Summary
Significantly higher plasma levels of IL6, IL1β, and sIL1Rα were associated with increased risk, whereas plasma IFNβ levels were associated with a decreased risk of TB recurrence in TB Recurrence upon Treatment with HAART (TRuTH) cohort, based in Durban, South Africa.
Keywords: Tuberculosis, HIV, ART, cytokine, chemokine
Abstract
Background
Immune correlates of tuberculosis (TB) risk in populations infected with human immunodeficiency virus (HIV) remain understudied, despite HIV being associated with a high burden of TB disease. Here we describe plasma cytokine correlates of TB recurrence in a well-characterized cohort of HIV-infected individuals on antiretroviral therapy (ART) with a history of prior TB cure.
Methods
Study participants were drawn from a prospective cohort study initiated at the conclusion of a randomized clinical trial in which individuals presented with untreated HIV infection and active pulmonary TB. At baseline, ART was initiated, and TB successfully cured. Participants were screened for TB recurrence quarterly for up to 4 years. TB recurrent cases (n = 63) were matched to controls (n = 123) on sex, study arm assignment in the original trial, and month of enrollment with a subset of cases sampled longitudinally at several time-points.
Results
Three cytokines were associated with increased rates of TB recurrence in univariate models: interleukin 6 (IL6) (odds ratio [OR] 2.66, 95% confidence interval [CI] 1.34–5.28, P = .005), IP10 (OR 4.62, 95% CI 1.69–12.65, P = .003), monokine induced by IFN-γ (MIG) (OR 3.11, 95% CI 1.10–8.82, P = .034). Conversely, interferon β (IFNβ) was associated with decreased TB risk (OR 0.34, 95% CI 0.13–0.87, P = .025). Following multivariate analyses adjusting for covariates IL6, interleukin 1β (IL1β), and interleukin 1Rα (IL1Rα) were associated with increased risk and IFNβ with decreased TB risk. Longitudinal analysis showed that levels of many TB-associated markers, including IL6, IP10, sCD14, and interferon γ (IFNγ) are reduced following TB treatment.
Conclusion
These data show that TB recurrence, in HIV-infected individuals on ART is predicted by biomarkers of systemic inflammation, many of which are implicated in more rapid HIV disease progression.
Clinical Trials Registration
South Africa has the highest burdens of both human immunodeficiency virus (HIV) and tuberculosis (TB) infections globally; these infections are frequently concomitant, suggesting the need for joint solutions to improve the health in this population. An estimated 1.2 million of the 9.6 million people that developed TB worldwide were HIV infected, with Africa accounting for 74% of coinfection cases. Given the scale of the HIV-associated TB epidemic, more effective TB prevention and control strategies, especially in those with HIV-related immune deficiency, are urgently needed.
The interaction between Mycobacterium tuberculosis (MTB) and the host evokes innate and adaptive immune responses during both quiescent and active replicating phases of the pathogen [1]. Although the immune mechanisms that drive the transition of TB from quiescence to active disease remain unclear, 2 of the main known risk factors for activation of latent TB infection (LTBI) are HIV infection and treatment with tumor necrosis factor (TNF) inhibitors [2–4]. Natural history studies suggest that most people infected by MTB do not develop TB disease, with an estimated 10% lifetime odds of reactivation. Risk increases dramatically to 10% annually in the context of HIV infection and is more pronounced as HIV disease progresses, with CD4 T-cell count being inversely associated with the incidence of TB disease [5]. Treatment with antiretroviral therapy (ART) and the associated immunological recovery are associated with a decreased TB incidence; however, despite ART treatment, the overall TB incidence rate for HIV-infected individuals remains 10-fold higher compared to individuals without HIV [6]. Although it has been established that the host immune system is critical to both containment and cure of TB infection, a greater understanding of the balance between protective and detrimental immune responses to MTB is needed, especially in the context of HIV coinfection.
Both TB and HIV infections have been associated with changes in the expression of various soluble plasma proteins, which either directly or indirectly contribute to the morbidity and/or mortality associated with these diseases. Several markers of systemic immune activation, including interleukin 6 (IL6), D-dimer, sCD14, and sCD163, have been associated with progression of HIV disease and an increased risk of HIV-related all-cause mortality while on HIV treatment [7–9]. Although immune activation and inflammation are elevated during untreated HIV infection, presumably due to increased levels of viral replication, most innate and adaptive immune responses remain dysfunctional despite effective ART and HIV viral suppression. Recent papers suggest this could be due to viral replication in distal tissues during ART [10, 11]. Independently of HIV infection, active TB infection has been associated with elevated levels of immune activation and inflammation, including elevated levels of tumor necrosis factor α (TNFα) and interleukin 17 (IL17) [12], interleukin 18 (IL18) [13, 14] and sCD14 [15]. As with HIV, inflammatory responses act as a double-edged sword in TB infection; although beneficial for containment of MTB and HIV in early infection, inflammation contributes to immune mediated pathology if left unregulated. Immune activation and systemic inflammation during HIV/TB coinfection have not been fully characterized; however, elevated T-cell activation was observed in HIV-infected individuals with both latent and active TB, whereas elevated levels of plasma sCD14, CRP, IL6, and IP10 were observed only in patients with active TB [16].
