The findings demonstrate the efficacy and safety of 3 single-dose loperamide-adjuncted antibiotic therapies. Comparable effectiveness of single-dose rifaximin was shown. Colonization with extended-spectrum β-lactamase–producing Escherichia coli was reported, but at a lesser rate compared with other observational studies.
Keywords: travelers’ diarrhea, randomized control trial, levofloxacin, rifaximin, azithromycin
Abstract
Background
Recommended treatment for travelers’ diarrhea includes the combination of an antibiotic, usually a fluoroquinolone or azithromycin, and loperamide for rapid resolution of symptoms. However, adverse events, postdose nausea with high-dose azithromycin, effectiveness of single-dose rifaximin, and emerging resistance to front-line agents are evidence gaps underlying current recommendations.
Methods
A randomized, double-blind trial was conducted in 4 countries (Afghanistan, Djibouti, Kenya, and Honduras) between September 2012 and July 2015. US and UK service members with acute watery diarrhea were randomized and received single-dose azithromycin (500 mg; 106 persons), levofloxacin (500 mg; 111 persons), or rifaximin (1650 mg; 107 persons), in combination with loperamide (labeled dosing). The efficacy outcomes included clinical cure at 24 hours and time to last unformed stool.
Results
Clinical cure at 24 hours occurred in 81.4%, 78.3%, and 74.8% of the levofloxacin, azithromycin, and rifaximin arms, respectively. Compared with levofloxacin, azithromycin was not inferior (P = .01). Noninferiority could not be shown with rifaximin (P = .07). At 48 and 72 hours, efficacy among regimens was equivalent (approximately 91% at 48 and 96% at 72 hours). The median time to last unformed stool did not differ between treatment arms (azithromycin, 3.8 hours; levofloxacin, 6.4 hours; rifaximin, 5.6 hours). Treatment failures were uncommon (3.8%, 4.4%, and 1.9% in azithromycin, levofloxacin, and rifaximin arms, respectively) (P = .55). There were no differences between treatment arms with postdose nausea, vomiting, or other adverse events.
Conclusions
Single-dose azithromycin, levofloxacin, and rifaximin with loperamide were comparable for treatment of acute watery diarrhea.
Clinical Trial Registration
Travelers’ diarrhea (TD) affects anywhere from 10% to 40% of short-term international travelers, resulting in 12%–46% having to change their itinerary [1]. To minimize the impact of TD, empiric treatment regimens are generally recommended, consisting of an oral antibiotic plus the antimotility agent loperamide, [2–4]. Fluoroquinolones have been considered a first-line antibiotic therapy; however, owing to increasing concerns associated with this drug class [5, 6], other antibiotics are now being used. Rifaximin is approved for use with Escherichia coli–associated, noninvasive TD and is recommended for travel to enterotoxigenic E. coli (ETEC)–predominant areas [7, 8]; however, it has lower efficacy in invasive bacterial infections [9, 10] and has never been evaluated in a single-dose regimen. Although azithromycin is also effective, there is concern about postdose nausea and vomiting associated with high doses [11, 12].
There is also concern about antibiotic treatment in combination with loperamide and increased rates of extended-spectrum β-lactamase–producing E. coli (ESBL-PE) [13, 14]. Colonization is typically transient and nonpathogenic in healthy travelers; however, potential spread of antimicrobial resistance warrants consideration [15, 16]. We conducted a study among military travelers to bolster the evidence of relative efficacy and safety of 3 available single-dose loperamide-adjuncted regimens for acute watery diarrhea (AWD).
METHODS
Trial Design
The study was a multisite, randomized, double-blind, controlled clinical trial among deployed US and UK military personnel. Patients presenting for care with suspected TD were clinically assessed for AWD. After treatment randomization and directly observed dosing, clinical follow-up assessments were performed at 1, 3, and 7 days. Subjects were given diaries to daily record data on timing of loperamide usage, the number and form of all stools passed, and the presence of symptoms and disability.
Participants
Eligible individuals included active-duty military personnel (or beneficiaries), deployed to Afghanistan, Djibouti, Kenya, or Honduras, aged ≥18 years, who presented with AWD and were ambulatory. Subjects had to meet the definition of TD (≥3 loose stools in 24 hours or ≥2 loose stools in 24 hours with associated symptoms, such as nausea, vomiting, abdominal cramps, or tenesmus) of ≤96 hours duration, be able to comply with follow-up procedures, and remain in country or be eligible for follow-up for ≥5 days after treatment. Stools were graded on a 5-point scale consistent with similar trials and defined as diarrheal (loose or liquid stool) if they took the shape of the container [11, 12]. Subjects were excluded if they reported an allergy to study drugs, took antibiotic therapy within 72 hours before presentation (excluding malaria prophylaxis), were taking concomitant medications with known drug-drug interactions with study drugs, reported a history of seizures, had a positive pregnancy test at presentation, used >4 mg loperamide (total) or any amount of loperamide for >24 hours before enrollment, or had dysentery or documented fever.
Randomization and Interventions
Randomly permuted block randomization (block size, 3–12 subjects) by site was performed to allocate treatment assignments across study interventions. Individually packaged treatment packs were labeled with random nonsequential treatment numbers following the randomization schedule. Antibiotics were overcapsuled into 6 individual pills to mask allocation. The 3 single-dose treatment assignments were rifaximin (1650 mg), azithromycin (500 mg), or levofloxacin (500 mg). In all arms, 4 mg of loperamide was given initially, followed by 2 mg after each unformed stool, not to exceed 16 mg/d for 2 days and self-administered by study subjects.
Outcomes
The primary efficacy end point was the proportion of patients in whom clinical cure was achieved 24 hours after the first treatment dose. Subjects met the end point if (1) they had no reported diarrheal stools >24 hours after initiation of therapy, (2) any diarrhea-associated symptoms present at 24 hours were no greater than mild in severity, and (3) the diarrhea had no impact on their activity, and they showed was no evidence of treatment failure. Treatment failure was defined as recurrence of diarrheal illness meeting TD case definition occurring within 72 hours after clinical cure, worsening of clinical symptoms (or failure to improve) after 24 hours of therapy, or illness continuing after 72 hours. A secondary outcome of time to last unformed stool (TLUS) was calculated as the time from the first dose of study medication until passage of the last loose or liquid stool that met diarrhea definition occurring in a 24-hour period.
The following symptoms were solicited at each clinic visit: abdominal cramping, fever, excessive gas, urgency to empty bowel, and malaise/fatigue, along with open-ended questions to capture other adverse events. If nausea or vomiting was not present before treatment but developed after treatment administration, it was considered to be associated with the intervention. Subjects indicated severity of symptoms in their diary based on functional impact on their duty performance (no impact, <50% impact, >50% impact, or inability to function). Constipation was assessed through daily diaries and during follow-up and defined as a change in baseline bowel movements characterized by a stool form that is mostly hard or lumpy and difficult to pass.
