Sequence type (ST) 131-H30 was responsible for 5.3% of all extraintestinal Escherichia coli infections and 43.3% of ESBL-producing extraintestinal E. coli infections among US children. The clinical and demographic correlates of infection with ST131-H30 differed between extended-spectrum cephalosporin-resistant and -sensitive isolates.
Keywords: E. coli infections, ST131, antimicrobial resistance, pediatric infections
Abstract
Background
Escherichia coli sequence type (ST) 131-H30 is a globally important pathogen implicated in rising rates of multidrug resistance among E. coli causing extraintestinal infections. Previous studies have focused on adults, leaving the epidemiology of H30 among children undefined.
Methods
We used clinical data and isolates from a case-control study of extended-spectrum cephalosporin-resistant E. coli conducted at 4 US children’s hospitals to estimate the burden and identify host correlates of infection with H30. H30 isolates were identified using 2-locus genotyping; host correlates were examined using log-binomial regression models stratified by extended-spectrum cephalosporin resistance status.
Results
A total of 339 extended-spectrum cephalosporin-resistant and 1008 extended-spectrum cephalosporin-susceptible E. coli isolates were available for analyses. The estimated period prevalence of H30 was 5.3% among all extraintestinal E. coli isolates (95% confidence interval [CI], 4.6%–7.1%); H30 made up 43.3% (81/187) of extended-spectrum β-lactamase (ESBL)–producing isolates in this study. Host correlates of infection with H30 differed by extended-spectrum cephalosporin resistance status: Among resistant isolates, age ≤5 years was positively associated with H30 infection (relative risk [RR], 1.83 [95% CI, 1.19–2.83]); among susceptible isolates, age ≤5 years was negatively associated with H30 (RR, 0.48 [95% CI, .27–.87]), while presence of an underlying medical condition was positively associated (RR, 4.49 [95% CI, 2.43–8.31]).
Conclusions
ST131-H30 is less common among extraintestinal E. coli collected from children compared to reported estimates among adults, possibly reflecting infrequent fluoroquinolone use in pediatrics; however, it is similarly dominant among ESBL-producing isolates. The H30 subclone appears to disproportionately affect young children relative to other extended-spectrum cephalosporin-resistant E. coli.
Extraintestinal Escherichia coli, a common cause of urinary tract and bloodstream infections across all ages, have displayed increasing rates of antimicrobial resistance over the past 2 decades [1]. This increase has been attributed to the emergence and rapid clonal expansion of E. coli sequence type (ST) 131, which has transformed the population structure of extraintestinal E. coli infections worldwide [2–5]. Molecular epidemiologic studies have shown that a subclone of ST131, termed H30, has driven the global dissemination of ST131 [6–9]. The clonal structure of ST131-H30 is tightly linked to antimicrobial resistance; the vast majority of H30 isolates are fluoroquinolone resistant due to mutations in the gyrA and parC chromosomal genes (isolates known as H30-R or clade C), while nested subclones are additionally associated with the production of CTX-M-type extended-spectrum β-lactamases (ESBLs) that confer resistance to extended-spectrum cephalosporins (Supplementary Figure 1) [7, 8, 10–12].
Although E. coli ST131-H30 (hereafter, H30) has been recognized as a clone of significant public health importance [5, 13], there is a lack of data about its epidemiology in children. Most studies that have included H30 isolates from children have occurred over short time periods at single centers and have accumulated few H30 isolates [14–16]. Among adults in the United States, H30 is estimated to comprise about 50% of ESBL-producing E. coli infections and 10%–20% of all extraintestinal E. coli infections, and has been linked to host factors including older age, healthcare contact, local or systemic compromise, and recent antibiotic use [6, 14–17]. Associations with adverse outcomes such as persistent infections, new infections, sepsis, and hospitalization have also been reported in adult populations [7, 14, 18]. Understanding the epidemiology of H30 in pediatric populations is important, as its dominance among multidrug-resistant (MDR) extraintestinal E. coli makes it a likely culprit of many difficult-to-treat infections in children. Proper treatment of urinary tract infections—the most common type of infection caused by extraintestinal E. coli—is especially critical in pediatric populations, as young children are more prone to upper urinary tract infection with potential short- and long-term complications such as renal scarring and decreased renal function [19, 20].