There is a continuing need for prospective studies of HIV-infected TB patients in order to better characterize the immune factors that predict the risk of TB recurrence in coinfected patients. Despite a large number of studies focusing on the relationship between markers of immune activation in HIV and TB only a few have assessed the impact of MTB infection and treatment completion on immune activation and microbial translocation in HIV-infected individuals on ART [16, 17]. Here we hypothesized that markers of inflammation would be predictive of active TB disease. We sought to characterize plasma cytokine/chemokine correlates of TB recurrence among HIV-infected patients on ART who had their previous episode of TB treated successfully.
METHODS
Study Group
Participants were enrolled from the CAPRISA 005 TB Recurrence upon Treatment with HAART (TRuTH) cohort, a prospective study, based at the CAPRISA eThekwini clinic in Durban, KwaZulu-Natal, South Africa. All TRuTH participants were previously enrolled in a CAPRISA 003 SAPiT trial investigating the timing of ART initiation during treatment for pulmonary TB [18, 19], and entry into TRuTH included a confirmed TB-negative status at the conclusion of TB treatment. The TRuTH study maintained participants on ART while screening quarterly for a maximum of 4 years for TB recurrence, defined as the first microbiologic confirmation of MTB by TB smear or culture. We conducted a nested case-control study with cases defined by TB recurrence with an available pre-recurrence sample matched 1:2 to individuals with no evidence of TB recurrence during the follow-up period. Written informed consent was obtained from all study participants prior to enrolment and the University of KwaZulu-Natal Biomedical Research Ethics Committee approved the study (ref: BF 051/09; Clinicaltrials.gov number NCT 01539005).
Case-control matching was carried out on the basis of study arm in the SAPiT trial, month of enrollment in that study, and sex. This ensured that cases and controls were on ART for a similar duration at sample time-point. Cases were sampled at a minimum of 3 and maximum of 9 months prior to TB recurrence, and controls at comparable time-points, ensuring no difference in length of sample cryopreservation between groups. A subset of 16 cases was followed longitudinally at following time points: Baseline (at the time of initial TB diagnosis in the SAPiT trial); Cure1 (following successful treatment of the first TB episode occurring 6 months post- SAPiT baseline); PreTB2 (a time point 18 to 6 months before TB recurrence); Recurrence/TB2 (2-month window before or after TB recurrence); Cure2 (capturing a 2-month window before and 3-month window after recurrent TB treatment completion date).
Sample Collection and Processing
Peripheral blood was collected in acid citrate dextrose (ACD) tubes. Following centrifugation plasma was collected and cryopreserved at −80°C for further analysis.
Cytokine/Chemokine Measurement
Cytokine/chemokine levels were measured using the Bio-RAD multiplex assays (Human Inflammation Panel and Human Cytokine/Chemokine Panel I and Panel III) and analyzed on a BioPlex-200 (Bio-Rad, Mississauga, ON, Canada). The Human Inflammation Panel includes following cytokines: IFNα2, IFNβ, IFNγ, IL8, IL12 (p40), IL12 (p70), IL27 (p28), IL28A/IFNλ2, IL29/IFNλ1. The Human Cytokine/Chemokine panel I included: (IL1ß, IL1Rα, IL2, IL6, IL7, IL10, IL15, IP10, TNFα). The Human Cytokine/Chemokine Panel III included: IL1α, IL18, and monokine induced by IFN-γ (MIG). Soluble CD14 (sCD14) levels were measured using the Human CD14 Quantikine ELISA Kit (R&D Systems Inc., Minneapolis, MN). I-FABP levels were measured using the Human FABP2/I-FABP DuoSet ELISA (R&D Systems Inc., Minneapolis, MN). All assays were performed following manufacturer’s instructions. Samples with values outside the range of the standard curve were assigned the value half the limit of detection in pg/mL, LOD/2.
Statistical Analysis
Wilcoxon signed ranks test, McNemar test, and Bowker test of symmetry were used to compare baseline characteristics of cases and controls. Data were modeled using univariate and multivariate conditional logistic regression to account for matching. P-values are reported and discussed as observed, and no corrections for the multiple comparisons were made [20].
