Abstract
We compared xenodiagnosis with quantitative polymerase chain reaction in skin biopsies from 3 patients with maculopapular or nodular post–kala-azar dermal leishmaniasis (PKDL). All patients infected sand flies. Parasite loads in skin varied from 1428 to 63 058 parasites per microgram. PKDL detection and treatment are important missing components of the kala-azar elimination program.
Keywords: leishmaniasis, post–kala-azar dermal leishmaniasis, control, xenodiagnosis
In 2005, Bangladesh, India, and Nepal launched an ambitious initiative to eliminate visceral leishmaniasis (VL or kala-azar) as a public health problem, setting a target incidence of <1 per 10 000 population at risk by 2020 [1]. Since 2005, estimated VL incidence has fallen by 79% and the target incidence has been achieved for 3 consecutive years in all endemic districts of Nepal, 96% of subdistricts of Bangladesh, and 72% of blocks in India. The strategy to decrease transmission relies on early VL diagnosis and treatment and vector control. However, the intiative included no measures to address post–kala-azar dermal leishmaniasis (PKDL), a skin condition usually affecting individuals after kala-azar treatment [2]. In the Indian subcontinent, an estimated 5%–15% of kala-azar patients develop PKDL 1–5 years after treatment [2, 3].
The primary importance of PKDL derives from its role as an infection reservoir [4]. PKDL patients are not systemically ill, and may remain untreated for years. Treatment requires long courses of sodium stibogluconate (SSG) or, more recently, miltefosine [2]. Although many investigators hypothesize that SSG treatment increases risk, PKDL has occurred after liposomal amphotericin, paromomycin, and miltefosine treatment, and also occurs in individuals with no prior VL treatment [3]. With kala-azar incidence close to the elimination target, there is increased urgency to better define PKDL infectivity and quantify its role in maintaining transmission.
Since 1928, 3 studies in India examined infectivity by feeding uninfected sand flies on PKDL patients and measuring infection rates in fed flies (xenodiagnosis) [4–6]. The paucity of studies is due to the difficulty of maintaining stable sand fly colonies and the impracticality of conducting xenodiagnosis on large numbers of patients. Ideally, xenodiagnosis could be replaced by a surrogate marker, for example, molecular quantification of parasites in different PKDL forms at various stages of evolution. This proof-of-concept study included 3 PKDL patients, and aimed to provide a preliminary comparison of direct xenodiagnosis with quantitative polymerase chain reaction (qPCR) in skin biopsies and peripheral blood.
MATERIALS AND METHODS
The protocol was approved by the ethical review committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) (number PR-14010). Patients provided written informed consent. Procedures were conducted at the Surja Kanta Kala-azar Research Centre (SK KRC), Mymensingh Medical College, under the supervision of 2 physicians. No adverse events occurred. Patients were referred for treatment following national protocols.
Procedures
PKDL patients aged ≥18 years were identified through active community searches. Eligibility required confirmation by microscopy or polymerase chain reaction (PCR). Exclusion criteria included any other illness or history of allergy to insect bites. Lesions were classified as macules, papules, or nodules, and the affected area was quantified using published methods [7]. Blood was collected and separated into serum, buffy coat, and red cells. Sera were tested by rK39 rapid test (InBios, Seattle Washington) and direct agglutination test. Following antisepsis, a 2 × 2-mm skin snip was collected by scalpel from an area with lesions. One-half was used for molecular assays; the other was used to prepare an impression smear, stained with Giemsa and examined by light microscopy. Quantification followed practices for cutaneous leishmaniasis impression smears; 1+ corresponded to 1 parasite in 100 fields.
Sand Fly Colony
A colony was established at SK KRC starting with wild blood-fed Phlebotomus argentipes females. Twenty randomly chosen first- and second-generation females were analyzed by reverse-transcription PCR (RT-PCR) to rule out flavivirus and phlebovirus infection; all were negative. Sand flies used in xenodiagnosis belonged to these generations.
Xenodiagnosis
Each participant placed his hand into a cage containing 7-day-old sand flies (Figure 1E) [8]. Flies were also fed on lesions in a 3-cm diameter tube topped with gauze. Engorged flies were kept at 26°C and 85%–95% humidity, and (Figure 1B) fed exclusively on 30% sucrose. Dissection was planned for 72 hours after blood feeding. Guts were individually examined by microscopy, and when negative, were processed by qPCR.
