Risk-based hepatitis C virus (HCV) testing is recommended for young adults. We used simulation modeling to show that routine rapid HCV testing during clinical visits might be cost-effective in settings with a high prevalence of HCV and injection drug use.
Keywords: computer simulation model, cost-effectiveness, hepatitis C testing, adolescents and young adults, injection drug use
Abstract
Background
High hepatitis C virus (HCV) rates have been reported in young people who inject drugs (PWID). We evaluated the clinical benefit and cost-effectiveness of testing among youth seen in communities with a high overall number of reported HCV cases.
Methods
We developed a decision analytic model to project quality-adjusted life years (QALYs), costs (2016 US$), and incremental cost-effectiveness ratios (ICERs) of 9 strategies for 1-time testing among 15- to 30-year-olds seen at urban community health centers. Strategies differed in 3 ways: targeted vs routine testing, rapid finger stick vs standard venipuncture, and ordered by physician vs by counselor/tester using standing orders. We performed deterministic and probabilistic sensitivity analyses (PSA) to evaluate uncertainty.
Results
Compared to targeted risk-based testing (current standard of care), routine testing increased the lifetime medical cost by $80 and discounted QALYs by 0.0013 per person. Across all strategies, rapid testing provided higher QALYs at a lower cost per QALY gained and was always preferred. Counselor-initiated routine rapid testing was associated with an ICER of $71000/QALY gained. Results were sensitive to offer and result receipt rates. Counselor-initiated routine rapid testing was cost-effective (ICER <$100000/QALY) unless the prevalence of PWID was <0.59%, HCV prevalence among PWID was <16%, reinfection rate was >26 cases per 100 person-years, or reflex confirmatory testing followed all reactive venipuncture diagnostics. In PSA, routine rapid testing was the optimal strategy in 90% of simulations.
Conclusions
Routine rapid HCV testing among 15- to 30-year-olds may be cost-effective when the prevalence of PWID is >0.59%.
(See the Editorial Commentary by Chhatwal on pages 385–6.)
Currently, nearly all hepatitis C virus (HCV) transmission in the United States occurs among young persons who inject drugs (PWID) [1]. National guidance recommends therapy for infected PWID to prevent HCV transmission [2]. Similarly, the Institute of Medicine and the World Health Organization cite treatment for PWID as critical to elimination goals [3, 4]. Risk-based testing is recommended for PWID [5], and one-time testing is recommended for individuals born between 1945 and 1965 [6]. HCV screening among this birth cohort may delay the pending burden of liver disease but it does not address HCV transmission.
Experience from human immunodeficiency virus (HIV) testing suggests that healthcare providers are ineffective at identifying high-risk patients [7]. Routine testing may identify more cases; however, it requires testing a larger pool of individuals to identify 1 case. Standard venipuncture HCV antibody followed by confirmatory HCV RNA testing is the diagnostic gold standard, but this 2-test process limits linkage to care. In contrast, rapid point-of-care testing provides immediate initial results and could improve linkage to care. Finally, the best implementation model within primary care sites such as community health centers is unknown. One common approach is for physicians to initiate HCV testing orders. Another option is a dedicated counselor/tester with standing orders.
We used decision analysis to evaluate testing strategies among 15- to 30-year-olds at urban community health centers to answer the following questions: Should testing target PWID or should routine testing be implemented in areas with a high prevalence of HCV? What is the estimated prevalence of PWID at which routine testing should be favored? Should testing be performed using rapid or standard venipuncture testing? Should testing be offered by a physician during a routine outpatient visit or by a dedicated counselor/tester? Our findings provide projections of long-term costs, benefits, and cost-effectiveness and can inform local testing protocols and guidelines for testing.
METHODS
Analytic Overview
We used TreeAge Pro 2014 to develop a decision analytic model to evaluate the cost-effectiveness of HCV testing among 15- to 30-years-olds who do not already know their HCV status and are seen in primary care settings in community health centers (TreeAge Software, Inc., Williamstown, Massachusetts). We used the model to compare 9 strategies that differed in the following 3 ways (Table 1):
Table 1.
Strategies Considered
| Test Type | Person Initiating the Test | Strategy |
|---|---|---|
| Standard venipuncture | Physician | Current practice |
| Routine physician ordered, phlebotomist performed venipuncture testing | ||
| Targeteda physician ordered, phlebotomist performed venipuncture testing | ||
| Counselor/tester | Routine counselor initiated venipuncture testing | |
| Targeted counselor initiated venipuncture testing | ||
| Rapid | Physician | Routine physician initiated, counselor performed rapid testing |
| Targeted physician initiated, counselor performed rapid testing | ||
| Counselor/tester | Routine counselor initiated rapid testing | |
| Targeted counselor initiated rapid testing |
aProviders used a validated hepatitis C virus screening checklist.