All specimens from matched cases and controls were analyzed blinded to clinical status with longitudinal specimens from the same individual and strata ran on the same multiplex plate. In the primary comparison, TB recurrence status was used as the dependent variable, and each cytokine modeled individually as an independent variable. All biomarkers with more than 60% of samples above the limit of detection were analyzed as continuous variables and log-transformed to adjust for skewness (IL12P40, IFNβ, IFNγ, IFNα2, IL6, IL1α, IL10, IL1β, TNFα, IL7, IL1Rα, MIG, IP10, IL18, sCD14); those below 60% detectability were analyzed binary variables (IL15, IFNλ1, IL2, IL27, IL8, IFNλ2, IL12P70, I-FAB). Multivariate analyses adjusted for a wide range of clinical and demographic covariates at the sample time point, including: age, body mass index, CD4 count, and viral load (VL). In addition, baseline covariates included lung cavities (both vs. no and one vs. no), previous history of TB (yes vs. no), history of IRIS, World Health Organization (WHO) stage (4 vs. 3). Longitudinal analyses were performed using paired t-tests. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Graphs were made using the GraphPad Prism (V 5, La Jolla, CA).
RESULTS
Study Participants Characteristic
The original study design included 63 and 126 controls; however, the main analysis included 63 cases and 123 controls, with 3 controls missing due to sample unavailability. The median age was 32 (interquartile range [IQR] 29–38) years for cases and 35 (IQR 28–41) years for controls (P = .719). The median CD4 counts were 145 cells/mm3 (IQR 57–234) for cases and a median of 151 cells/mm3 (IQR 77–247) for controls (P = .159). Despite all participants being maintained on ART and a high rate of treatment success, viral loads were detectable in 40% of cases and 22.5% of controls. Only 3 cases and 1 control had a new AIDS-defining illness within 3 months of sample time point. Additional clinical and demographic cohort characteristics are listed in Table 1.
Table 1.
Clinical and Demographic Cohort Characteristics
| Variable | TB recurrence (N = 65) | No TB recurrence (N = 130) | P-Value |
|---|---|---|---|
| SAPiT enrollment | |||
| Randomization arm, n (%) | |||
| Early integrated arm | 17 (26.1) | 34 (26.1) | |
| Late integrated arm | 25 (38.5) | 50 (38.5) | |
| Sequential arm | 23 (35.4) | 46 (35.4) | |
| Age (y), median (IQR) | 32 (29–38) | 35 (28–41) | .719 |
| Body mass index (kg/m2), median (IQR) | 22.35 (20.2–24.7) | 22 (19.7–24.7) | .235 |
| CD4 count (cells/mm3), median (IQR) | 145 (57–234) | 151 (77–247) | .159 |
| Viral load (log copies/mL), mean (SD)a | 5.1 (0.8) | 5.0 (0.8) | .201 |
| Sex, n (%) | |||
| Male | 30 (46.2) | 60 (46.2) | - |
| Female | 35 (53.8) | 70 (53.8) | |
| WHO stage, n (%)b | |||
| 3 | 59 (93.7) | 116 (96.7) | .180 |
| 4 | 4 (6.3) | 4 (3.3) | |
| History of TB, n (%) | |||
| Yes | 28 (43.1) | 39 (30.0) | .024 |
| No | 37 (56.9) | 91 (70.0) | |
| Lung cavities, n (%)c | |||
| Both | 8 (13.3) | 11 (9.4) | .635 |
| No | 33 (55.0) | 70 (59.8) | |
| One | 19 (31.7) | 36 (30.8) | |
| SAPiT ART initiation | |||
| CD4 count (cells/mm3), median (IQR) | 119 (51–249) | 172 (88–264) | .159 |
| Viral load (log copies/ml), mean (SD)d | 5.0 (0.8) | 4.9 (0.9) | .272 |
| End of TB treatment in SAPiT | |||
| CD4 count (cells/mm3), median (IQR) | 212.5 (101.5–445.5) | 233.5 (127.5–353.5) | .634 |
| Viral load, n (%)e | |||
| Detectable | 22 (37.9) | 53 (44.9) | .096 |
| Undetectable | 36 (62.1) | 65 (55.1) | |
| Variables at sample collection | |||
| Age (y), median (IQR) | 34 (31–40) | 37 (31–43) | .789 |
| CD4 count (cells/mm3), median (IQR) | 344 (127–551) | 402 (257–547) | .011 |
| Viral load, n (%)f | |||
| Detectable | 26 (40.0) | 29 (22.5) | .03 |
| Undetectable | 39 (60.0) | 100 (77.5) | |
| History of IRIS, n (%) | |||
| IRIS | 12 (18.5) | 17 (13.1) | .281 |
| No IRIS | 53 (81.5) | 113 (86.9) | |
Missing data: a8, b12, c18, d2, e19, f1.
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; IRIS, immune reconstitution inflammatory syndrome; SD, standard deviation; TB, tuberculosis; WHO, World Health Organization.