Laboratory Methods
Microbiological methods included detection of common bacterial, viral, and parasitic etiological agents and ESBL-PE characterization from stool samples collected on days 0 (baseline) and 21. Specimen portions were either immediately plated on selective and differential media or transferred into modified Cary-Blair bacterial transport medium for later plating. Up to 5 E. coli isolates from Maconkey agar plates were picked and evaluated with multiplex polymerase chain reaction (PCR) for key virulence factors often associated with ETEC, enteroaggregative E. coli (EAEC), enteroinvasive E. coli, enteropathogenic E. coli, and enterohemorrhagic E. coli [17–19]. Single colonies exhibiting proper characteristics on various media were identified on standard automated platforms via biochemical algorithms. Additional aliquots of stool were fixed by 10% formalin in buffered saline for stool parasite microscopy, as well as aliquots kept frozen at −70°C for real-time PCR of enteric viruses. All nonpathogenic E. coli isolates identified from days 0 and 21 stool samples were screened for ESBL phenotype by means of disk diffusion [20].
Sample Size
Sample size calculations were based on comparing treatment arms with respect to the difference in proportion of subjects experiencing 24-hour clinical cure between less-studied loperamide-adjuncted single-dose regimens (azithromycin, rifaximin) and that of levofloxacin. From a comparable TD trial in active-duty populations [12], the cumulative probability of passing last diarrheal stool in a levofloxacin (500 mg) plus loperamide arm by the end of the first 24 hours was 0.79 (95% confidence interval, .70–.87), compared with 0.65 (.55–.74) for azithromycin (albeit at 1000 mg) combined with loperamide. A sample size of 120 subjects per arm (assuming a 25% drop-out rate) was estimated based on noninferiority with a margin of 0.15, using a large-sample approximation statistic for the 1-sided test conducted at a significance level of .025 [21].
Statistical Analysis
A modified intent-to-treat analysis was performed to include randomized subjects with primary outcome data available. For the primary end point, azithromycin and rifaximin arms were independently compared with the levofloxacin arm (“active control”) within a stepwise noninferiority/superiority testing framework involving 2 distinct sets of hypotheses. Two coprimary hypotheses involved the loperamide-adjuncted single-dose active control antibiotic, levofloxacin, versus the rifaximin and azithromycin arms. For the secondary end point, TLUS, differences in recovery times were evaluated using Kaplan–Meier survival analyses. Safety outcomes, microbiological identification, and ESBL-PE frequencies were evaluated for differences between treatment arms and tested for significance using χ2 tests, or exact counterparts as appropriate.
Human Research Protections
The study protocol was approved by the Uniformed Services University’s Infectious Disease Institutional Review Board, the UK Ministry of Defence Research Ethics Committee, and the Kenyan Medical Research Institute’s Institutional Review Board. The study is registered at ClinicalTrials.gov (NCT01618591).
RESULTS
Study Population
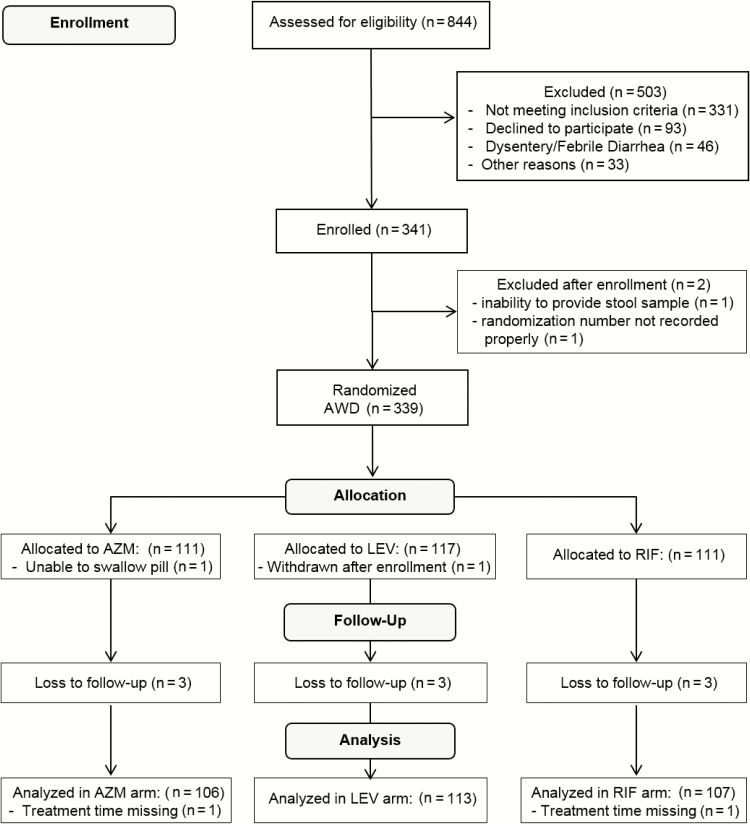
Among 844 patients assessed for eligibility, 339 were determined to have AWD and randomized to 1 of 3 interventions (Figure 1). Primary reasons for noneligibility included inability to provide a diarrheal stool sample (n = 111), illness for >96 hours (n = 80), leaving country in <5 days (n = 66), and already taking antibiotics or loperamide at screening (n = 36). Among those randomized, 106, 111, and 107 subjects in the azithromycin, levofloxacin, and rifaximin arms, respectively, were able to take the full treatment dose and had outcome data available for evaluation. Subject enrollment was distributed from Kenya (n = 130; 40%), Djibouti (n = 104; 32%), Afghanistan (n = 50; 15%), and Honduras (n = 42; 13%). The mean duration of deployment to country of illness was 84.7 days (median, 49.5 days; interquartile range, 27–112 days) and differed between sites (median for Kenya, 31 days; Honduras, 134 days; Djibouti, 101.5 days; Afghanistan, 68 days).
Figure 1.
Flow chart for the Trial Evaluating Ambulatory Therapy of Travelers’ Diarrhea (TrEAT TD) study. Abbreviations: AWD, acute watery diarrhea; AZM, azithromycin; LEV, levofloxacin; RIF, rifaximin.
Subjects presented for care with a mean duration of diarrhea of 26.8 hours (standard deviation, 21.0 hours). A minority of participants presented with diarrhea between 48 and 72 hours (12.6%) or between 72 and 96 hours (4.7%), and these proportions did not differ between treatment arms. Among those with ≥24 hours of diarrheal illness, the maximum number of loose or liquid stools in a 24-hour period was 6.9 and did not differ between treatment arms (Table 1). Cramping was the most frequent associated symptom, reported in 74.3%. Vomiting, fecal incontinence, and fever were reported in 18.0%, 12.7%, and 9.6%, respectively. Overall, 76.5% of subjects reported some impact of illness on their ability to function, with 30% significantly or completely disabled.
Table 1.