We sought to address this knowledge gap using data from a multiyear, multicenter prospective case-control study of extraintestinal E. coli infections to quantify the burden and identify clinical and demographic correlates of infection with H30 in a US pediatric population. In addition, we describe and compare the antimicrobial resistance characteristics of H30 and non-H30 E. coli isolates.
METHODS
Patients and Isolates
All isolates and clinical data came from a multicenter case-control study that prospectively collected isolates and is described in detail elsewhere [21]. In brief, between 1 September 2009 and 30 September 2013, 4 freestanding US children’s hospitals (referred to here as West, Midwest 1, Midwest 2, and East) used standard clinical microbiology techniques to identify and collect all extended-spectrum cephalosporin-resistant (ESC-R) E. coli collected from urine or other normally sterile sites during routine clinical care of both inpatient and outpatient children <22 years of age. ESC-R isolates were defined as those nonsusceptible to ceftriaxone, cefotaxime, ceftazidime, cefepime, or aztreonam. Patients could contribute multiple ESC-R isolates if the subsequent isolate was collected ≥15 days after the previous ESC-R isolate. For each resistant isolate, 3 consecutive E. coli isolates that were susceptible to the aforementioned agents, referred to here as extended-spectrum cephalosporin-susceptible (ESC-S) isolates, were collected without respect to any patient or microbiological characteristics beyond temporal proximity to the ESC-R isolates and prior enrollment in the study (patients could only contribute 1 ESC-S isolate). Demographic and clinical data were collected from the medical records; methods for categorizing underlying medical conditions, capturing antibiotic exposure, and characterizing the clinical significance of urine isolates (likely urinary tract infection vs not) were described previously [21, 22]. The institutional review board at each hospital approved the study protocol.
Laboratory Methods
Methods for antibiotic susceptibility testing and typing of resistance phenotypes and determinants were described previously [21]. In brief, ESC-R phenotypes (ESBL vs AmpC) were characterized using a combination of disk diffusion and E-tests. Genetic determinants of extended-spectrum cephalosporin resistance were identified by polymerase chain reaction (PCR) using primers for genes encoding common extended-spectrum cephalosporinases [21]. H30 isolates were identified using the fumC/fimH genotyping scheme [23]. Isolates belonging to the H30Rx sublineage were identified by PCR detection of sublineage-specific single-nucleotide polymorphisms [7].
Statistical Analyses
Prevalence Estimates
The period prevalence of H30 was estimated by calculating a weighted average of the ESC-R and ESC-S stratum-specific prevalence estimates (details in Supplementary Methods).
Host Correlates of Infection
Only the first isolate from each unique individual was considered in the host factor analyses. Host factors were compared between patients with H30 vs non-H30 isolates, stratified by ESC-R status and adjusting for study hospital where sample size allowed. The magnitude of the association between each predictor of interest and H30 infection was then assessed using univariable and multivariable log-binomial regression models. For each predictor of interest, the relative risk (RR) and 95% confidence intervals (CIs) from 3 models are presented: (1) a univariable model that estimates the crude (unadjusted) total effect of the predictor of interest on the outcome; (2) a multivariable model that estimates the total effect of the predictor of interest on the outcome, adjusted for potential confounders; and (3) a multivariable model that estimates the direct effect of the predictor of interest on the outcome, adjusted for potential confounders as well as for potential mediators. All multivariable models adjusted for study hospital; additional potential confounders and mediators were selected according to the conceptual frameworks found in the supplementary material (Supplementary Figures 3 and 4). Finally, we conducted post hoc analyses of the interaction between age and underlying medical condition (details in Supplementary Methods).