Plasma Cytokine/Chemokine Predictors of Tuberculosis Recurrence
To define the plasma cytokine/chemokine expression patterns that correlate with TB recurrence, we examined the expression of 21 cytokines/chemokines known to be important in immune activation and IFN signaling. Because immune activation in HIV is believed to be driven by microbial translocation, we further studied an intestinal fatty-acid binding protein (I-FABP/FABP2), a marker of gut damage, and soluble sCD14 (sCD14), a marker of lipopolysaccharide (LPS) bioactivity and monocyte activation. Univariate conditional logistic regression analysis revealed that 3 cytokines were associated with increased rates of TB recurrence, including IL6 (OR 2.66, 95% CI 1.34–5.27, P = .005), IP10 (OR 4.62, 95% CI 1.69–12.65, P = .003), MIG (OR 3.11, 95% CI 1.10–8.82, P = .034), whereas IFNβ was associated with decreased risk (OR 0.34, 95% CI 0.13–0.87, P = .025) of TB recurrence (Figure 1. and Table 2). IL1β (OR 1.89, 95% CI 0.97–3.68, P = .064) and IL1Rα (OR 1.52, 95% CI 0.95–2.45, P = .084) showed statistical trends toward increased rates of TB recurrence (Figure 1 and Table 2). Multivariate analysis, correcting for age, body mass index (BMI), CD4 count, VL, lung cavities, previous TB, history of IRIS, WHO stage confirmed the results for IL6 (adjusted odds ratio [aOR] 4.79, 95% CI 1.66–13.81, P = .004, Table 2). IL1β and IL1Rα that showed a trend toward significance in the univariate model were significant in the multivariate model (IL1β: aOR 3.41, 95% CI 1.26–9.24, P = .016; IL1Rα: aOR 2.04, 95% CI 1.04–3.98, P = .038). The interferon gamma-induced chemokines IP10 and MIG were no longer significantly associated with TB outcome following adjustments for covariates (IP10: aOR 3.74, 95% CI .90–15.53, P = .069; MIG: aOR 2.92, 95% CI .81–10.57, P = .103), likely due to the fact that they are influenced by HIV viral load, as described previously (Table 2) [21]. IFNβ (aOR 0.27, 95% CI .07–.99, P = .049) remained significantly associated with decreased risk of TB recurrence.
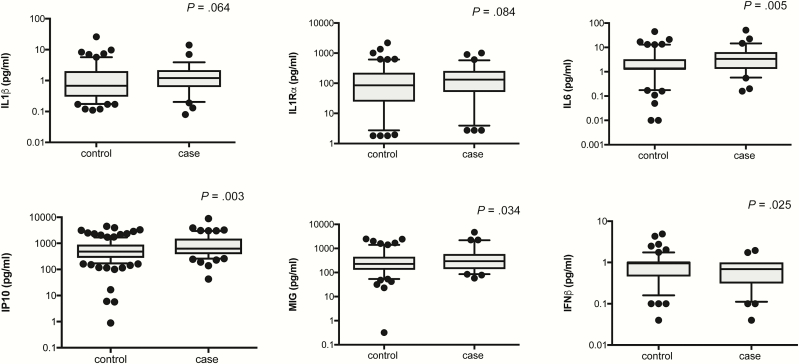
Figure 1.
Cytokines/chemokines differentially expressed between controls (n = 123) and cases (n = 63) by univariate conditional logistic regression. Five cytokines were associated with increased rates of TB recurrence, including IL6 (OR 2.66, 95% CI 1.34–5.27, P = .005), IP10 (OR 4.62, 95% CI 1.69–12.65, P = .003), MIG (OR 3.11, 95% CI 1.10–8.82, P = .034) and IL1β (OR 1.89, 95% CI 0.97–3.68, P = .064) and IL1Rα (OR 1.52, 95% CI 0.95–2.45, P = .084, whereas IFNβ was associated with decreased risk (OR 0.34, 95% CI 0.13–0.87, P = .025). Cytokines were plotted on log scale (Log 10), Box and Whiskers (5–95%). P-values indicated in the figures are result of a univariate conditional logistic regression (Table 1). Abbreviations: CI, confidence interval; IL, interleukin; IFN, interferon; IP, interferon gamma induced protein; MIG, monokine induced by IFN-γ; OR, odds ratio; TB, tuberculosis.
Table 2.