Demographic and Baseline Clinical Characteristics of Subjects Analyzed in the Modified Intent-to-Treat Analysis
| Characteristic | Azithromycin (500 mg; n = 106) | Levofloxacin (500 mg; n = 113) | Rifaximin (1650 mg) (n = 107) | Total (n = 326) |
|---|---|---|---|---|
| Age, median (IQR), y | 27 (23–33) | 28 (23–33) | 28 (23–34) | 27 (23–34) |
| Male, No. (%) | 100 (94.3) | 103 (91.2) | 97 (90.7) | 300 (92.0) |
| Enlisted rank, No. (%) | 86 (81.1) | 91 (80.5) | 83 (77.6) | 260 (77.6) |
| Race, No. (%) | ||||
| White | 89 (84.0) | 87 (77.0) | 93 (86.9) | 269 (82.5) |
| Black | 10 (9.4) | 16 (14.2) | 6 (5.6) | 32 (9.8) |
| Other | 7 (6.6) | 10 (8.8) | 8 (7.5) | 25 (7.7) |
| Service, No. (%) | ||||
| Army | 78 (73.6) | 73 (64.6) | 79 (73.8) | 230 (73.8) |
| Navy | 13 (12.3) | 18 (15.9) | 15 (14.0) | 46 (14.1) |
| Marines | 2 (1.9) | 2 (1.8) | 3 (2.8) | 7 (2.2) |
| Air Force | 9 (8.5) | 17 (15.0) | 7 (6.5) | 33 (10.0) |
| Other | 4 (3.8) | 3 (2.7) | 3 (2.8) | 10 (3.1) |
| Baseline clinical characteristicsa | ||||
| Maximum LLS in 24 hours (all cases), mean (SD), No. of stools | 6.3 (3.5) | 6.3 (4.2) | 6.3 (3.5) | 6.3 (3.7) |
| Subjects with ≥24 hours of diarrhea pretreatment, No. (%) | 46 (43.8) | 53 (47.3) | 48 (45.3) | 147 (45.5) |
| Maximum LLS in 24 hours (limited), mean (SD), No. of stoolsb | 6.8 (3.9) | 7.5 (5.0) | 6.5 (3.4) | 6.9 (4.2) |
| Diarrhea duration, mean (SD), h | 27 (22) | 27 (21) | 26 (19) | 27 (21) |
| Vomiting, No. (%) | 17 (16.2) | 20 (17.9) | 21 (19.8) | 58 (18.0) |
| Fever, No. (%) | 8 (7.6) | 11 (9.8) | 12 (11.3) | 31 (9.6) |
| Abdominal cramps, No. (%) | 76 (72.4) | 84 (75.0) | 80 (75.5) | 240 (74.3) |
| Fecal incontinence, No. (%) | 14 (13.3) | 13 (11.6) | 14 (13.2) | 41 (12.7) |
| Impact of illness on activity level/ability to function in primary duty assignment, No. (%) | ||||
| Normal | 28 (26.7) | 28 (25.0) | 20 (18.9) | 76 (23.5) |
| Decreased ≤50% | 51 (48.6) | 46 (41.1) | 52 (49.1) | 149 (46.1) |
| Decreased >50% | 24 (22.9) | 28 (25.0) | 30 (28.3) | 82 (25.4) |
| Complete inability to function | 2 (1.9) | 10 (8.9) | 4 (3.8) | 16 (5.0) |
Abbreviations; IQR, interquartile range; LLS, loose or liquid stools; SD, standard deviation.
aData missing for 3 subjects (1 from each treatment group).
bAnalysis limited to subjects with ≥24 hours of illness before presentation for treatment.
During the study, a freezer failure occurred at the Djibouti site, preventing etiological work-up on stool samples from 50 subjects. Among the remaining subjects (n = 274) from whom specimens were collected, ≥1 cause for TD was found in 57.7% (Tables 2 and 3). Multipathogen (mixed) infections were common, occurring in 16.1% of all cases. Among individuals with an identified pathogen, EAEC was most common, followed by ETEC and enteropathogenic E. coli. Norovirus was identified in 19 cases (6.9% among all samples tested, 16.7% among those with a solo pathogen, and 20.5% in mixed infections). Differences in pathogen recovery and etiological proportions across enrollment sites were noted, with higher rates of identification in Djibouti (76.3%) and Honduras (61.9%) than in Kenya (51.2%) or Afghanistan (47.7%) (data not shown).
Table 2.
Number of Pathogens by Treatment Arm
| No. of Pathogens | Samples, No. (%) | |||
|---|---|---|---|---|
| Azithromycin | Levofloxacin | Rifaximin | All | |
| 0 | 43 (50.0) | 33 (33.7) | 40 (44.0) | 116 (42.3) |
| 1 | 33 (38.4) | 43 (44.3) | 38 (42.2) | 114 (41.6) |
| 2 | 9 (10.5) | 20 (20.4) | 10 (11.1) | 39 (14.2) |
| 3 | 1 (1.2) | 1 (1.0) | 2 (2.2) | 4 (1.5) |
| 4 | 0 | 1 (1.0) | 0 | 1 (0.4) |
Table 3.
Pathogen Types by Treatment Arm
| Pathogen Type | Samples, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Azithromycin | Levofloxacin | Rifaximin | All | |||||
| Solo Pathogen (n = 33) | Multipathogena (n = 10) | Solo Pathogen (n = 43) | Multipathogena (n = 22) | Solo Pathogen (n = 38) | Multipathogena (n = 12) | Solo Pathogen (n = 114) | Multipathogena (n = 44) |
|
| Campylobacter spp. | 0 | 0 | 0 | 0 | 0 | 1 (8.3) | 0 | 1 (2.3) |
| C. jejuni | 0 | 0 | 0 | 1 (4.6) | 0 | 0 | 0 | 1 (2.3) |
| Vibrio spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shigella spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. flexneri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. sonnei | 0 | 0 | 0 | 1 (4.6) | 0 | 0 | 0 | 1 (2.3) |
| S. boydii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. dysenteriae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Salmonella spp. | 0 | 0 | 1 (2.3) | 0 | 0 | 0 | 1 (0.9) | 0 |
| ETEC | 7 (21.2) | 8 (80.0) | 8 (18.6) | 10 (45.5) | 13 (34.2) | 6 (50.0) | 28 (24.6) | 24 (54.6) |
| EAEC | 19 (57.6) | 7 (70.0) | 15 (34.9) | 14 (63.6) | 10 (26.3) | 9 (75.0) | 44 (38.6) | 30 (68.2) |
| EHEC | 0 | 1 (10.0) | 0 | 3 (13.6) | 1 (2.6) | 2 (16.7) | 1 (0.9) | 6 (13.6) |
| EPEC | 0 | 2 (20.0) | 6 (14.0) | 6 (27.3) | 2 (5.3) | 3 (25.0) | 8 (7.0) | 11 (25.0) |
| EIEC | 0 | 0 | 1 (2.3) | 2 (9.1) | 1 (2.6) | 1 (8.3) | 2 (1.8) | 3 (6.8) |
| Cryptospordium parvum | 2 (6.1) | 0 | 0 | 0 | 2 (5.3) | 1 (8.3) | 4 (3.5) | 1 (2.3) |
| Giardia lamblia | 0 | 0 | 0 | 0 | 2 (5.3) | 0 | 2 (1.8) | 0 |
| Norovirus strain GI | 2 (6.1) | 1 (10.0) | 2 (4.7) | 3 (13.6) | 1 (2.6) | 0 | 5 (4.4) | 4 (9.1) |
| Norovirus strain GII | 1 (3.0) | 0 | 7 (16.3) | 3 (13.6) | 5 (13.2) | 2 (16.7) | 13 (11.4) | 5 (11.4) |
| Norovirus non-GI/GII strains | 0 | 0 | 1 (2.3) | 0 | 0 | 0 | 1 (0.9) | 0 |
| Rotavirus | 2 (6.1) | 2 (20.0) | 2 (4.7) | 4 (18.2) | 1 (2.6) | 1 (8.3) | 5 (4.4) | 7 (15.9) |
Abbreviations: EAEC, enteroaggregative Escherichia coli; EHEC, enterohemorrhagic E. coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli.
aBecause fecal samples have >1 pathogen, the numbers add up to more than the total.