Approach to screening—Current practice vs expanded targeted risk-based screening vs routine screening. For current practice, testing was offered only to individuals perceived as high risk. The offer rate was based on data from 3 community health centers in Boston neighborhoods with a high number of reported HCV cases among 15- to 30-year-olds. In expanded targeted risk-based testing, we used a validated HCV screening checklist to identify high-risk individuals [8]. For routine testing, there was an attempt to approach all individuals regardless of perceived risk.
Ordering provider—Physician vs. counselor/tester. When physicians ordered testing as part of routine visits, there were no additional costs beyond the test itself. In strategies involving counselor/testers, we included labor and HCV test costs [9, 10].
Type of test—Rapid finger stick vs standard serum venipuncture. In rapid testing, pre- and post-test counseling was provided by a counselor/tester, and test results were available within 20 minutes. All rapid tests were US Food and Drug Administration approved, with high sensitivity and specificity. Venipuncture testing requires phlebotomy, and results are available in 2 to 3 days. A trained phlebotomist performed venipuncture testing ordered by a physician, while counselor/testers completed blood draws for the patients they tested. Patients with reactive tests are required to return to the clinic for RNA testing.
Model Structure
The model began with a decision analytic tree that simulated HCV testing at the community health center and the cascade of HCV care. The decision tree identified and categorized all patients at entry by HCV status. HCV-infected individuals were then assigned probabilities of linking to care. Final branches of the tree ended with a Monte Carlo simulation of the individuals’ remaining life expectancies based on their status at the beginning of the simulation. The lifetime simulation calculated lifetime costs and quality-adjusted life years (QALYs) [11, 12].
Decision Tree
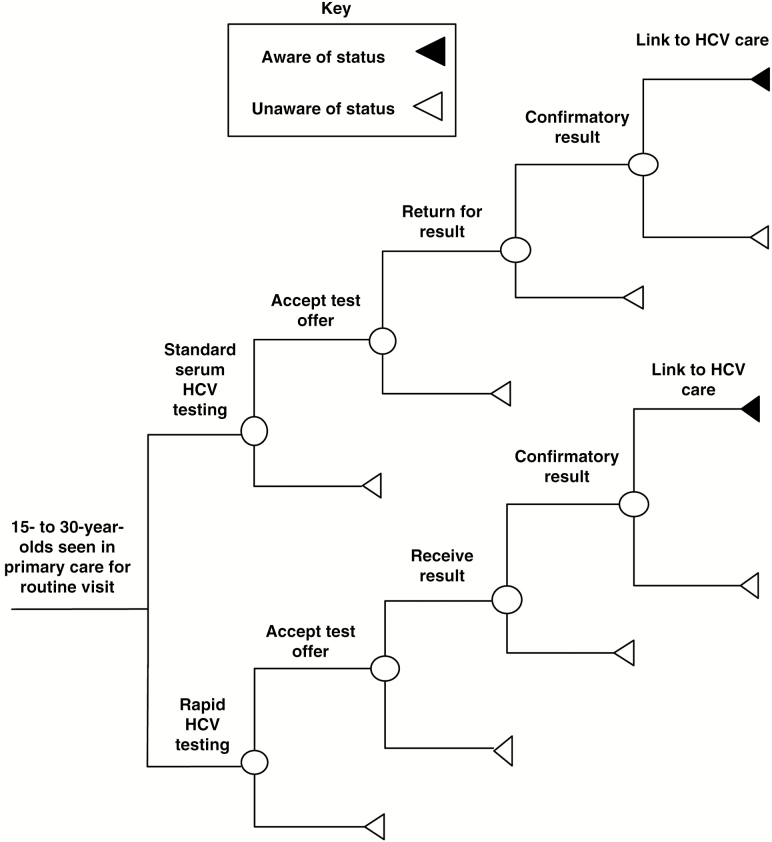
Each strategy in the decision tree included the HCV cascade of care: test offer, result delivery, linkage to care, and confirmatory HCV RNA testing (Figure 1). Linkage to care was defined as a visit with a health provider for confirmatory testing.
Figure 1.
Decision analytic model schematic. Abbreviation: HCV, hepatitis C virus. Adapted from Schackman et al [12].
Monte Carlo Simulation
We adapted the hepatitis C cost-effectiveness model (HEP-CE) [12, 13] to simulate individuals’ lifetime progression with or without chronic HCV. HEP-CE is an individual-level state transition Monte Carlo simulation of HCV screening, linkage to care, and treatment. Following is an overview of the model.
HCV Disease Progression
The model assigned a METAVIR fibrosis stage at simulation start based on the patient’s age and presumed age at HCV infection [14]. Then, fibrosis progression was simulated as a series of probabilistic transitions. There was no HCV-attributable mortality or decrease in quality of life related to HCV until patients reached cirrhosis. Cirrhotic patients faced risks of liver-related death and decompensation events.