Influence of Different Cytokines on Tuberculosis Recurrence
| Cytokine | Univariate | Multivariateb | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| IL1β | 1.89 (0.97–3.68) | .064 | 3.41 (1.26–9.24) | .016 |
| IL1Rα | 1.52 (0.95–2.45) | .084 | 2.04 (1.04–3.98) | .038 |
| IL6 | 2.66 (1.34–5.27) | .005 | 4.79 (1.66–13.81) | .004 |
| IL7 | 1.27 (0.53–3.03) | .591 | 1.73 (0.48–6.31) | .403 |
| IL10 | 1.08 (0.58–2.01) | .814 | 1.61 (0.63–4.12) | .317 |
| IP10 | 4.62 (1.69–12.65) | .003 | 3.74 (0.90–15.53) | .069 |
| TNFα | 0.98 (0.57–1.67) | .934 | 1.04 (0.48–2.27) | .916 |
| IL1α | 0.95 (0.33–2.69) | .916 | 1.26 (0.32–4.97) | .739 |
| MIG | 3.11 (1.10–8.82) | .034 | 2.92 (0.81–10.57) | .103 |
| IFNβ | 0.34 (0.13–0.87) | .025 | 0.27 (0.07–0.99) | .049 |
| IFNα2 | 0.99 (0.48–2.02) | .967 | 0.59 (0.19–1.84) | .369 |
| IFNγ | 0.83 (0.57–1.22) | .349 | 0.69 (0.42–1.16) | .167 |
| IL18 | 1.38 (0.80–2.38) | .249 | 1.53 (0.75–3.11) | .241 |
| IL12P40 | 1.83 (0.66–5.09) | .244 | 3.44 (0.79–15.02) | .101 |
| sCD14 | 0.90 (0.08–10.42) | .931 | 0.83 (0.04–17.41) | .902 |
| IL15a | 0.20 (0.02–1.64) | .133 | 0.39 (0.03–4.69) | .460 |
| IFNλ1a | 0.80 (0.26–2.51) | .706 | 0.56 (0.14–2.29) | .419 |
| IL2a | 0.73 (0.29–1.84) | .500 | 0.64 (0.16–2.52) | .519 |
| IL27a | 0.64 (0.28–1.46) | .288 | 0.72 (0.22–2.31) | .580 |
| IL8a | 1.10 (0.53–2.28) | .803 | 1.14 (0.45–2.88) | .780 |
| IFNλ2a | 1.49 (0.72–3.11) | .284 | 1.69 (0.60–4.72) | .319 |
| IL12P7a | 1.15 (0.56–2.35) | .713 | 1.72 (0.62–4.78) | .298 |
| I-FABa | 1.27 (0.66–2.44) | .476 | 1.00 (0.44–2.27) | .993 |
Abbreviations: CI, confidence interval; IFN, interferon; IL, interleukin; IP, interferon gamma induced protein; MIG, monokine induced by IFN-γ; OR, odds ratio; sCD, soluble CD; TNF, tumor necrosis factor.
aAnalyzed as binary variables.
bMultivariate analyses adjusted for WHO stage of the disease, body mass index, lung cavities, age, CD4+ count, viral load, previous history of TB, history of IRIS. Viral load closer to sample collection was predictive of TB recurrence in all multivariate models.
Longitudinal Changes in Plasma Cytokine/Chemokine Expression
To determine the impact of TB treatment on cytokine markers, we included a small subset of cases (n = 16) who were followed longitudinally, starting with the first TB episode until the end of treatment of the second TB episode (Sup. Table 2). Expression levels of 2 analytes were decreased following completion of treatment after the first TB episode: IP10 (mean difference [MD] 0.27, 95% CI 0.062–0.480, P = .015) and sCD14 (MD 0.085, 95% CI 0.019–0.151, P = .016), (Figure 2A). Expression levels of 4 analytes were decreased after completion of treatment following TB reoccurrence including IP10 (MD 0.281, 95% CI −0.0167 to 0.5788, P = .063), sCD14 (MD 0.129, 95% CI 0.0249–0.2328, P = .019), IL6 (MD 0.535, 95% CI 0.191–0.879, P = .005) and IFNγ (MD 0.682, 95% CI 0.131–1.233, P = .019) (Figure 2B). Second TB episode occurred a median of 26 (IQR 11.7–44.3) months following the ART initiation. There was a significant increase in sCD14 between PreTB2 (time point 18–6 months before TB recurrence) and the second TB episode (MD −0.101, 95% CI −0.154 to −0.048, P = .001, Sup. Table 1.), suggesting sCD14 is the biomarker of active TB, as has been previously described [15, 16]. There was a significant increase in IFNγ between completion of treatment for the first TB episode and TB recurrence (MD −0.8999, 95% CI −1.661 to −0.138, P = .024), likely reflecting immune response to the increase in bacterial load. Additionally, there was a significant decrease in IL10 (MD 0.214, 95% CI 0.021–0.407, P = .0321), IP10 (MD 0.419, 95% CI 0.186–0.653, P = .002) and IFNα (MD = 0.529, 95% CI 0.089–0.969, P = .0218) between the baseline TB and recurrent TB episode (Sup. Table 1), potentially reflecting the effect of ART duration, as the expression of all 3 cytokines is known to decrease with ART [22, 23].
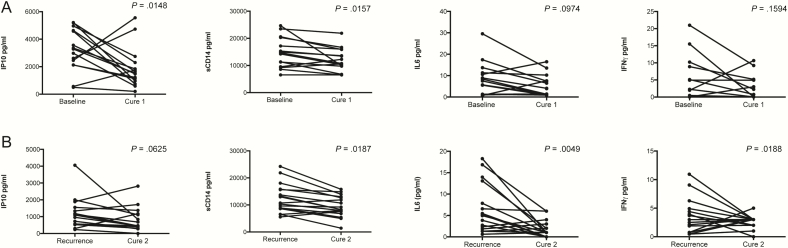
Figure 2.