Antimicrobial Resistance Characteristics
We examined co-resistance to commonly used antimicrobial agents in the first E. coli isolate collected per individual, stratifying by ESC-R and ESC-S status to maintain consistency with the sampling scheme of the parent study. H30 isolates were additionally stratified into H30Rx and H30-non-Rx (Supplementary Figure 1) and compared to non-H30 isolates. Among ESC-R isolates, ESC-R-associated resistance mechanisms and determinants were also identified and compared. All analyses were conducted using R version 3.3.1 (R Core Team, 2016).
RESULTS
Isolates and Prevalence Estimates
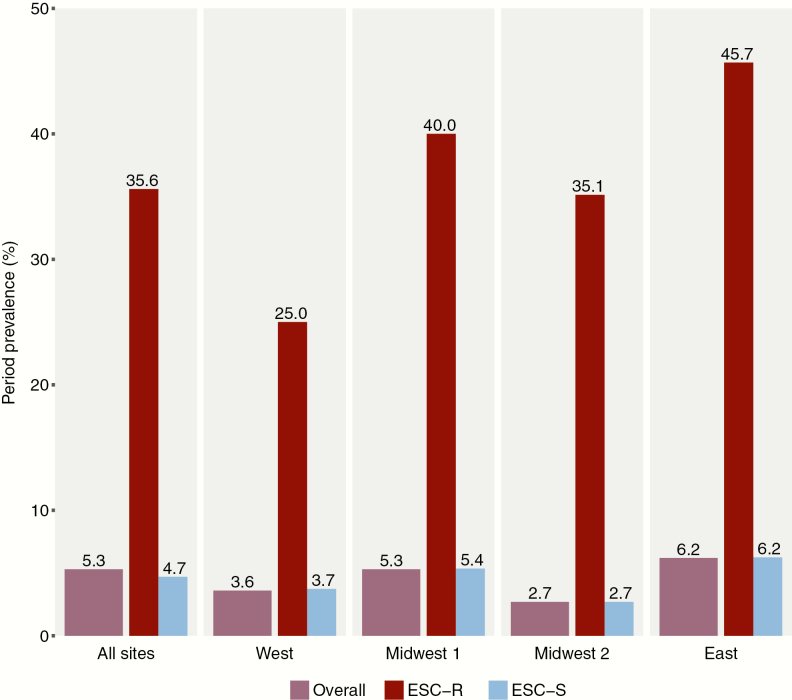
A total of 339 ESC-R isolates from 278 patients and 1008 ESC-S isolates from 1008 patients were available for analyses (Supplementary Figure 2). The estimated prevalence of H30 among all clinical E. coli isolates at all study hospitals was 5.3% (95% CI, 4.6%–7.1%), while the hospital-specific prevalence ranged from 2.7% to 6.2% (Figure 1). The estimated overall prevalence of H30Rx was 0.87% (95% CI, .70%–1.7%).
Figure 1.
Estimated prevalence of ST131-H30 among extraintestinal Escherichia coli infections overall and by study hospital. The raw numbers that generated these estimates can be found in Supplementary Table 2. Abbreviations: ESC-R, extended-spectrum cephalosporin-resistant; ESC-S, extended-spectrum cephalosporin-susceptible.
Host Correlates of Infection by Extended-Spectrum Cephalosporin Resistance Status
The first ESC-R isolate from each of the 278 patients with an ESC-R isolate collected during the study period was included in the host correlates analyses (Supplementary Figure 2). Among these patients, patient age was associated with H30 infection and further examined as a predictor of interest (Table 1). Our sample size precluded multilevel predictors, so age was categorized into ages 0–5 vs 6–21 years in regression models. After adjusting for potential confounders, age 0–5 was associated with an 83% increased risk of the infecting organism being H30 (RR, 1.83 [95% CI, 1.19–2.83]). There was no evidence that this association was mediated through factors related to underlying illness (Table 2), or that underlying illness interacted with age (Table 3 and Supplementary Table 3). When restricting the outcome to H30Rx infection only (vs non-H30 infection) and adjusting for potential confounders, the effect size was stronger (RR, 2.25 [95% CI, 1.33–3.80]).