HCV Treatment
Only those who were tested, diagnosed, and linked to care were eligible for treatment. Genotype distribution was based on recent surveys of PWID, with higher prevalence of genotype 3 compared to the cohort born between 1945 and 1965 [15]. We used treatment with 8 to 12 weeks of sofosbuvir/ledipasvir for genotype 1 and with 12 weeks of sofosbuvir/velpatasvir for genotypes 2 and 3. When patients achieved sustained virologic response (SVR), costs attributable to HCV infection decreased by 50%, and quality of life returned to that of an age- and sex-matched person without infection. When patients with cirrhosis attained SVR, their liver-related mortality decreased by 94% [16]. Those who attained SVR, but continued to inject drugs, faced reinfection. We assumed potential for retreatment for individuals who were reinfected. Individuals who had cirrhosis at the time of HCV cure could still develop hepatocellular carcinoma.
Future HCV Screening and Linkage
Individuals who were not tested or who failed to link to care, as well as those who became reinfected, had a future opportunity to be identified and linked to care.
Model Data
Analyses
Strategies were compared using incremental cost-effectiveness ratios (ICERs), calculated as the additional cost divided by the additional health benefit of the next most costly strategy, and expressed as the cost per QALY gained [17]. We used the commonly cited US threshold of $100000/QALY. Strategies were defined as dominated if they were more costly and less effective than a competing strategy or if they were associated with an ICER greater than that of a more effective strategy. Outcome measures were assessed from a healthcare sector perspective, and QALYs and costs were discounted at 3% annually. Costs were in 2016 US dollars. We performed deterministic sensitivity analyses to identify parameters with the greatest impact on cost-effectiveness. We also conducted a sensitivity analysis in which confirmatory HCV RNA testing was automatically performed for reactive serology with venipuncture testing (“reflex RNA testing”). We then performed a probabilistic sensitivity analysis in which we defined key parameter values as distributions and used second-order Monte Carlo simulation to perform 100000 cohort simulations. We also calculated the additional number of individuals who need to be evaluated to identify a new HCV case when comparing routine to targeted testing and provided the incremental cost of testing from a clinic budgetary perspective.
Base Case Inputs
Clinical Data
Table 2 summarizes model inputs used for the base case analysis. Data obtained from the literature included background mortality rate, natural history of HCV infection, HCV prevalence, cascade-of-care inputs, test characteristics, treatment effectiveness, HCV-related mortality rate, and cost associated with testing, confirmatory testing, and treatment. We also modeled increased risk of death in PWID by using published standardized mortality ratios (SMRs) for PWID [18]. Throughout the simulation, individuals could transition between drug use states with appropriate adjustment to their SMR such that long-term cessation and relapse behavior influenced life expectancy [19].
Table 2.
Model Input Parameter
| Variable | Base Case Value | Range Evaluated | Source |
|---|---|---|---|
| Cohort characteristics | |||
| Mean age, y | 20 | 19–21 | [24] |
| Proportion male | 0.4 | 0.3–0.5 | [24] |
| Baseline prevalence of PWID among 15- to 30-year-olds, % |
1.2 | 0.6–12 | [25, 26] |
| Average age at infection, y | 19 | 15–30 | [27] |
| Monthly probability of recovery from drug use | 0.0139 | 0.0104–0.0174 | [19] |
| Monthly probability of relapse to drug use | 0.032 | 0.024–0.04 | [19] |
| Baseline prevalence of HCV in young PWID, % | 34 | 25–42 | [28, 29] |
| HCV reinfection in young PWID, infections/100 person-years |
15.6 | 1.9–26.7 | [30] |
| Probability of clearing acute infection | 0.26 | 0.19–0.32 | [31, 32] |
| Proportion with genotype 1, % | 55 | 41–69 | [15] |
| Proportion with genotype 2, % | 14 | 11–18 | [15] |
| Proportion with genotype 3, % | 31 | 23–39 | [15] |
| Screening test approach | |||
| Screening test offer rate | |||
| Current practice: | |||
| PWID, % | 5 | 4–6 | [33] |
| Non-PWID, % | 3 | 2–4 | Assumption |
| Expanded risk-targeted approach: | |||
| Physician offer rate among PWID, % | 20 | 15–25 | [8] |
| Counselor offer rate among PWID, % | 44 | 30–50 | Assumption |
| Non-PWID, % | 5 | 4–6 | [8] |
| Routine approach, % | |||
| Physician offer rate | 36 | 25–45 | [10] |
| Counselor offer rate | 80 | 60–99 | [10] |
| Cascade of care variables | |||
| Proportion accepting screening offer, % | 56 | 42–70 | [33–35] |
| Proportion receiving screening test results, % | |||
| HCV standard serum antibody testing | 74 | 56–93 | [36, 37] |
| HCV rapid testing | 99 | 74–99 | [21] |
| Proportion identified with HCV and linked to care, % |