Effect of treatment completion on cytokine/chemokine expression following active TB (n = 15). A, Effect of treatment following the first TB episode, during early ART. Expression of 2 analytes was decreased IP10 (mean difference (MD) 0.271, 95% CI 0.0618 to 0.4803, P = .0148), sCD14 (MD = 0.0847, 95% CI 0.0186–0.1508, P = .0157), whereas IL6 (MD = 0.222, 95% CI −0.0461 to 0.4909, P = .097) and IFNγ (MD = 0.4687, 95% CI −0.2097 to 1.1472. P = .159) expression was not significantly affected. B, Effect of treatment following the recurrent TB episode. Expression of 4 analytes was decreased following TB treatment completion, including IP10 (MD = 0.281, 95% CI −0.0167 to 0.5788 P = .0625), sCD14 (MD = 0.1289, 95% CI 0.0249–0.2328, P = .0187), IL6 (MD = 0.5352, 95% CI 0.1913–0.8791, P = .0049), IFNγ (MD = 0.6821, 95% CI 0.1313–1.233, P = .0188). Paired t-test was used to evaluate changes in analyte expression between different time points. (Cytokines significantly affected at either of the time-points are shown here. Information for the rest of the measured cytokines can be found in Sup. Table 1). Abbreviations: ART, antiretroviral therapy; CI, confidence interval; IL, interleukin; IFN, interferon; IP, interferon gamma induced protein; sCD, soluble CD; TB, tuberculosis.
DISCUSSION
There is an urgent need for the identification and validation of TB diagnostic markers in HIV-positive individuals as this is key for timely patient management and for the development of new therapeutics and vaccines. Because all HIV-infected individuals now qualify for ART, but ART does not completely ameliorate HIV-associated TB risk, the population for this study is important for defining TB risk factors. Significantly higher plasma levels of IL6, IL1β and sIL1Rα were associated with increased risk of TB recurrence. Interestingly, plasma IFNβ levels were associated with a decreased risk of TB recurrence. Active TB correlated with markers of systemic immune activation (IL6 and sCD14), known to correlate with more rapid HIV disease progression and mortality [8, 24, 25].
Significantly higher plasma IL6 and IL1β levels, known to exert pro-inflammatory effects, were found to be the strongest predictors of TB recurrence in both univariate and multivariate models. IL6 was previously described as a strong predictor of active TB irrespective of HIV status [16, 26]. TB treatment completion in the longitudinal subanalysis was associated with decrease in IL6 expression, further suggesting that IL6 could be a useful biomarker of both active TB disease and treatment success [26]. IL1β-induced immune responses have the contrasting potential to both clear bacterial infection [27] and to promote disease by inflicting tissue damage [28]. Here we show that increased plasma IL1β levels associate with TB disease recurrence. TB treatment did not have any effect on IL1β plasma levels suggesting that IL1β likely reflects the sub-clinical TB effects and could be used as a biomarker of latent TB. In addition to IL6 and IL1β, the interleukin 1 receptor antagonist (IL1Rα) was found to be significantly associated with TB recurrence independent of CD4 count, VL, and other adjusted covariates. Increase in serum sIL1Rα levels was previously associated with TB disease activity [29]. Soluble IL1Rα binds to IL1 receptors but does not induce any intracellular response, thereby acting as a regulatory mechanism for IL1-induced responses. Both IL6 and IL1β are known to be potent inducers of sIL1Rα and could be driving the increased sIL1Rα levels observed here [30, 31]. As with IL1β, TB treatment had no effect on the sIL1Rα levels, further indicating that sIL1Rα may directly reflect the IL1β cytokine response.
A previous study performed in the TRuTH cohort showed that elevated production of IL1β from monocytes following TLR-2, TLR-4, and TLR-7/8 stimulation was associated with reduced odds of TB recurrence, whereas production of IL-1β from both monocytes and mDCs following Bacillus Calmette-Guérin (BCG) stimulation was associated with increased odds of TB recurrence [32]. Here we report that increased IL1β plasma levels are associated with higher risk of TB recurrence. Although increased IL1β production is required for proper development of antimicrobial adaptive immunity during initial MTB infection, if left uncontrolled, chronic IL1β production can be detrimental in MTB immunity. As seen with systemic pDC IFNα production during chronic HIV infection, increased, chronic plasma cytokine levels correlate with decreased ability of cells to produce the same cytokine upon further stimulation [33, 34], reflecting the anergy that can be associated with chronic infection.