Outcomes
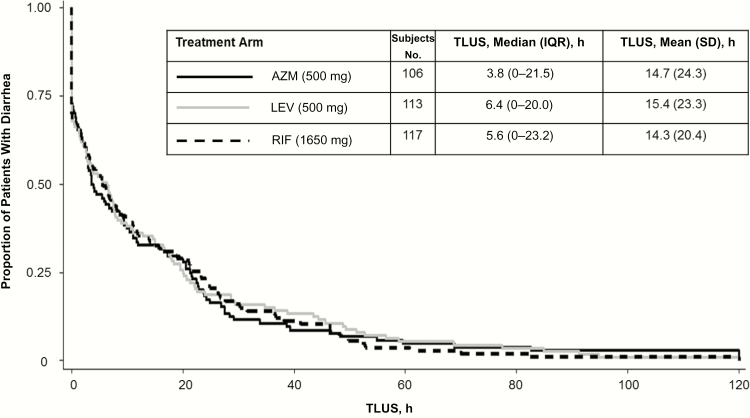
For the primary outcome of clinical cure at 24 hours, the referent levofloxacin arm was 81.4%, with comparator cure rates of 78.3% and 74.8% in the azithromycin and rifaximin arms, respectively. Azithromycin was found to be noninferior (P = .01) and nonsuperior (P = >.99). Noninferiority to levofloxacin could not be shown for rifaximin (P = .07). At 48 and 72 hours, azithromycin (both P < .001) and rifaximin (both P = .003) were noninferior to levofloxacin (clinical cure rate, 90.8% at 48 and 96.0% at 72 hours). Clinical failures were infrequent, occurring in 3 subjects (2.8%) for azithromycin, 1 (0.9%) for levofloxacin, and 1 for (0.9%) rifaximin (P = .46). Four (3.6%) subjects in the levofloxacin arm and 1 each (0.9%) in the azithromycin and rifaximin arms had disease lasting >72 hours (P = .36). For the median TLUS outcome (Figure 2), there were no differences among treatment arms (azithromycin, 3.8 [interquartile range, 0–21.5] hours; levofloxacin, 6.4 [0–20.0] hours; rifaximin, 5.6 [0–23.2] hours; P = .96).
Figure 2.
Comparative effectiveness of azithromycin (AZM), levofloxacin (LEV), and rifaximin (RIF) for treatment of travelers’ diarrhea (proportion remaining with diarrhea after initiation of therapy). Abbreviations: IQR, interquartile range; SD, standard deviation; TLUS, time to last unformed stool.
There were no statistically significant differences between treatment arms with respect to clinical outcomes when subjects were stratified by baseline severity. However, 24-hour clinical cure rates were lower and TLUS periods longer among those with more severe baseline disease (Table 4). When the analysis was limited to solo pathogen infections, rifaximin was significantly less efficacious than azithromycin and levofloxacin against any diarrheagenic E. coli infection, with clinical cure attained in 59.3% of subjects at 24 hours, compared with 84.6% for azithromycin (P = .04) and 86.7% for levofloxacin (P = .02) (Table 5). Treatment effects were consistent for both ETEC and EAEC infections, and TLUS outcomes were similar. When we evaluated differences in efficacy by etiological agent inclusive of copathogen infections, differences between treatment arms for diarrheagenic E. coli were no longer apparent for primary or secondary clinical outcomes. There were no observed differences in efficacy outcomes based on the number of pathogens identified (data not shown).
Table 4.
Primary, Secondary, and Safety Outcomes
| Outcome | Azithromycin (n = 106) | Levofloxacin (n = 113) | Rifaximin (n = 107) | Total (n = 326) |
|---|---|---|---|---|
| Overall clinical cure, No. (%) | ||||
| 24 h | 83 (78.3) | 92 (81.4) | 80 (74.8) | 255 (78.2) |
| 48 h | 96 (90.6) | 101 (89.4) | 99 (92.5) | 296 (90.8) |
| 72 h | 100 (94.3) | 108 (95.6) | 105 (98.1) | 313 (96.0) |
| No more LLS after treatment | 28 (26.4) | 36 (32.4) | 32 (29.9) | 96 (29.5) |
| Clinical cure at 24 h by tertile of LLS in past 8 h at presentation, No./total (%) | ||||
| 0–2 | 32/38 (84.2) | 29/34 (85.3) | 31/39 (79.5) | 92/111 (79.5) |
| 3–4 | 31/41 (75.6) | 48/60 (80.0) | 28/38 (73.7) | 107/139 (77.0) |
| ≥5 | 20/27 (74.1) | 15/19 (79.0) | 21/30 (70.0) | 56/76 (73.7) |
| Clinical cure at 24 h by self-reported function at presentation, No./total (%)a | ||||
| No impact | 24/28 (85.7) | 24/28 (85.7) | 17/20 (85.0) | 65/76 (85.5) |
| ≤50% impact | 41/51 (80.4) | 39/46 (84.8) | 43/52 (82.7) | 123/149 (82.6) |
| >50% impact | 17/24 (70.8) | 24/28 (85.7) | 17/30 (56.7) | 58/82 (70.7) |
| Inability to function | 0/2 | 5/10 (50.0) | 2/4 (50.0) | 7/16 (43.8) |
| TLUS by self-reported function at presentation, median (IQR). h | ||||
| No impact | 3.5 (0.1–10.7) | 6.1 (0.0–20.1) | 0.0 (0.0–9.2) | 3.1 (0.0–12.0) |
| ≤50% impact | 2.7 (0.0–20.5) | 2.7 (0.0–15.0) | 3.8 (0.0–15.9) | 2.8 (0.0–17.6) |
| >50% impact | 13.0 (2.6–23.8) | 7.2 (0.0–18.9) | 17.3 (4.3–27.6) | 10.9 (1.3–24.1) |
| Inability to function | 30.3 (26.8–33.8) | 26.6 (11.0–49.3) | 17.5 (3.9–38.1) | 28.3 (8.2–45.0) |
| Postdose nausea or vomiting, No. (%) | ||||
| Nausea | 3 (2.9) | 2 (1.8) | 5 (4.7) | 10 (3.1) |
| Vomiting | 2 (1.9) | 1 (0.9) | 1 (0.9) | 4 (1.2) |
| Nausea or vomiting | 4 (3.8) | 2 (1.8) | 5 (4.7) | 11 (3.4) |
| Treatment failure | 4 (3.8) | 5 (4.4) | 2 (1.9) | 11 (3.4) |
| Worsening or failure to improveb | 3 (2.8) | 1 (0.9) | 1 (0.9) | 5 (1.5) |
| Disease >72 h | 1 (0.9) | 4 (3.6) | 1 (0.9) | 6 (1.9) |
| Relapses after cure | 0 | 0 | 0 | 0 |
| Rebound constipation, No. (%) | ||||
| Symptoms only | 12 (11.3) | 14 (12.3) | 15 (14.0) | 41 (12.6) |
| Symptoms concurrent with formed stool | 5 (4.7) | 2 (1.8) | 7 (6.5) | 14 (4.3) |
| Adjunctive loperamide use after initial dose | ||||
| Mean (SD) | 1.5 (1.9) | 1.7 (2.6) | 1.9 (3.0) | 1.7 (2.5) |
| Median (IQR) | 1 (0–2) | 1 (0–2) | 0 (0–3) | 1 (0–2) |
Abbreviations: IQR, interquartile range; LLS, loose or liquid stools; SD, standard deviation; TLUS, time to last unformed stool.
aOne subject in each group was missing self-reported function at presentation.
bRescue therapy was initiated.