53 | 40–66 | [36, 38, 39] |
| 10-year probability of re-engaging with care after being lost to follow-up, %a |
27 | 20–34 | Assumption; see footnote |
| HCV disease progression | |||
| Median time from infection to cirrhosis, y | 25 | 19–31 | [40] |
| Median time from cirrhosis to first decompensation, y | 11 | 8–13 | [41, 42] |
| Liver-related mortality with cirrhosis, deaths/100 person-years | 3 | 2–3 | [43] |
| HCV therapy efficacy | |||
| Genotype 1 without cirrhosis Probability of SVRb |
0.98 | 0.74–0.99 | [44, 45] |
| Genotype 1 with cirrhosis Probability of SVRb |
0.97 | 0.73–0.99 | [44, 45] |
| Genotype 2 without cirrhosis Probability of SVRb |
0.99 | 0.74–0.99 | |
| Genotype 2 with cirrhosis Probability of SVRb |
0.93 | 0.70–0.99 | [46] |
| Genotype 3 without cirrhosis Probability of SVRb |
0.98 | 0.74–0.99 | [46] |
| Genotype 3 with cirrhosis Probability of SVRb |
0.93 | 0.70–0.99 | [46] |
| Costs | |||
| Select rapid testing program-related costsc | |||
| Counselor time, ( $)/hour | 21 | 15–25 | [47] |
| Rapid HCV antibody test, ($)/test | 13 | 6–20 | [12] |
| Routine medical costs per month without HCV,d ($) | 142–1064 | 71–1596 | [48] |
| Routine medical costs per month with HCV, no cirrhosis,d ($) | 248 | 187–309 | [48, 49] |
| Routine medical costs per month with HCV, mild to moderate cirrhosis,d ($) | 446 | 319–557 | [48, 49] |
| Routine medical costs per month with HCV, decompensated cirrhosis,d ($) | 841 | 628–1064 | [48, 49] |
| HCV therapy costs per month, ($) | |||
| Provider visit costs,e ($) | 122 | 62–184 | [9, 50] |
| Sofosbuvir/Ledipasvir | 30400 | 15200–45600 | [51] |
| Sofosbuvir/Velpatasvir | 30400 | 15200–44600 | Assumptionf [51] |
| Complete course genotype 1 infection (weighted average 8-week and 12-week), ($) No cirrhosis Cirrhosis |
71950g 90190g |
36480–108430 45600–135790 |
Assumption [9, 50, 51] |
| Complete course genotype 2 infection, ($) No cirrhosis Cirrhosis |
137790 137820 |
68910–206730 68910–206730 |
Assumption [9, 50, 51] |
| Complete course genotype 3 infection, ($) No cirrhosis Cirrhosis |
137820 137820 |
68910–206730 68910–206730 |
Assumption [9, 50, 51] |
| Managing treatment ending toxicity, ($) | 240 | 120–370 | [9, 50] |
| Quality of life | |||
| Without HCV infectionh | 0.90 | 0.80–1.0 | [52–54] |
| HCV with cirrhosis | 0.62 | 0.55–0.75 | [55–57] |
| HCV after first decompensation event | 0.48 | 0.40–0.60 | [55–57] |
| On HCV treatmenti | 0.99 | 0.84–0.99 | [58] |
| Major toxicity decrementj | 0.16 | 0.09–0.25 | [59] |
Costs are in 2016 US dollars.
Abbreviations: HCV, hepatitis C Virus; PWID, person who injects drugs; SVR, sustained virologic response.
aTen-year probability of re-engaging with care assumed to be half of original linkage probability.
bProbability of SVR given reaching end of treatment.
cSelect rapid testing program-related costs are presented in Table 2. Additional information is provided in Supplementary Table S1.
dCosts varied as a function of age and sex.
eTreatment visit costs are higher in the first month compared to other months.
fAt the time of this analysis, the cost of velpatasvir was not available, and we made some assumptions on its likely cost.
gWeighted average 8-week and 12-week.
hThe less than 1.0 utility for those living without HCV infection reflects lower quality of life for individuals with HCV risk factors such as substance use.
iThis utility was combined with an individual’s health state utility during the months that a patient was receiving HCV therapy without major toxicity. For example, a patient with HCV and mild to moderate fibrosis who underwent HCV treatment had a utility = 0.801 (0.90 × 0.89) during the months that (s)he was on medications.
jThis utility “toll” was subtracted from a patient’s health state utility during the month of a major toxicity event.
As there was limited information for young adults during the interferon-free era, test acceptance, linkage to care, and treatment initiation were mostly derived from studies performed among PWID during the interferon era. For linkage to care rates, we included a recent study during the interferon-free era [20]. For strategies involving a counselor/tester, we used the offer rate reported in a randomized controlled trial of HIV testing in an emergency department as there was no comparable HCV-specific information [10].
Health-Related Quality of Life and Cost
Health outcomes were expressed in QALYs to incorporate mortality and morbidity associated with a particular health state (Table 2). We derived the direct medical costs of HCV care (clinic visits and monitoring of tests) by estimating healthcare utilization and applying unit costs. Estimates of time and labor costs for counselor/tester-initiated strategies were derived from a clinical trial [12, 21]. Treatment costs were derived from RedBook online. The costs of laboratory tests were from Medicare fee schedules.