Table 1.
Selected Demographic and Clinical Characteristics of Patients With H30 and Non-H30 Isolates, Stratified By Extended-Spectrum Cephalosporin Resistance Status
| Characteristic | ESC-R (n = 278) | ESC-S (n = 1008) | ||||
|---|---|---|---|---|---|---|
| H30 (n = 83) | Non-H30 (n = 195) | P Valuea | H30 (n = 47) | Non-H30 (n = 961) | P Valuea | |
| Age, y | .008* | <.001* | ||||
| 0–5 | 60 (72.3) | 98 (50.3) | 504 (52.5) | |||
| 6–10 | 10 (12.1) | 40 (20.5) | 4 (8.5) | 190 (19.8) | ||
| 11–15 | 6 (7.2) | 31 (15.9) | 12 (25.5) | 126 (13.1) | ||
| 16–21 | 7 (8.4) | 26 (13.3) | 15 (31.9) | 141 (14.7) | ||
| Sex | .407 | .440 | ||||
| Male | 18 (21.7) | 53 (27.2) | 8 (17.0) | 130 (13.5) | ||
| Female | 65 (78.3) | 142 (72.8) | 39 (83.0) | 831 (86.5) | ||
| Ethnicityb | .110 | .312 | ||||
| Hispanic | 8 (10.0) | 36 (19.3) | 4 (8.9) | 135 (14.6) | ||
| Non-Hispanic | 72 (90.0) | 151 (80.7) | 41 (91.1) | 791 (85.4) | ||
| Raceb,c | .087 | .314 | ||||
| White | 39 (49.4) | 116 (62.0) | 29 (63.0) | 629 (68.2) | ||
| African-American | 12 (15.2) | 29 (15.5) | 12 (26.1) | 219 (23.7) | ||
| Asian | 22 (27.9) | 32 (17.1) | 2 (4.4) | 51 (5.5) | ||
| Native American | 4 (5.1) | 2 (1.1) | 1 (2.2) | 6 (0.7) | ||
| Pacific Islander | 2 (2.5) | 7 (3.7) | 1 (2.2) | 10 (1.1) | ||
| >1 race | 0 (…) | 1 (0.5) | 1 (2.2) | 8 (0.9) | ||
| Site of culture | .233 | .753 | ||||
| Urinec,d | 78 (94.0) | 173 (88.7) | 45 (95.7) | 923 (96.1) | ||
| Blood | 2 (2.4) | 15 (7.7) | 2 (4.3) | 32 (3.3) | ||
| Other | 3 (3.6) | 7 (3.6) | 0 (0.0) | 6 (0.6) | ||
| Type of acquisitionb,e | .832 | <.001* | ||||
| Community-associated | 28 (33.7) | 65 (33.3) | 14 (29.8) | 599 (62.7) | ||
| Healthcare-associated | 45 (54.2) | 103 (52.8) | 30 (63.8) | 297 (31.1) | ||
| Hospital-associated | 10 (12.0) | 27 (13.8) | 3 (6.4) | 60 (6.3) | ||
| Hospitalized in past 6 mob | .429 | .017* | ||||
| Yes | 25 (30.1) | 69 (35.4) | 13 (27.7) | 143 (15.0) | ||
| No | 58 (69.9) | 126 (64.6) | 34 (72.3) | 813 (85.0) | ||
| Underlying medical conditionb | .854 | <.001* | ||||
| Urologicf | 30 (36.1) | 75 (38.7) | 26 (55.3) | 185 (19.3) | ||
| Malignancy | 4 (4.8) | 13 (6.7) | 1 (2.1) | 26 (2.7) | ||
| Other condition | 16 (19.3) | 35 (18.0) | 6 (12.8) | 104 (10.8) | ||
| No condition | 33 (39.8) | 71 (36.6) | 14 (29.8) | 644 (67.2) | ||
| Antibiotic use in the past 30 db | .548 | .006* | ||||
| Yes | 34 (41.0) | 85 (43.6) | 16 (34.0) | 176 (18.4) | ||
| No | 49 (59.0) | 110 (56.4) | 31 (66.0) | 781 (81.6) | ||
| History of transplantationb | .108 | <.001* | ||||
| Yes | 3 (3.6) | 19 (9.7) | 5 (10.6) | 22 (2.3) | ||
| No | 80 (96.4) | 175 (90.2) | 42 (89.4) | 937 (97.7) | ||
| Received immunosuppressants in last yearb,g | .100 | .071 | ||||
| Yes | 9 (10.8) | 37 (19.1) | 6 (12.8) | 62 (6.5) | ||
| No | 74 (89.2) | 157 (80.9) | 41 (87.2) | 897 (93.5) | ||
| Device type | .157 | <.001* | ||||