Table 5.
Primary and Secondary Clinical Outcomes by Pathogen and Treatment Arm
| Pathogen | Cure at 24 h, No./Total (%) | TLUS, Median (IQR), h | ||||
|---|---|---|---|---|---|---|
| Azithromycin | Levofloxacin | Rifaximin | Azithromycin | Levofloxacin | Rifaximin | |
| No pathogens identified | 35/43 (81.4) | 26/33 (78.8) | 33/40 (82.5) | 3.8 (0.8–20.1) | 6.6 (0.0–20.0) | 2.3 (0.0–19.9) |
| Limited to solo pathogens | ||||||
| Any bacteria | 22/26 (84.6) | 27/31 (87.1) | 16/27 (59.3) | 2.8 (0.0–8.7) | 6.8 (0.0–19.6) | 11.1 (2.5–38.1) |
| Diarrheagenic Escherichia coli | 22/26 (84.6) | 26/30 (86.7) | 16/27 (59.3) | 2.8 (0.0–8.7) | 5.8 (0.0–19.5) | 11.1 (2.5–38.1) |
| ETEC | 6/7 (85.7) | 7/8 (87.5) | 8/13 (61.5) | 2.9 (0.0–11.7) | 3.4 (0.0–13.9) | 9.0 (0.0–41.2) |
| EAEC | 16/19 (84.2) | 12/15 (80.0) | 6/10 (60.0) | 1.4 (0.0–8.7) | 7.5 (0.0–20.7) | 18.6 (5.3–31.5) |
| EHEC | 0/0 | 0/0 | 0/1 | … | … | 29.7a |
| EPEC | 0/0 | 6/6 (100) | 1/2 (50.0) | … | 3.4 (0.0–19.5) | 24.9 (0.0–49.8) |
| EIEC | 0/0 | 1/1 (100) | 1/1 (100) | … | 0a | 3.0a |
| Campylobacter | 0/0 | 0/0 | 0/0 | … | … | … |
| Shigella | 0/0 | 0/0 | 0/0 | … | … | … |
| Salmonella | 0/0 | 1/1 (100) | 0/0 | … | 19.6a | … |
| Any parasite | 2/2 (100) | 0/0 | 2/4 (50.0) | 0.9 (0.0–1.8) | … | 29.0 (11.8–36.6) |
| Cryptosporidium parvum | 2/2 (100) | 0/0 | 0/2 | 0.9 (0.0–1.8) | … | 36.6 (36.4–36.8) |
| Giardia lamblia | 0/0 | 0/0 | 2/2 (100) | … | … | 11.8 (1.9–21.7) |
| Any virus | 3/5 (60.0) | 9/12 (75.0) | 6/7 (85.7) | 23.8 (0.9–27.5) | 8.1 (1.3–28.5) | 4.4 (0.0–7.3) |
| Norovirus | 1/3 (33.3) | 7/10 (70.0) | 5/6 (83.3) | 27.5 (23.8–59.5) | 8.1 (1.3–34.8) | 5.7 (0.0–7.3) |
| Rotavirus | 2/2 (100) | 2/2 (100) | 1/1 (100) | 0.5 (0.0–0.9) | 11.8 (1.4–22.2) | 0 a |
| Including coinfections | ||||||
| Any bacteria | 28/36 (77.8) | 46/53 (86.8) | 27/39 (69.2) | 3.4 (0.0–21.3) | 5.6 (0.0–19.5) | 8.0 (0.0–29.7) |
| Diarrheagenic E. coli | 28/36 (77.8) | 45/52 (86.5) | 27/39 (69.2) | 3.4 (0.0–21.3) | 5.1 (0.0–18.6) | 8.0 (0.0–29.7) |
| ETEC | 10/15 (66.7) | 17/18 (94.4) | 13/19 (68.4) | 5.8 (0.2–27.5) | 5.1 (0.0–9.9) | 9.0 (0.0–41.2) |
| EAEC | 20/26 (76.9) | 25/29 (86.2) | 14/19 (73.7) | 2.8 (0.0–11.9) | 7.2 (0.0–17.8) | 11.9 (0.7–24.1) |
| EHEC | 0/1 | 2/3 (66.7) | 2/3 (66.7) | 27.5a | 3.6 (0.0–68.7) | 18.9 (0.0–29.7) |
| EPEC | 1/2 (50.0) | 11/12 (91.7) | 4/5 (80.0) | 35.0 (21.6–48.3) | 5.2 (0.0–13.5) | 0.0 (0.0–14.9) |
| EIEC | 0/0 | 3/3 (100) | 2/2 (100) | … | 1.5 (0.0–22.5) | 1.5 (0.0–3.0) |
| Campylobacter | 0/0 | 0/1 | 1/1 (100) | … | 28.7a | 07a |
| Shigella | 0/0 | 1/1 (100) | 0/0 | … | 1.5a | … |
| Salmonella | 0/0 | 1/1 (100) | 0/0 | … | 19.6a | … |
| Any parasite | 2/2 (100) | 0/0 | 2/5 (40.0) | 0.9 (0.0–1.8) | … | 36.4 (21.7–36.8) |
| C. parvum | 2/2 (100) | 0/0 | 0/3 | 0.9 (0.0–1.8) | … | 36.8 (36.4–82.5) |
| G. lamblia | 0/0 | 0/0 | 2/2 (100) | … | … | 11.8 (1.9–21.7) |
| Any virus | 6/8 (75.0) | 17/22 (77.3) | 9/10 (90.0) | 15.5 (0.5–25.7) | 4.7 (0.0–22.5) | 2.2 (0.0–7.0) |
| Norovirus | 2/4 (50.0) | 11/16 (68.8) | 7/8 (87.5) | 25.7 (22.7–43.5) | 8.1 (0.6–42.0) | 5.5 (0.0–7.2) |
| Rotavirus | 4/4 (100) | 6/6 (100) | 2/2 (100) | 0.5 (0.0–5.2) | 0.7 (0.0–2.6) | 0a |
Abbreviations: EAEC, enteroaggregative E. coli; EHEC, enterohemorrhagic E. coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; IQR, interquartile range; TLUS, time to last unformed stool.
aIQR not determinable owing to small sample size.