RESULTS
Base Case
The average person lived for 79 years and accumulated $122080 in discounted lifetime medical costs (Table 3). As we were evaluating a screening intervention, the benefits were limited to the relatively small population of at-risk individuals (ie, PWID who are HCV infected). Although the population level average effects appeared to be small (0.0002–0.0013 incremental discounted QALYs, $10–80 incremental discounted cost), HCV-infected PWID who initiated therapy were projected to live approximately 2 years longer (50.18 vs 48.09 years) and accrued substantial additional discounted costs ($214000 vs $145000) compared with individuals who did not receive therapy.
Table 3.
Base Case Cost-Effectiveness Results
| Strategy | Undiscounted Life Expectancy (y) | Total Discounted Cost per Person ($) | Remaining QALY per Person (QALY) | Incremental Cost- Effectiveness Ratio ($/QALY) |
|---|---|---|---|---|
| Current practice | 79.0458 | 122080 | 23.7700 | --- |
| Physician-ordered, targeted venipuncture testing | 79.0460 | 122090 | 23.7702 | Dominated |
| Physician-ordered, counselor-performed targeted rapid testing | 79.0461 | 122090 | 23.7702 | 40000 |
| Counselor-initiated, targeted venipuncture testing | 79.0465 | 122100 | 23.7705 | Dominated |
| Physician-ordered, routine venipuncture testing | 79.0463 | 122110 | 23.7704 | Dominated |
| Counselor-initiated, targeted rapid testing | 79.0467 | 122110 | 23.7706 | 44000 |
| Physician ordered, counselor-performed routine rapid testing | 79.0465 | 122110 | 23.7705 | Dominated |
| Counselor-initiated, routine venipuncture testing | 79.0471 | 122140 | 23.7709 | Dominated |
| Counselor-initiated, routine rapid testing | 79.0475 | 122160 | 23.7713 | 71000 |
Costs are rounded to the nearest $10, QALYs to nearest 0.0001, incremental cost-effectiveness ratios to nearest $1000/QALY. Small inconsistencies may be present due to rounding.
Abbreviation: QALY, quality adjusted life-year.
Rapid testing always provided greater life expectancy than venipuncture testing at either a lower lifetime medical cost or a lower cost/QALY gained. Thus, none of the venipuncture-based strategies were an efficient use of resources. Compared to physician-ordered targeted rapid testing, counselor/tester-initiated, targeted rapid testing provided an additional 0.0004 QALY at an incremental cost of $20 for an ICER of $44000/QALY gained. Compared to counselor/tester-initiated targeted rapid testing, counselor/tester-initiated routine rapid testing provided an additional 0.0006 QALY at an incremental cost of $40 for an ICER of $71000/QALY gained.
Compared to current practice, counselor-initiated routine rapid testing identified more cases (20% vs 5%) with a larger proportion of individuals reaching SVR (18% vs. 2%; Figure 2). Counselor-initiated routine rapid testing also reduced the proportion of deaths attributable to HCV (34% vs 31%) when compared to current practice. When comparing routine rapid to targeted testing performed by a counselor, an additional 464 individuals need to be evaluated in the clinic to identify 1 new HCV case. The incremental cost of testing to identify 1 case of HCV by routine testing compared to targeted testing was $8720.
Figure 2.
Outcomes for strategies that were not dominated. The bar graph shows the percentage of hepatitis C virus (HCV)-infected individuals who reached HCV cascade of care outcomes and the percentage of HCV-related deaths. Each bar represents a specific strategy. Abbreviation: HCV, hepatitis C virus.
Sensitivity Analysis
Deterministic Sensitivity Analysis
HCV and Injection Drug Use Prevalence
Both the prevalence of PWID and the prevalence of HCV among PWID influenced cost-effectiveness results, but our overall conclusions did not change within a range of reasonable estimates for either prevalence value. For example, counselor-initiated routine rapid testing was the optimal strategy unless the prevalence of PWID among 15- to 30-year-olds was <0.59% (base case 1.2%; Figure 3) or the HCV prevalence among PWID was <16% (base case 34%).
Figure 3.
Incremental cost-effectiveness ratios (ICERs) of increased percentage of young people who inject drugs in the population. The line graph illustrates the ICER of the counselor-initiated routine rapid hepatitis C virus antibody testing compared to the next best alternative across a range of percentages of young people who inject drugs in the population. Abbreviations: ICER, incremental cost-effectiveness ratio; PWID, person who injects drugs; QALY, quality adjusted life-year; WTP, willingness-to-pay.
Testing Program Characteristics
The cost-effectiveness of routine testing depended on the effectiveness of targeting testing to those at high risk. When we increased the probability that PWID aged 15 to 30 years were correctly identified and offered tests in the targeting strategies, routine testing became less attractive. However, only when targeted testing correctly identified at least 63% of PWID who were aged <30 years (base case 44%) did the ICER of routine rapid testing become >$100000/QALY. With this very effective targeting, counselor-initiated targeted rapid testing was the favored strategy with an associated ICER of $44000/QALY gained.
