antigenically different from the previously circulating clade 3 (B/Wisconsin/1/2010 Yam-like) viruses [25]. Again, in 2014, Yamagata lineage viruses accounted for 86% of influenza B viruses in Hong Kong and resulted in moderately high hospitalization rates in children. This might be due to the circulation of clade 3 viruses that were antigenically distinguishable from the vaccine strain B/Massachusetts/2/2012, which was a clade 2 virus [26]. Clade analysis on 30 Yamagata lineage viruses in that year showed that 27 indeed belonged to clade 3 (Lo et al, unpublished data).
There are a number of limitations to our study. Our study is based on children hospitalized with influenza B, and we have no information on influenza B circulation in children in the general community. We included children who met our case definition of fever (or history of fever) plus at least 1 respiratory symptom and therefore may have excluded a small number of influenza B hospitalizations with atypical presentation. Furthermore, we do not have antigenic characterization of the individual strains from patients. However, viral circulation and antigenic analysis derived from the overall surveillance data in Hong Kong from the Department of Health provides some indication of viruses, lineages, and antigenic variants circulating in the Hong Kong community overall.
In conclusion, we report one of the first population-based studies of pediatric hospitalization for influenza B by lineage. We found that hospitalization rates varied from year to year, largely corresponding to overall virus circulation within the community. We found that higher hospitalization rates correlated with changes in lineage. We documented that Victoria lineage viruses were associated with greater excess hospitalization than might have been expected based on the proportion of viruses in circulation within the community (Figure 3). This study highlights the importance of interpreting influenza B disease burden in the context of antigenic changes and background viral activity.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the nurses at ward B6 at Pamela Youde Nethersole Eastern Hospital and ward K7N at Queen Mary Hospital for research support.
Financial support. This work was supported by Area of Excellence Scheme of the University Grants Committee of Hong Kong (grant number AoE/M-12/06) and the Research Grants Council of Hong Kong (project number 17105414) of the Hong Kong Special Administrative Region Government. B. J. C. is supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant number U54 GM088558).
Potential conflicts of interest. B. J. C. has received research funding from MedImmune Inc and Sanofi Pasteur and has consulted for Crucell NV. All other authors report no other potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59–68. [DOI] [PubMed] [Google Scholar]
- 2. Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health 2013; 103:e43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glezen WP. Editorial commentary: changing epidemiology of influenza B virus. Clin Infect Dis 2014; 59:1525–6. [DOI] [PubMed] [Google Scholar]
- 4. Chiu SS, Chan KH, Chen H, et al. Virologically confirmed population-based burden of hospitalization caused by influenza A and B among children in Hong Kong. Clin Infect Dis 2009; 49:1016–21. [DOI] [PubMed] [Google Scholar]
- 5. Chiu SS, Lo JY, Chan KH, et al. Population-based hospitalization burden of influenza A virus subtypes and antigenic drift variants in children in Hong Kong (2004–2011). PLoS One 2014; 9:e92914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan KH, Maldeis N, Pope W, et al. Evaluation of the Directigen FluA+B test for rapid diagnosis of influenza virus type A and B infections. J Clin Microbiol 2002; 40:1675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo JY, Tsang TH, Leung YH, Yeung EY, Wu T, Lim WW. Respiratory infections during SARS outbreak, Hong Kong, 2003. Emerg Infect Dis 2005; 11:1738–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biere B, Bauer B, Schweiger B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J Clin Microbiol 2010; 48:1425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu SS, Feng S, Chan KH, et al. Hospital-based vaccine effectiveness against influenza B lineages, Hong Kong, 2009-14. Vaccine 2016; 34:2164–9. [DOI] [PubMed] [Google Scholar]
- 10. Yuen CYS, Fong DYT, Lee ILY, Chu S, Sau-mei Siu E, Tarrant M. Prevalence and predictors of maternal seasonal influenza vaccination in Hong Kong. Vaccine 2013; 31:5281–8. [DOI] [PubMed] [Google Scholar]
- 11. Lau JT, Cai Y, Tsui HY, Choi KC. Prevalence of influenza vaccination and associated factors among pregnant women in Hong Kong. Vaccine 2010; 28:5389–97. [DOI] [PubMed] [Google Scholar]
- 12. Monto AS, Kioumehr F. The Tecumseh Study of respiratory illness. IX. occurrence of influenza in the community, 1966–1971. Am J Epidemiol 1975; 102:553–63. [DOI] [PubMed] [Google Scholar]
- 13. Hite LK, Glezen WP, Demmler GJ, Munoz FM. Medically attended pediatric influenza during the resurgence of the Victoria lineage of influenza B virus. Int J Infect Dis 2007; 11:40–7. [DOI] [PubMed] [Google Scholar]
- 14. Zhao B, Qin S, Teng Z, et al. Epidemiological study of influenza B in Shanghai during the 2009–2014 seasons: implications for influenza vaccination strategy. Clin Microbiol Infect 2015; 21:694–700. [DOI] [PubMed] [Google Scholar]
- 15. Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis 2003; 36:299–305. [DOI] [PubMed] [Google Scholar]
- 16. Poehling KA, Edwards KM, Weinberg GA, et al. ; New Vaccine Surveillance Network The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 17. Anders KL, Nguyen HL, Nguyen NM, et al. Epidemiology and virology of acute respiratory infections during the first year of life: a birth cohort study in Vietnam. Pediatr Infect Dis J 2015; 34:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. Recommended composition of influenza virus vaccines for use in the 1997–1998 season. Wkly Epidemiol Rec 1997; 72:57–61. [PubMed] [Google Scholar]
- 19. Tan Y, Guan W, Lam TT, et al. Differing epidemiological dynamics of influenza B virus lineages in Guangzhou, southern China, 2009–2010. J Virol 2013; 87:12447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vijaykrishna D, Holmes EC, Joseph U, et al. The contrasting phylodynamics of human influenza B viruses. Elife 2015; 4:e05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chi CY, Wang SM, Lin CC, et al. Clinical features of children infected with different strains of influenza B in southern Taiwan. Pediatr Infect Dis J 2008; 27:640–5. [DOI] [PubMed] [Google Scholar]
- 22. Barr IG, Vijaykrishna D, Sullivan SG. Differential age susceptibility to influenza B/Victoria lineage viruses in the 2015 Australian influenza season. Euro Surveill 2016; 21. doi:10.2807/1560-7917.ES.2016.21.4.30118. [DOI] [PubMed] [Google Scholar]
- 23. Horthongkham N, Athipanyasilp N, Pattama A, et al. Epidemiological, clinical and virological characteristics of influenza B virus from patients at the hospital tertiary care units in Bangkok during 2011–2014. PLoS One 2016; 11:e0158244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2008 influenza season. Wkly Epidemiol Rec 2007; 82:351–6. [PubMed] [Google Scholar]
- 25. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2013–2014 Northern Hemisphere influenza season. Wkly Epidemiol Rec 2013; 88:101–14. [PubMed] [Google Scholar]
- 26. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2015 Southern Hemisphere influenza season. Wkly Epidemiol Rec 2014; 89:441–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.