Induction of type I interferons has been associated with bacterial virulence and TB pathogenesis in a number of previous studies [35, 36]. We did not observe any difference in IFNα2 expression; however, IFNβ was the only examined cytokine shown to be protective against TB reactivation in this study. Despite sharing the type I IFN receptor (IFNAR1), the responses induced by IFNα and IFNβ differ significantly, likely due to differences in binding affinity for IFNAR1, where IFNβ out-competes IFNα2 at >30-fold affinity [37]. One hypothesis that could explain how IFNβ could provide protection against TB is that it averts the negative effects of IFNα2 signaling by blocking receptor affinity. Furthermore, IL1 and type I IFN pathways counter-regulate each other [38], and IFNβ is known to limit pro-IL1β and pro-IL18 transcription and has opposing effects on NLRP3- and AIM2- inflammosome activation [39]. Validation and further examination of potential mechanisms that explain this observation could have important implications for managing TB infection in HIV coinfected individuals.
Results from the longitudinal analysis suggest that sCD14 is the biomarker of active TB, as has been previously described [15, 16]. Completion of treatment after baseline and recurrent TB led to significant decreases in sCD14 expression, and there was a significant increase in sCD14 between 18 and 6 months before TB recurrence and active TB. We observed no differences between cases and controls when it came to I-FAB, a marker of intestinal epithelial barrier damage, further indicating that the observed changes in sCD14 are due to active TB and monocyte activation and are unlikely to be the result of HIV-associated microbial translocation and the gastrointestinal tissue damage [40].
Observed differences in IP10 and MIG, previously characterized as markers of TB disease [41, 42], were found to be primarily driven by HIV viral load in the context of HIV/TB coinfection. Expression of IP10, IL10, and IFNα decreased between the two TB disease episodes likely due to the duration of ART and the associated immune-reconstitution.
As strain typing was not available we were unable to delineate new infections from relapse in the context of TB recurrence. Future studies should address this issue as immunological mechanisms in these 2 scenarios are likely to differ. Here we show that in HIV-infected individuals on ART, TB recurrence is predicted by biomarkers of soluble, systemic inflammation, many of which are also implicated in more rapid HIV disease progression and non-AIDS mortality in the context of effective HIV treatment. Some of the identified markers decreased following TB treatment completion, implicating these as surrogates of subclinical TB disease activity and associated immune responses.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Authors’ contributions. A. S., L. R. M., and K. N. designed the project. A. S. performed the experiments and wrote the manuscript with critical review from all the authors. Data analysis was done by A. S., L. R. M., and N. Y.; K. N. provided clinical and technical advice, and S. G. and N. S. assisted in subject recruitment and characterization. S. S. A. K. and K. N. provided oversight to study implementation.
Acknowledgments. The authors thank all of the research participants and the staff of the CAPRISA eThekwini clinic in KwaZulu-Natal, South Africa, for their dedication to these studies.
Disclaimer. The funding sources listed here did not have any role in the analysis or preparation of the data in this manuscript, nor was any payment received by these or other funding sources for this manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of either the Howard Hughes Medical Institute or the Centers for Disease Control and Prevention (CDC).
Financial support. The TRuTH study was supported by the Howard Hughes Medical Institute, grant 55007065, as well as the Centers for Disease Control and Prevention (CDC) cooperative agreement UY2G/PS001350-02. The research infrastructure to conduct this trial, including the data management, laboratory and pharmacy cores were established through the US National Institutes for Health’s Comprehensive International Program of Research on AIDS grant (CIPRA, grant AI51794). K. N. was supported by the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, grant D43 TW000231). Patient care was supported by the KwaZulu-Natal Department of Health and the US President’s Emergency Plan for AIDS Relief (PEPFAR). Research reported in this publication was supported by the Strategic Health Innovation Partnership (SHIP) Unit of the South African Medical Research Council, a grantee of the Bill & Melinda Gates Foundation, through the University of Cape Town. L. R. M. is supported by Canadian Institute of Health Research (CIHR) New Investigator Award.