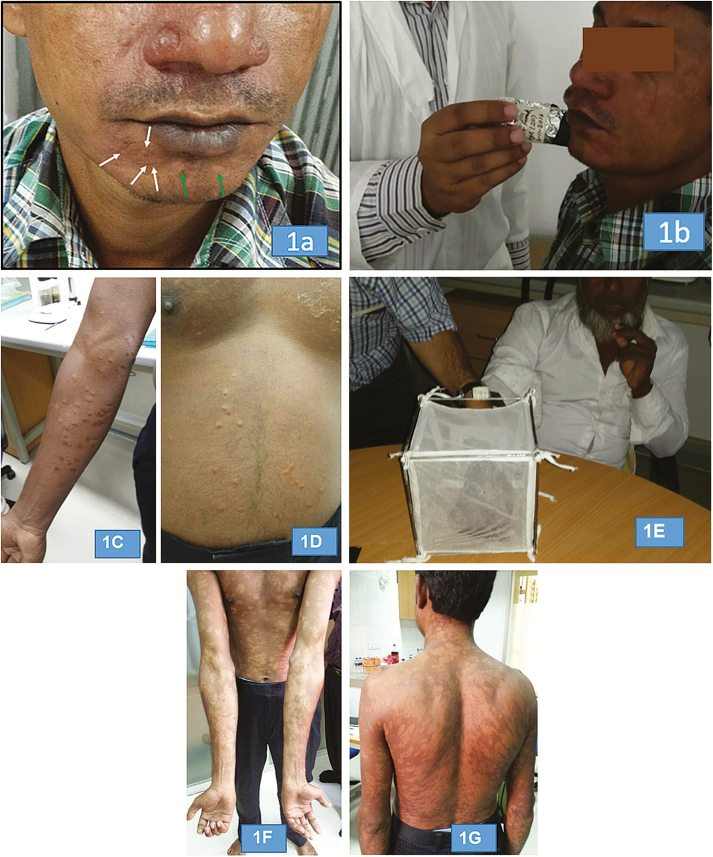
Figure 1.
Post–kala-azar dermal leishmaniasis patients included in the xenodiagnosis experiments. A, Patient 1: macular (green arrows) and nodular (white arrows) lesions. B, Direct xenodiagnosis using a small tube. C and D, Patient 2: nodular lesions on forearm and abdomen. E, Direct xenodiagnosis placing hand in cage with sand flies. F and G, Patient 3: extensive macular lesions on forearms, abdomen, and back.
Quantitative PCR
Tissue, buffy coat, and sand fly midgut specimens were processed using Qiagen kits. Real-time PCR used R223/ R333 primers (Sigma-Aldrich) for small-subunit ribosomal RNA and LightCycler FastStart DNA Master SYBR Green I (Roche Applied Science, Switzerland) [9]. Cycle thresholds were extrapolated in a standard curve to estimate parasite load.
RESULTS
PKDL manifestations ranged from mild perioral macules (patient 1) to localized nodules (patient 2) to extensive chronic maculopapular disease (patient 3) (Table 1 and Figure 1). All 3 patients had positive serology, microscopy, and qPCR in skin, but buffy coat specimens were negative by qPCR. The proportion of engorged flies per experiment ranged from 14% to 58%. Patient 1 was a smoker with limited perioral macules; despite washing to remove traces of tobacco, only 3 of 18 flies fed (17%). Fly dissection was conducted at 60 hours after feeding, 12 hours earlier than planned, reducing the probability of detecting promastigotes. In all 3 cases, at least 1 sand fly developed detectable infection. In patients 2 and 3, 50% of engorged flies in the cage experiments were positive by PCR.
Table 1.