Extended-Spectrum β-Lactamase Carriage
Evaluation of baseline and posttreatment ESBL-PE identification was limited to individuals from whom nonenteropathogenic E. coli isolates were isolated (Table 6). Data are reported for all individuals with isolates collected before treatment and on day 21. Baseline rates of ESBL-PE recovery were 5.1%, with rates higher in the azithromycin (8.5%) arm and lower in the rifaximin arm (1.4%). Among all individuals with isolates characterized at day 21, the overall rate of recovery was 9.7%, with similar rates across each treatment arm. For subjects with paired samples, pretreatment rates were similar (2.5%–5.4%) and increased at day 21 for all drug arms (7.5%–13.5%). Across all treatments, recovery rates increased from 3.5% to 9.7% (P = .11), with no differences between regimens. There were notable differences in overall day 0 ESBL-PE rates, with Afghanistan having higher rates than other enrollment sites. When analyses were limited to individuals with paired stool samples and recovery of nonpathogenic E. coli, rates were found to increase across all arms for each country. No differences were noted when treatment arms were stratified by country (data not shown).
Table 6.
Extended-Spectrum β-Lactamase-Producing Escherichia coli at Presentation and 21 days After Therapy
| Subjects and Timing | Subjects Positive for ESBL-Producing E. coli/Total No. (%) | |||
|---|---|---|---|---|
| Azithromycin | Levofloxacin | Rifaximin | Overall | |
| All subjects | ||||
| Day 0 | 6/71 (8.5) | 4/74 (5.4) | 1/72 (1.4) | 11/217 (5.1) |
| Day 21 | 5/57 (8.8) | 5/54 (9.3) | 5/44 (11.4) | 15/155 (9.7) |
| Subjects with paired samplesa | ||||
| Day 0 | 1/40 (2.5) | 2/37 (5.4) | 1/37 (2.7) | 4/114 (3.5) |
| Day 21 | 3/40 (7.5) | 3/37 (8.1) | 5/37 (13.5) | 11/114 (9.6) |
Abbreviation: ESBL, extended-spectrum β-lactamase.
aFor analysis of subjects with paired samples, day 21 data represent incident cases only (eg, excluding subjects who were positive for ESLB-producing E. coli on both day 0 and day 21).
Safety and Tolerance
All treatment regimens were equivalently well tolerated, and no serious adverse events occurred. Postdose nausea was reported in 3.1% of subjects, without differences across treatment arm (P = .44), and vomiting occurred in 2 subjects in the azithromycin arm and 1 each in the levofloxacin and rifaximin arms (P = .70). Self-reported rebound constipation symptoms occurred in 12.6% of subjects, with 4.3% reporting symptoms associated with a formed stool without differences by treatment arm (Table 4). Constipation most often happened within 24 hours of treatment and was nontreatment emergent, self-resolved, and not associated in frequency or severity with the total number of loperamide doses taken (data not shown). The blinding was broken in 2 subjects involving cases of transient mild diplopia and nonanaphylactic urticaria, both of whom had received levofloxacin.
DISCUSSION
Our findings complement existing studies demonstrating effectiveness in rapid cure of single-dose, loperamide-adjuncted antibiotic regimens [22]. Rapid resolution of symptoms and clinical cure with combined therapy significantly reduces lost time whether one is traveling for business, pleasure, or military deployment. Although no placebo arm was included, the median TLUS for each regimen (4–6 hours) compares favorably to TLUS measures in historical placebo-controlled studies (approximately 60 hours) and a single loperamide-only trial (approximately 33 hours if failures with antibiotic rescue therapy are included) [23–25]. Along with optimizing an individual’s return to health, such rapid cure should also contribute to a decreased risk of dehydration through cessation of fluid losses. Reducing the need for multiple doses simplifies treatment recommendations and should result in fewer negative consequences associated with antibiotic use, including resistance acquisition and dysbiosis.
Although prior studies have shown the effectiveness of single-dose fluoroquinolone and azithromycin regimens, this study represents the first demonstration of efficacy for single-dose rifaximin as therapy for TD. Although noninferiority relative to levofloxacin was not demonstrated, when the entirety of the clinical results are examined, the findings suggest that single-dose rifaximin (1650 mg) is a comparable alternative to other first-line agents. Although caution should be taken with comparing results across trials from different periods, countries, and populations, it seems that high-dose rifaximin combined with loperamide has superior efficacy compared with multiday dosing regimen (TLUS, approximately 27 hours for multiday dosing regimen in an analysis of US students in Mexico [26] vs 6 hours in the current study). A possible explanation may involve increased luminal concentration and drug contact time effects in high-dose regimens with loperamide.
Antibiotic treatment of TD and associated increased rates of ESBL-PE has garnered concern about potential adverse consequences for treating what is often considered a nonserious, self-limited illness [13, 14]. Relative to previously published studies, we found a lower post-TD recovery of ESBL-PE. Similar to other observational studies, we found that antibiotic treatment increases recovery of ESBL-PE 21 days after treatment compared with baseline at a 2–3-fold rate. Furthermore, the rates of ESBL-PE recovery (at acute illness) were lower than one might anticipate for a population that had been living in a developing world country for an average of 3 months before their enrollment. A number of features of the current study differ from those in the published literature and could explain the differences in results. First, the food and water on a US or UK military base meets the standards of quality in the country of origin. Service members who get an acute diarrheal illness are usually exposed in similar means as the average traveler, through eating a contaminated meal off base or other fecal-oral exposures. One might therefore expect a baseline rate of ESBL-PE analogous to that seen in the host country. Furthermore, ours is the only study to report ESBL-PE recovery during acute TD, which may have decreased the recovery of nonpathogenic Enterobacteriaceae owing to competitive colonization by the infectious pathogen overgrowth. The observed lower postinfective diarrhea rates need to be put in context with findings from observational studies [14, 16].
In a study of in Finnish travelers to multiple geographic regions, the incident colonization rate was 21% among those without treatment, 20% for those who took only loperamide, 40% among those who took only antibiotics, and 71% who took both [14]. The overall rates of ESBL-PE colonization in the trial were relatively low compared with those reported in a recent review of observational studies, which found incident colonization rates among returning travelers ranging between 21% and 51% [27]. Differences in collection and processing methods could account for lower rates of ESBL-PE recovery in our study and preclude a direct comparison. It is also possible that single-dose antibiotics had a reduced effect on enhancing colonization with resistant organisms compared with multidose regimens, which are still commonly used [28]. Nevertheless, the attributable fraction of single-dose antibiotic treatment for TD in contribution to multidrug-resistance acquisition needs to be put into appropriate perspective and balanced against the clear benefit of antibiotics for use as empiric TD therapy, and it requires further study.
Postdose nausea and vomiting have been a concern with high-dose azithromycin [11, 12]. In our study, there were 4 vomiting episodes (2 in the azithromycin arm). Although the trend is toward azithromycin having increased gastrointestinal intolerance at a 500-mg dose, these results should be interpreted with caution given the high pill burden (6 large capsules) used in this trial. Self-reported posttreatment constipation was identified, although it was transient, self-limited, and not associated with a dose response, and it most often represented absence of stooling after a treated bout of TD. Proper education on traveler self-administration of loperamide and expectations of changes in stool frequency remain important.