Potential conflict of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Barry CE 3rd, Boshoff HI, Dartois V et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009; 7:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24:351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005; 191:150–8. [DOI] [PubMed] [Google Scholar]
- 4. Keane J, Gershon S, Wise RP et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–104. [DOI] [PubMed] [Google Scholar]
- 5. McShane H. Co-infection with HIV and TB: double trouble. Int J STD AIDS 2005; 16:95–100– quiz 101. [DOI] [PubMed] [Google Scholar]
- 6. Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; 23:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandler NG, Wand H, Roque A et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuller LH, Tracy R, Belloso W et al. ; INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knudsen TB, Ertner G, Petersen J et al. Plasma CD163 independently predicts all-cause mortality from HIV-1 infection. J Infect Dis 2016: jiw263. [DOI] [PubMed] [Google Scholar]
- 10. Deleage C, Schuetz A, Alvord WG et al. Impact of early cART in the gut during acute HIV infection. JCI Insight 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Ghneim K, Sok D et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 2016; 353:1045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sutherland JS, de Jong BC, Jeffries DJ, Adetifa IM, Ota MO. Production of TNF-alpha, IL-12(p40) and IL-17 can discriminate between active TB disease and latent infection in a West African cohort. PLoS One 2010; 5:e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sutherland JS, Hill PC, Adetifa IM et al. Identification of probable early-onset biomarkers for tuberculosis disease progression. PLoS One 2011; 6:e25230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada G, Shijubo N, Shigehara K, Okamura H, Kurimoto M, Abe S. Increased levels of circulating interleukin-18 in patients with advanced tuberculosis. Am J Respir Crit Care Med 2000; 161:1786–9. [DOI] [PubMed] [Google Scholar]
- 15. Lawn SD, Labeta MO, Arias M, Acheampong JW, Griffin GE. Elevated serum concentrations of soluble CD14 in HIV- and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin Exp Immunol 2000; 120:483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR. Latent and active tuberculosis infection increase immune activation in individuals co-infected with HIV. EBioMedicine. EBIOM 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawn SD, Labeta MO, Arias M, Acheampong JW, Griffin GE. Elevated serum concentrations of soluble CD14 in HIV- and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin Exp Immunol 2000; 120:483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gengiah TN, Holford NH, Botha JH, Gray AL, Naidoo K, Abdool Karim SS. The influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosis. Eur J Clin Pharmacol 2012; 68:689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abdool Karim SS, Naidoo K, Grobler A et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–6. [PubMed] [Google Scholar]
- 21. Simmons RP, Scully EP, Groden EE et al. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 2013; 27:2505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stylianou E, Aukrust P, Kvale D, Müller F, Frøland SS. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression–down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol 1999; 116:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardy GA, Sieg S, Rodriguez B et al. Interferon-α is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One 2013; 8:e56527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts L, Passmore JA, Williamson C et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 2010; 24:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandler NG, Wand H, Roque A et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattos AMM, de S Almeida C, Franken KLMC et al. Increased IgG1, IFN-γ, TNF-α and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int. Immunol 2010; 22:775–82. [DOI] [PubMed] [Google Scholar]
- 27. Jayaraman P, Sada-Ovalle I, Nishimura T et al. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol 2013; 190:4196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang G, Zhou B, Li S et al. Allele-specific induction of IL-1β expression by C/EBPβ and PU.1 contributes to increased tuberculosis susceptibility. PLoS Pathog 2014; 10:e1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juffermans NP, Verbon A, van Deventer SJH, van Deutekom H, Speelman P, van der Poll T. Tumor necrosis factor and interleukin-1 inhibitors as markers of disease activity of tuberculosis. Am J Respir Crit Care Med 2012; 157:1328–31. [DOI] [PubMed] [Google Scholar]
- 30. Bargetzi MJ, Lantz M, Smith CG et al. Interleukin-1 beta induces interleukin-1 receptor antagonist and tumor necrosis factor binding protein in humans. Cancer Res 1993; 53:4010–3. [PubMed] [Google Scholar]
- 31. Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood 1994; 83:113–8. [PubMed] [Google Scholar]
- 32. Thobakgale C, Naidoo K, McKinnon LR et al. Interleukin 1-beta (IL-1β) production by innate cells following TLR stimulation correlates with TB recurrence in ART-treated HIV infected patients. J Acquir Immune Defic Syndr 2016; 74:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamga I, Kahi S, Develioglu L et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis 2005; 192:303–10. [DOI] [PubMed] [Google Scholar]
- 34. Tilton JC, Manion MM, Luskin MR et al. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol 2008; 82:3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berry MP, Graham CM, McNab FW et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010; 466:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 2007; 178:3143–52. [DOI] [PubMed] [Google Scholar]
- 37. Jaitin DA, Roisman LC, Jaks E et al. Inquiring into the differential action of interferons (IFNs): an IFN-alpha2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-beta. Mol Cell Biol 2006; 26:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayer-Barber KD, Yan B. Clash of the cytokine titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell Mol Immunol 2017; 14:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Briken V, Ahlbrand SE, Shah S. Mycobacterium tuberculosis and the host cell inflammasome: a complex relationship. Front Cell Infect Microbiol 2013; 3:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toossi Z, Funderburg NT, Sirdeshmuk S et al. Systemic immune activation and microbial translocation in dual HIV/tuberculosis-infected subjects. J Infect Dis 2013; 207:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hong JY, Lee HJ, Kim SY, Chung KS, Kim EY. Efficacy of IP-10 as a biomarker for monitoring tuberculosis treatment. J Infect 2014; 68:252–8. [DOI] [PubMed] [Google Scholar]
- 42. Yang Q, Cai Y, Zhao W et al. IP-10 and MIG are compartmentalized at the site of disease during pleural and meningeal tuberculosis and are decreased after antituberculosis treatment. Clin Vaccine Immunol 2014; 21:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.