The cost-effectiveness of rapid testing compared to venipuncture depended on loss to follow-up before receiving rapid test results. When we decreased the proportion of result delivery for rapid screening from 99% (base case) to 75%, routine venipuncture testing initiated by a counselor/tester was the strategy that provided higher QALYs at a lower cost per QALY gained. The associated ICER was $82000/QALY gained.
Rates of Reinfection With HCV After Treatment and Post-Treatment Costs
Compared to the base case of 15 cases per 100 person-years, a reinfection rate of 1.9 cases per 100 person-years yielded an ICER of $34000/QALY gained for routine rapid testing initiated by a counselor/tester. In contrast, a reinfection rate of 26.7 cases per 100 person-years was associated with an ICER of $104000/QALY gained. In addition, our conclusions were unchanged when we assumed that SVR resulted in a more substantial reduction in HCV-attributable cost.
Reflex HCV RNA Testing
We evaluated a scenario in which confirmatory HCV RNA testing was automatically performed after reactive venipuncture testing. With reflex HCV RNA testing, routine testing was still the favorable approach, but venipuncture testing initiated by a counselor/tester provided higher QALYs at a lower cost per QALY gained (Supplementary Table S4).
Probabilistic Sensitivity Analysis
We performed 100000 simulations in which input values for offer rate, test results, test characteristics, treatment cost, treatment efficacy, and quality weights were varied simultaneously. We found that counselor/tester-initiated routine rapid testing was the optimal strategy in 90% of simulations, with counselor/tester-initiated targeted rapid screening preferred in the remaining 10%.
DISCUSSION
We demonstrate that routine HCV testing improved life expectancy and is cost-effective in 15- to 30-year-olds in primary care settings serving communities with a PWID prevalence exceeding 0.59%. This core finding was robust across broad ranges of loss to follow-up along the HCV cascade of care and the risk of HCV reinfection after cure. Our analysis shows that there is value in routinely testing 15- to 30-year-olds seen at health centers in areas with high reported cases of HCV, even with ongoing HCV testing in competing venues such as substance use treatment centers. This finding does not imply that testing at health centers should replace testing in other venues. Previous work demonstrates that testing in sites such as substance use treatment centers is cost-effective [12]. Given the frequency with which PWID access services in such nonmedical settings, these venues play a central role in any national strategy to eliminate HCV. Instead, we demonstrate that routine testing at health centers is an appealing approach to add to the portfolio of strategies for eliminating HCV. Fortunately, in the United States, it is possible to screen in multiple locations, and our findings demonstrate that it is cost-effective to do so. In light of high rates of HCV infection among young adults [22], our findings provide information to inform recommendations for HCV testing among persons aged 15–30 years.
Beyond the conclusion that “screening is cost-effective,” our analysis implies an ordered list of priorities for expanding testing in urban primary care settings. Our findings suggest that rapid testing should be considered instead of venipuncture diagnostics, or centers should ensure reflex RNA testing due to the reduction in loss to follow-up. Reflex RNA testing is not feasible in all settings, as it is associated with some logistical challenges. These include the possible risk of contamination if reflex testing uses the same tube collected for serology and the need to collect 2 tubes of blood upfront but only run RNA testing if the antibody is reactive.
In addition, centers in areas with high HCV case rates among those aged <30 years should consider using a dedicated counselor/tester to provide either targeted testing or routine testing to all. In our analysis, this strategy provides the best outcomes within commonly cited willingness-to-pay thresholds in the United States.
Despite our evidence that routine opt-out HCV testing using a dedicated counselor/tester is cost-effective, budgetary constraints may hinder implementation. Leveraging an existing HIV testing infrastructure with dedicated counselors/testers is a potential solution to reduce those costs and facilitate HCV testing expansion. Given that HIV and HCV share modes of transmission and HIV testing is recommended for all adults [23], this approach would use the existing infrastructure to increase HCV diagnosis.
It is also notable that routine testing remained cost-effective, even when we assumed very high incidence of HCV reinfection after HCV cure. Though perhaps counterintuitive, this finding reflects the reality that HCV therapy is itself cost-effective, with ICERs substantially below $1000000/QALY gained. Thus, as long as retreatment remains possible, screening continues to provide value. Essentially, each course of therapy is highly cost-effective and therefore allows for ratios to remain below the threshold. Although we found that screening remained cost-effective despite high rates of reinfection, preventing reinfection with harm reduction approaches could minimize reinfection rates.
There are limitations to our study. We do not currently have detailed information on current HCV testing rates for youth in primary care settings. We estimated our base case values by using rates of testing at 3 community health centers located in Boston neighborhoods with a high number of reported HCV cases. We then tested our assumptions in sensitivity analyses. Without information on the offer rate for rapid HCV testing compared to standard venipuncture testing, we used available information from HIV rapid testing. One might consider delaying testing until individuals are older to avoid possible reinfection. However, as demonstrated by our sensitivity analyses, conclusions remain robust despite very high rates of reinfection. Moreover, waiting until individuals reach older age would allow for ongoing HCV transmission. In addition, we only considered 1-time testing at age 15–30 years and did not evaluate strategies for repeat testing among those at high risk in this age range. Our analysis focused on strategies to find undiagnosed HCV cases by current guidance. Periodic, or repeat, testing is also important but it is intended to improve outcomes among a different population—individuals who are already known to be at high risk. We also did not include the influence of extrahepatic manifestations independent of fibrosis stage such as vasculitis or lymphomas.