The lack of loperamide-only and standard rifaximin regimen arms is a stated limitation and does not allow us to determine whether alternatives might have been equivalently effective in this trial. However, our study was specifically designed to assess single-dose antibiotic therapy, given the potential importance of its use in an operational military environment. In a study of students in Mexico presenting with acute TD, rifaximin and loperamide alone and in combination were compared [29]. Before treatment, the mean duration of illness was approximately 25 hours, and the median number of unformed stools was approximately 5 across all arms. In comparison, our subjects had a mean illness duration of 26.7 hours and a median of 7 unformed stools. The Mexico study reported comparable clinical outcomes as a mean TLUS of 32.5 hours for rifaximin alone, 27.3 hours for rifaximin plus loperamide, and 69.0 hours for the loperamide-only group. These data suggest that our population tended to have more severe disease, yet clinical outcomes were better with high-dose rifaximin combined with loperamide (mean TLUS, 14.9 hours overall and 12.5 hours in Honduras). It is possible that higher loading dose of rifaximin improved clinical outcomes related to antibacterial and nonbactericidal effects [30–33].
The generalizability of these results to nonmilitary travelers is worth consideration. Military travelers differ from others in that they are predominantly male, younger, and healthier; being healthier could conceivably result in a more rapid response to a given effective therapy. Nevertheless, travelers, whether military or civilian, do share many commonalities, including exposure to similar etiologic agents [34, 35] and exposures to contaminated food and beverages that result in acute illness. Consistent with the results of this trial, prior studies of combined loperamide adjunctive therapies in nonmilitary travelers with single-dose azithromycin and fluoroquinolones have demonstrated equivalently rapid clinical cures [36, 37]. Finally, consistent with findings of other trials and epidemiological studies, a substantial proportion of etiological agents could not be identified using our culture-based bacterial methods and PCR for viruses, and these probably represent bacterial agents based on the response to therapy. Future studies ought to consider implementation of culture-independent testing methods to improve diagnostic sensitivity, although issues in copathogen detection will confound attribution [38], and isolates for culture and sensitivity remain important in cases of treatment failure.
Overall, our findings provide strong evidence that loperamide-adjuncted, single-dose antibiotic therapy for AWD among travelers is safe and highly effective. Furthermore, these results highlight rapid clinically relevant improvement of illness and diarrheal symptoms associated with combination therapy.
Notes
Acknowledgments. The TrEAT TD study could not have been completed without the dedication of a team of clinical investigators, laboratory support personnel, and other study staff. Clinical investigators included the following: Sqn Ldr Matthew Adam, Sgt Ernest Akorli, Cpl Rachael Armstrong, Cpl Lucy Ashford-Brown, CPT Jaime Alvarado, Ricardo Aviles, MD, Cpl Charlotte Ayres, Lt Col Timothy Ballard, Sgt Liam Barry, CAPT Mary Bavaro, LCDR Catherine Berjohn, CDR Robert Bjoraker, Capt Peter Blenkinsop, LCDR Jason Blitz, LT Jeromy Boucher, CAPT Timothy Burgess, Maj Daniel Burns, Cpl Jenna Burns, Capt Shauna Butler, CPT Anthony Cancio, CPT Anthony Cardile, CPT Tarah Carnes, CPT Fongkuei Cheng, Maj Katherine Clay, LT David Cook, Robert Deiss, MD, Maj Charles Duffield, CDR Christopher Duplessis, MAJ Rhonda Dyer, MAJ Aaron Farmer, CDR Robert Gormley, Maj Antonia Hazlerigg, CPT Jewell Hemmings, Lt Col Neil Hill, CPT Emily Hollis, CPT Jack Hutter, Cpl Alshia Johnson, Maj Paul Kartchner, LT Fred Kency Jr., CDR Kelly Latimer, Maj Julian Lentaigne, LCDR Andrew Letizia, CAPT Jason Maguire, CPT Jennifer Masel, CDR Ryan Maves, Lt Aline Miura, CPT Lynette Moore, LT Olamide Oladipo, Capt Shane Patterson, CPT Mark Pence, Lt Cdr Adrian Proffitt, Sqn Ldr Joanna Rimmer, LCDR Benjamin Rodriguez, Capt Carlo Rossi, Lt Col Claire Royston, 1LT Melanie Sanders, MAJ Karen Santiago, Surg Lt Cdr Thomas Scorer, LCDR Amanda Self, CPT Akira Shishido, Cpl Mildred Sitonik, LT Daniel Snyder, CDR Garrick Stride, CDR Hamilton Tilley, CPT Matthew Timlin, CPT Melanie Trado, CPT Detonya Tulsie, Capt Lavanya Viswanathan, LCDR Tyler Warkentien, Maj J. T. A. Wedgwood, and Lt Col Samuel White. Key laboratoary and site staff included the following: Cliff Philips, Margaret Koech, Elizabeth Odundo, Giselle Soto, MD, Faviola Reyes, and Michelle Tisdale. Staff of the Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, included the following: Indrani Mitra, Josh Kumpf, Grier Greene, Sarah Roberts, Kalyani Telu, and Renee Bowers. In addition, gratitude is owed to John Sanders, MD (US Navy captain, retired) who was instrumental in garnering the institutional support necessary to execute this trial. Finally, we express our sincere appreciation to all the dedicated service members of the US and UK militaries for volunteering not only to serve their countries but also to participate in this study.
Disclaimer. The views expressed are those of the authors and do not necessarily reflect the official views or policies of the Uniformed Services University of the Health Sciences; the Henry M. Jackson Foundation for the Advancement of Military Medicine; the US Department of Defense or the Departments of the Army, Navy, or Air Force; or the UK Ministry of Defence.
Mention of trade names, commercial products, or organization does not imply endorsement by the US government. Some coauthors are military service members or employees of the US government; this work was prepared as part of their official duties.
Financial support. This work was supported by the Bureau of Medicine and Surgery, Uniformed Services University of the Health Sciences (USU grant agreement HU0001-11-1-0022; USU project G187V2) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (interagency agreement Y1-AI-5072).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
TrEAT TD Study Team:
Matthew Adam, Ernest Akorli, Rachael Armstrong, Lucy Ashford-Brown, Jaime Alvarado, Ricardo Aviles, Charlotte Ayres, Timothy Ballard, Liam Barry, Mary Bavaro, Catherine Berjohn, Robert Bjoraker, Peter Blenkinsop, Jason Blitz, Jeromy Boucher, Timothy Burgess, Daniel Burns, Jenna Burns, Shauna Butler, Anthony Cancio, Anthony Cardile, Tarah Carnes, Fongkuei Cheng, Katherine Clay, David Cook, Robert Deiss, Charles Duffield, Christopher Duplessis, Rhonda Dyer, Aaron Farmer, Robert Gormley, Antonia Hazlerigg, Jewell Hemmings, Neil Hill, Emily Hollis, Jack Hutter, Alshia Johnson, Paul Kartchner, Fred Kency, Jr., Kelly Latimer, Julian Lentaigne, Andrew Letizia, Jason Maguire, Jennifer Masel, Ryan Maves, Aline Miura, Lynette Moore, Olamide Oladipo, Shane Patterson, Mark Pence, Adrian Proffitt, Joanna Rimmer, Benjamin Rodriguez, Carlo Rossi, Claire Royston, Melanie Sanders, Karen Santiago, Thomas Scorer, Amanda Self, Akira Shishido, Mildred Sitonik, Daniel Snyder, Garrick Stride, Hamilton Tilley, Matthew Timlin, Melanie Trado, Detonya Tulsie, Lavanya Viswanathan, Tyler Warkentien, J T A Wedgwood, and Samuel White
References
- 1. Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA 2015; 313:71–80. [DOI] [PubMed] [Google Scholar]
- 2. Adachi JA, Ostrosky-Zeichner L, DuPont HL, Ericsson CD. Empirical antimicrobial therapy for traveler’s diarrhea. Clin Infect Dis 2000; 31:1079–83. [DOI] [PubMed] [Google Scholar]
- 3. Shlim DR. Update in traveler’s diarrhea. Infect Dis Clin North Am 2005; 19:137–49. [DOI] [PubMed] [Google Scholar]
- 4. Yates J. Traveler’s diarrhea. Am Fam Physician 2005; 71:2095–100. [PubMed] [Google Scholar]
- 5. Richard P, Delangle MH, Raffi F, Espaze E, Richet H. Impact of fluoroquinolone administration on the emergence of fluoroquinolone-resistant gram-negative bacilli from gastrointestinal flora. Clin Infect Dis 2001; 32:162–6. [DOI] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Safety announcement 2016. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf. Accessed 22 March 2017.