Patient Characteristics, Laboratory Parameters, and Xenodiagnosis Results From 3 Patients With Post–Kala-azar Dermal Leishmaniasis, Bangladesh
| Characteristic or Finding | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Sex | Male | Male | Male |
| Age, y | 24 | 40 | 35 |
| Profession | Tailor | Business | Construction |
| History of VL | No | Yes | Yes |
| VL treatment drug | NA | SSG | SSG |
| Time since VL treatment, mo | NA | 180 | 120 |
| Previous PKDL treatment | Yes | No | Yes |
| PKDL treatment drug | AmBisome | NA | SSG |
| Date of PKDL treatment | April 2014 | NA | July 2006 |
| Duration of current lesions, mo | 7 | 174 | 108 |
| Rash type | |||
| Macular | Minor | No | Extensive |
| Papular | Minor | No | No |
| Nodular | No | Abundant | No |
| Score for extent of rash | 2 | 62 | 558 |
| DAT titer | 12 800 | 12 800 | 12 800 |
| rK39 | Positive | Positive | Positive |
| Buffy coat qPCR | Negative | Negative | Negative |
| Skin biopsy results | |||
| Microscopy | Positive 1+ | Positive 1+ | Positive 1+ |
| Cycle thresholda | 25 | 30 | 22 |
| Parasites per micrograma | 21 621 | 1428 | 63 058 |
| Xenodiagnosis results, no./No. (%) | |||
| Hand in cage of sand flies | |||
| Fed/exposed flies | 2/11 (18.2) | 4/15 (26.7) | 6/13 (46.2) |
| Microscopy positive/dissected flies | 0/2 (0) | 0/4 (0) | 0/4 (0) |
| PCR positive/processed flies | 0/2 (0) | 2/4 (50) | 3/6 (50) |
| Tube of sand flies applied to skin | |||
| Fed/exposed flies | 1/7 (14.3) | 7/12 (58.3) | 2/9 (22.2) |
| Microscopy positive/dissected flies | 1/1 (100) | 0/5 (0) | 0/0 (0) |
| PCR positive/processed flies | Not done | 1/7 (14.2) | 0/2 (0) |
| Positive by any method | 1/5 (20) | 3/20 (15) | 3/12 (25) |
Abbreviations: DAT, direct agglutination test; NA, not applicable; qPCR, quantitative polymerase chain reaction; PKDL, post–kala-azar dermal leishmaniasis; SSG, sodium stibogluconate; VL, visceral leishmaniasis.
aMean of 2 assays by qPCR.
DISCUSSION
Researchers have long observed VL epidemic cycles lasting approximately 10 years and recurring every 10–20 years; these patterns are hypothesized to result from increasing herd immunity followed by a nadir period in which new susceptibles join the population through birth and in-migration [3]. The peak in kala-azar incidence (most recently 2005–2007) may be followed by a smaller peak in PKDL [3]. Previous cycles peaked in the late 1970s and early 1990s. An investigation in West Bengal in 1992 suggested that PKDL patients comprised the interepidemic reservoir and introduced transmission into new areas [4]. Assuming treatment access, kala-azar cases can be tracked using facility-based data, but PKDL cases often remain undetected [3, 10]. To quantify the contribution of PKDL to transmission and model control measures needed to consolidate elimination, data on distribution of cases in the community, including clinical characteristics, duration, and care-seeking behavior, must be combined with infectivity data. This study represents a first step toward that goal.
The 1933 xenodiagnosis study used wild flies and prolonged survival by feeding them with mouse blood after the human blood meal [5]. Although only 1 of 38 flies showed promastigotes after a single feed, the proportions rose to 2 of 10 and 13 of 45 flies after 2 and 3–4 blood meals, respectively [5]. The 1992 study, like ours, used laboratory-reared flies [4]; advantages include standardization and ability to ensure that the colony is free of pathogens. However, laboratory-reared P. argentipes have high mortality within a few days after human blood feeding and their survival cannot be prolonged with extra blood meals (personal observation, R. Molina). In the 1992 study [4], the feeding rate was 26%, and 30% of fed flies died within 48 hours of blood feeding, proportions similar to those in our experiments. Therefore, our results provide a minimum estimate of infectivity. The use of cage feeding and qPCR appear to yield the best results, at least for patients with extensive lesions.
We found few amastigotes by microscopy, even in patients with high parasite loads by qPCR, but all 3 patients were infective to sand flies and had positive qPCR in skin. Previous molecular studies demonstrated parasite DNA in all lesion types, with higher parasite loads in nodular than maculopapular PKDL [11]. As in our data, loads were highest in the most longstanding cases. PKDL may play an important role due both to high infectivity and the prolonged periods such patients are available to infect flies. Buffy coat qPCR results were negative, demonstrating that skin parasite loads are the crucial issue.