We show that routine testing provides the most clinical benefit and best economic value in an urban community health setting where HCV prevalence is high. Centers should consider either routine rapid testing by a counselor/tester or provide reflex HCV RNA following venipuncture testing. Future studies are needed to define the programmatic effectiveness of HCV treatment among youth and the testing and treatment acceptability in this population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This project was supported by grants from the National Institute on Drug Abuse (R01DA031059, R01DA031059-03S1, P30DA040500, R25DA035163) and the National Institute of Allergy and Infectious Diseases (P30AI042853).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Statistics and surveillance Available at http://www.cdc.gov/hepatitis/statistics/index.htm. Accessed on 30 June 2016.
- 2. AASLD/IDSA/IAS–USA. Recommendations for testing, managing, and treating hepatitis C Available at: http://www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed 24 December.
- 3. The National Academies of Sciences, Engineering, and Medicine Health and Medicine Division. National strategy on the elimination of hepatitis B and C meetings 1–3 Available at: http://nationalacademies.org/hmd/Activities/PublicHealth/NationalStrategyfortheEliminationofHepatitisBandC/2015-DEC-16.aspx. Accessed 1 May.
- 4. World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief Available at: http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf. Accessed 7 September.
- 5. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47(RR-19):1–39. [PubMed] [Google Scholar]
- 6. Moyer VA; U.S. Preventive Services Task Force Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 159:349–57. [DOI] [PubMed] [Google Scholar]
- 7. Van Hande M, Dietz P. Missed opportunities for HIV testing during routine doctor visits, BRFSS, 2011–2013. Conference on Retroviruses and Opportunistic Infections (CROI) Boston, Massachusetts, 2016. [Google Scholar]
- 8. Drainoni ML, Litwin AH, Smith BD et al. Effectiveness of a risk screener in identifying hepatitis C virus in a primary care setting. Am J Public Health 2012; 102:e115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. United States Department of Health and Human Services Center for Medicare Services. Clinical diagnostic laboratory fee schedule Available at: http://www.cms.gov/ClinicalLabFeesched/. Accessed 22 December.
- 10. Walensky RP, Reichmann WM, Arbelaez C et al. Counselor- versus provider-based HIV screening in the emergency department: results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med 2011; 58(1 Suppl 1):S126–32 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linas BP, Barter DM, Leff JA et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 2014; 9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schackman BR, Leff JA, Barter DM et al. Cost-effectiveness of rapid hepatitis C virus (HCV) testing and simultaneous rapid HCV and HIV testing in substance abuse treatment programs. Addiction 2015; 110:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linas BP, Barter DM, Morgan JR et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med 2015; 162:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996; 24:289–93. [DOI] [PubMed] [Google Scholar]
- 15. Dias PT, Hahn JA, Delwart E et al. Temporal changes in HCV genotype distribution in three different high risk populations in San Francisco, California. BMC Infect Dis 2011; 11:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Meer AJ, Veldt BJ, Feld JJ et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–93. [DOI] [PubMed] [Google Scholar]
- 17. Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG.. Cost-Effectiveness in Health and Medicine, 2nd Edition Oxford, UK: Oxford University Press; 2016. [Google Scholar]
- 18. Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ 2013; 91:102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort, 1988–2000: description and determinants. Am J Epidemiol 2003; 158:695–704. [DOI] [PubMed] [Google Scholar]
- 20. Coyle C, Viner K, Hughes E et al. Identification and linkage to care of HCV-infected persons in five health centers—Philadelphia, Pennsylvania, 2012–2014. MMWR Morb Mortal Wkly Rep 2015; 64:459–63. [PMC free article] [PubMed] [Google Scholar]
- 21. Metsch LR, Feaster DJ, Gooden L et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health 2012; 102:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Notes from the field: risk factors for hepatitis C virus infections among young adults—Massachusetts, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1457–8. [PubMed] [Google Scholar]
- 23. Branson BM, Handsfield HH, Lampe MA et al. ; Centers for Disease Control and Prevention Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 24.[HCV testing rates at Boston health centers]. Unpublished raw data.