- 7. Diemert DJ. Prevention and self-treatment of traveler’s diarrhea. Clin Microbiol Rev 2006; 19:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DuPont HL. Travellers’ diarrhoea: contemporary approaches to therapy and prevention. Drugs 2006; 66:303–14. [DOI] [PubMed] [Google Scholar]
- 9. Adachi JA, DuPont HL. Rifaximin: a novel nonabsorbed rifamycin for gastrointestinal disorders. Clin Infect Dis 2006; 42:541–7. [DOI] [PubMed] [Google Scholar]
- 10. Taylor DN, Bourgeois AL, Ericsson CD et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg 2006; 74:1060–6. [PubMed] [Google Scholar]
- 11. Tribble DR, Sanders JW, Pang LW et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis 2007; 44:338–46. [DOI] [PubMed] [Google Scholar]
- 12. Sanders JW, Frenck RW, Putnam SD et al. Azithromycin and loperamide are comparable to levofloxacin and loperamide for the treatment of traveler’s diarrhea in United States military personnel in Turkey. Clin Infect Dis 2007; 45:294–301. [DOI] [PubMed] [Google Scholar]
- 13. Barreto Miranda I, Ignatius R, Pfuller R et al. High carriage rate of ESBL-producing Enterobacteriaceae at presentation and follow-up among travellers with gastrointestinal complaints returning from India and Southeast Asia. J Travel Med 2016; 23:tav024. [DOI] [PubMed] [Google Scholar]
- 14. Kantele A, Lääveri T, Mero S et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis 2015; 60:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruppé E, Armand-Lefèvre L, Estellat C et al. High rate of acquisition but short duration of carriage of multidrug-resistant Enterobacteriaceae after travel to the tropics. Clin Infect Dis 2015; 61:593–600. [DOI] [PubMed] [Google Scholar]
- 16. Kantele A, Mero S, Kirveskari J, Lääveri T. Increased risk for ESBL-producing bacteria from co-administration of loperamide and antimicrobial drugs for travelers’ diarrhea. Emerg Infect Dis 2016; 22:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nada RA, Shaheen HI, Touni I et al. Design and validation of a multiplex polymerase chain reaction for the identification of enterotoxigenic Escherichia coli and associated colonization factor antigens. Diagn Microbiol Infect Dis 2010; 67:134–42. [DOI] [PubMed] [Google Scholar]
- 18. Lüscher D, Altwegg M. Detection of shigellae, enteroinvasive and enterotoxigenic Escherichia coli using the polymerase chain reaction (PCR) in patients returning from tropical countries. Mol Cell Probes 1994; 8:285–90. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen TV, Le Van P, Le Huy C, Gia KN, Weintraub A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J Clin Microbiol 2005; 43:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement CLSI document M100-S18. Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 21. Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials 1982; 3:345–53. [DOI] [PubMed] [Google Scholar]
- 22. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler’s diarrhea: a systematic review and meta-analysis. Clin Infect Dis 2008; 47:1007–14. [DOI] [PubMed] [Google Scholar]
- 23. De Bruyn G, Hahn S, Borwick A. Antibiotic treatment for travellers’ diarrhoea. Cochrane Database Syst Rev 2000; 3:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson PC, Ericsson CD, DuPont HL, Morgan DR, Bitsura JA, Wood LV. Comparison of loperamide with bismuth subsalicylate for the treatment of acute travelers’ diarrhea. JAMA 1986; 255:757–60. [PubMed] [Google Scholar]
- 25. DuPont HL, Flores Sanchez J, Ericsson CD et al. Comparative efficacy of loperamide hydrochloride and bismuth subsalicylate in the management of acute diarrhea. Am J Med 1990; 88:15S–9S. [DOI] [PubMed] [Google Scholar]
- 26. Dupont HL, Jiang ZD, Belkind-Gerson J et al. Treatment of travelers’ diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone. Clin Gastroenterol Hepatol 2007; 5:451–6. [DOI] [PubMed] [Google Scholar]
- 27. Hassing RJ, Alsma J, Arcilla MS, van Genderen PJ, Stricker BH, Verbon A. International travel and acquisition of multidrug-resistant Enterobacteriaceae: a systematic review. Euro Surveill 2015; 20: doi: 10.2807/1560-7917.ES.2015.20.47.30074. [DOI] [PubMed] [Google Scholar]
- 28. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016; 111:602–22. [DOI] [PubMed] [Google Scholar]
- 29. Dupont HL, Jiang ZD, Belkind-Gerson J et al. Treatment of travelers’ diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone. Clin Gastroenterol Hepatol 2007; 5:451–6. [DOI] [PubMed] [Google Scholar]
- 30. Acosta A, Camilleri M, Shin A et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016; 7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DuPont HL. Therapeutic effects and mechanisms of action of rifaximin in gastrointestinal diseases. Mayo Clin Proc 2015; 90:1116–24. [DOI] [PubMed] [Google Scholar]
- 32. Schrodt C, McHugh EE, Gawinowicz MA, Dupont HL, Brown EL. Rifaximin-mediated changes to the epithelial cell proteome: 2-D gel analysis. PLoS One 2013; 8:e68550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cottreau J, Baker SF, DuPont HL, Garey KW. Rifaximin: a nonsystemic rifamycin antibiotic for gastrointestinal infections. Expert Rev Anti Infect Ther 2010; 8:747–60. [DOI] [PubMed] [Google Scholar]
- 34. Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg 2006; 74:891–900. [PubMed] [Google Scholar]
- 35. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 2009; 80:609–14. [PubMed] [Google Scholar]
- 36. Ericsson CD, DuPont HL, Mathewson JJ. Single dose ofloxacin plus loperamide compared with single dose or three days of ofloxacin in the treatment of traveler’s diarrhea. J Travel Med 1997; 4:3–7. [DOI] [PubMed] [Google Scholar]
- 37. Ericsson CD, DuPont HL, Okhuysen PC, Jiang ZD, DuPont MW. Loperamide plus azithromycin more effectively treats travelers’ diarrhea in Mexico than azithromycin alone. J Travel Med 2007; 14:312–9. [DOI] [PubMed] [Google Scholar]
- 38. Vila J. New molecular diagnostic tools in traveller’s diarrhea. J Travel Med 2017; 24:23–8. [DOI] [PubMed] [Google Scholar]