The data in this proof-of-concept study are limited. However, our data raise doubts regarding the conventional belief that macular and papular forms are significantly less infective than nodular PKDL; for now we should assume that all PKDL patients can be infective. Data from a much larger number of patients with varying lesion types, extent, and chronicity are needed to characterize the distribution of infectivity and model transmission in populations. Our findings support the current norm of treatment of all PKDL patients in the Indian subcontinent [12], and raise questions about the practice of withholding treatment for mild PKDL in Sudan [12]. Active PKDL case finding will be necessary to enable to early detection, and a short, safe, efficacious treatment regimen would greatly facilitate universal treatment of PKDL.
Xenodiagnosis is the gold standard for infectivity but the technique is complex, requiring specialized expertise and generation of large numbers of flies, and has potential biases. One colony can be a better transmitter than another of the same species. Flies are starved to force blood meals and have high mortality after feeding. Some patients are more attractive than others; tobacco residue, as in patient 1, may repel the flies. Having more than 1 colony per region will contribute to more robust knowledge. Three groups are now conducting xenodiagnosis in the Indian subcontinent, with planned studies of >100 PKDL patients over the next 24 months. Of equal importance is the validation of surrogate measures of infectivity, such as qPCR, to enable testing of many more people than xenodiagnosis, and testing of other potential infection reservoirs, such as asymptomatic leishmanial infection. Exchange of information between groups will be crucial to allow more accurate modeling of transmission and better predict the future of the elimination program.
Notes
Acknowledgments. We gratefully acknowledge Drs Maribel Jiménez and Vishal Goyal for technical support, and Mymensingh Medical College Hospital and the Government of Bangladesh for providing infrastructure and clinical support for the study.
Disclaimer. The funding sources had no role in the study design, collection, analysis and interpretation of the data, preparation of the manuscript, or the decision to submit for publication.
Financial support. This work was supported by the World Health Organization Special Programme for Research and Training in Tropical Diseases, Switzerland; Spanish Foundation for lnternational Cooperation, Health and Social Affairs; and icddr,b core donors.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Regional strategic framework for elimination of Kala-azar from the South-East Asia region (2005–2015). New Delhi, India: WHO, 2005. [Google Scholar]
- 2. Mondal D, Khan MG. Recent advances in post-kala-azar dermal leishmaniasis. Curr Opin Infect Dis 2011; 24:418–22. [DOI] [PubMed] [Google Scholar]
- 3. Islam S, Kenah E, Bhuiyan MA, et al. Clinical and immunological aspects of post-kala-azar dermal leishmaniasis in Bangladesh. Am J Trop Med Hyg 2013; 89:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Addy M, Nandy A. Ten years of kala-azar in west Bengal, Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ 1992; 70:341–6. [PMC free article] [PubMed] [Google Scholar]
- 5. Napier L, Smith R, Das-Gupta C, Mukerji S. The infection of Phlebotomus argentipes from dermal leishmanial lesions. Indian J Med Res 1933; 21:173–7. [Google Scholar]
- 6. Shortt H, Swaminath CS. Note on dermal leishmanoid. Indian J Med Res 1928; 16:239–40. [Google Scholar]
- 7. Mondal D, Hasnain MG, Hossain MS, et al. Study on the safety and efficacy of miltefosine for the treatment of children and adolescents with post-kala-azar dermal leishmaniasis in Bangladesh, and an association of serum vitamin E and exposure to arsenic with post-kala-azar dermal leishmaniasis: an open clinical trial and case-control study protocol. BMJ Open 2016; 6:e010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molina R, Lohse JM, Pulido F, Laguna F, López-Vélez R, Alvar J. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. Am J Trop Med Hyg 1999; 60:51–3. [DOI] [PubMed] [Google Scholar]
- 9. Cunha J, Carrillo E, Sánchez C, Cruz I, Moreno J, Cordeiro-da-Silva A. Characterization of the biology and infectivity of Leishmania infantum viscerotropic and dermotropic strains isolated from HIV+ and HIV- patients in the murine model of visceral leishmaniasis. Parasit Vectors 2013; 6:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mondal D, Nasrin KN, Huda MM, et al. Enhanced case detection and improved diagnosis of PKDL in a kala-azar-endemic area of Bangladesh. PLoS Negl Trop Dis 2010; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma S, Kumar R, Katara GK, et al. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One 2010; 5:e10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis 2003; 3:87–98. [DOI] [PubMed] [Google Scholar]