- 25. Chatterjee S, Tempalski B, Pouget ER, Cooper HL, Cleland CM, Friedman SR. Changes in the prevalence of injection drug use among adolescents and young adults in large U.S. metropolitan areas. AIDS Behav 2011; 15:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tempalski B, Pouget ER, Cleland CM et al. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PloS One 2013; 8: e64789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology 2004; 15:543–9. [DOI] [PubMed] [Google Scholar]
- 28. Hagan H, Campbell J, Thiede H et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep (Washington, DC: 1974). 2006; 121: 710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shafer KP, Hahn JA, Lum PJ, Ochoa K, Graves A, Moss A. Prevalence and correlates of HIV infection among young injection drug users in San Francisco. J Acquir Immune Defic Syndr (1999). 2002; 31:422–31. [DOI] [PubMed] [Google Scholar]
- 30. Sacks-Davis R, Grebely J, Dore GJ et al. ; InC3 Study Group Hepatitis C virus reinfection and spontaneous clearance of reinfection—the InC3 study. J Infect Dis 2015; 212:1407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006; 13:34–41. [DOI] [PubMed] [Google Scholar]
- 32. Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol 2008; 103:1283–97; quiz 98. [DOI] [PubMed] [Google Scholar]
- 33. Cullen BL, Hutchinson SJ, Cameron SO et al. Identifying former injecting drug users infected with hepatitis C: an evaluation of a general practice-based case-finding intervention. J Public Health (Oxf) 2012; 34:14–23. [DOI] [PubMed] [Google Scholar]
- 34. Anderson EM, Mandeville RP, Hutchinson SJ et al. Evaluation of a general practice based hepatitis C virus screening intervention. Scott Med J 2009; 54:3–7. [DOI] [PubMed] [Google Scholar]
- 35. Serfaty MA, Lawrie A, Smith B et al. Risk factors and medical follow-up of drug users tested for hepatitis C—can the risk of transmission be reduced?Drug Alcohol Rev 1997; 16:339–47. [DOI] [PubMed] [Google Scholar]
- 36. Mark KE, Murray PJ, Callahan DB, Gunn RA. Medical care and alcohol use after testing hepatitis C antibody positive at STD clinic and HIV test site screening programs. Public Health Rep (Washington, DC: 1974) 2007; 122:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Page K, Hahn JA, Evans J et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009; 200:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr (1999) 2004; 37:1367–75. [DOI] [PubMed] [Google Scholar]
- 39. Strathdee SA, Latka M, Campbell J et al. ; Study to Reduce Intravenous Exposures Project Factors associated with interest in initiating treatment for hepatitis C virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis 2005; 40(Suppl 5):S304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–32. [DOI] [PubMed] [Google Scholar]
- 41. Pineda JA, Aguilar-Guisado M, Rivero A et al. ; Grupo para el Estudio de las Hepatitis Víricas (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis 2009; 49:1274–82. [DOI] [PubMed] [Google Scholar]
- 42. Sangiovanni A, Prati GM, Fasani P et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology 2006; 43:1303–10. [DOI] [PubMed] [Google Scholar]
- 43. Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol 2010; 8:280–8, 8 e1. [DOI] [PubMed] [Google Scholar]
- 44. Afdhal N, Zeuzem S, Kwo P et al. ; ION-1 Investigators Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 45. Kowdley KV, Gordon SC, Reddy KR et al. ; ION-3 Investigators Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 46. Foster GR, Afdhal N, Roberts SK et al. ; ASTRAL-2 Investigators; ASTRAL-3 Investigators Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 47. National Compensation Survey. United States Department of Labor. [Google Scholar]
- 48. Agency for Healthcare Research and Quality. Total health services mean and median expenses per person with expense and distribution of expenses by source of payment: medical expenditure panel survey household component data Generated interactively. Available at: http://meps.ahrq.gov/mepsweb/. Accessed 15 July.
- 49. Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol 2011; 45:e17–24. [DOI] [PubMed] [Google Scholar]
- 50. Centers for Medicare & Medicaid Services. Medicare physician fee schedule, 2015. [Google Scholar]
- 51. Micromedex 2.0. Drug Topics Red Book Online Available at: http://www.micromedexsolutions.com. Accessed January 13.
- 52. Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction 2001; 96:1267–78. [DOI] [PubMed] [Google Scholar]
- 53. Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med 2010; 153:778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vickerman P, Platt L, Hawkes S. Modelling the transmission of HIV and HCV among injecting drug users in Rawalpindi, a low HCV prevalence setting in Pakistan. Sex Transm Infect 2009; 85(Suppl 2): ii23–30. [DOI] [PubMed] [Google Scholar]
- 55. Chong CA, Gulamhussein A, Heathcote EJ et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003; 98:630–8. [DOI] [PubMed] [Google Scholar]
- 56. Grieve R, Roberts J, Wright M et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006; 55:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stein K, Dalziel K, Walker A et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess 2002; 6:1–122. [PubMed] [Google Scholar]
- 58. Stepanova M, Nader F, Cure S, Bourhis F, Hunt S, Younossi ZM. Patients’ preferences and health utility assessment with SF-6D and EQ-5D in patients with chronic hepatitis C treated with sofosbuvir regimens. Aliment Pharmacol Ther 2014; 40:676–85. [DOI] [PubMed] [Google Scholar]
- 59. Schackman BR, Teixeira PA, Weitzman G, Mushlin AI, Jacobson IM. Quality-of-life tradeoffs for hepatitis C treatment: do patients and providers agree?Med Decis Making 2008; 28:233–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.