| Central venous catheter | 7 (8.4) | 28 (14.4) | 3 (6.4) | 53 (5.5) | ||
| Foley catheter | 6 (7.2) | 5 (2.6) | 3 (6.4) | 11 (1.1) | ||
| Other device | 14 (16.9) | 26 (13.3) | 10 (21.3) | 55 (5.7) | ||
| No device | 56 (67.5) | 136 (69.7) | 31 (66.0) | 842 (87.6) | ||
| Hospital | .156 | .349 | ||||
| West | 22 (26.5) | 78 (40.0) | 13 (27.7) | 341 (35.5) | ||
| East | 24 (28.9) | 51 (26.2) | 16 (34.0) | 284 (29.6) | ||
| Midwest 1 | 11 (13.3) | 23 (11.8) | 3 (6.4) | 108 (11.2) | ||
| Midwest 2 | 26 (31.3) | 43 (22.1) | 15 (31.9) | 228 (23.7) | ||
Abbreviations: ESC-R, extended-spectrum cephalosporin-resistant; ESC-S, extended-spectrum cephalosporin-susceptible.
a P values generated via Mantel-Haenzel χ2 tests (adjusting for study hospital) unless otherwise indicated.
bNumber does not add to n because of missing data.
c P values generated via (unadjusted) Fisher exact test.
dAll isolates collected from urine and without missing data were characterized as likely urinary tract infection (UTI); 7 isolates with missing data could not be classified (3 extended-spectrum cephalosporin-resistant and 4 extended-spectrum cephalosporin-susceptible).
eType of acquisition was defined as follows: community associated, culture obtained in an outpatient setting or <48 hours after hospital admission from an otherwise healthy patient without hospitalization in the previous 6 months; healthcare associated, culture obtained in an outpatient setting or <48 hours after hospital admission from a patient who had been hospitalized in the previous 6 months and/or had a chronic medical condition requiring frequent healthcare or prolonged/recurrent antibiotic courses; and hospital associated, culture obtained >48 hours after hospital admission or <48 hours after hospital discharge from a patient without signs or symptoms of infection on admission.
fDiagnoses included in the urologic category are congenital urological abnormality, neurogenic bladder, and vesicoureteral reflux.
gImmunosuppressants included antineoplastic agents, high-dose glucocorticoids (≥2 mg/kg of body weight), tumor necrosis factor inhibitors, calcineurin inhibitors, and mycophenolate mofetil.
*P value <.05.
Table 3.
Analysis of Interaction Between Age and Underlying Medical Condition on the Risk of H30 Infection Versus Infection With Other Escherichia coli Types Using Log-Binomial Regression Models
| Condition | Age | RRs (95% CI)a for Age 0–5 vs Age 6–21 Within Strata of Underlying Medical Condition | |
|---|---|---|---|
| 0–5 y | 6–21 y | ||
| RR (95% CI)a | RR (95% CI)a | ||
| Presence of an underlying medical condition | 2.80 (.90–8.70) | 8.66 (3.38–22.2) | 0.32 (.14–.72) |
| No underlying medical condition | 1.52 (.51–4.50) | 1.0 (ref) | 1.51 (.50–4.53) |
| Presence of an underlying medical condition vs no underlying medical condition within strata of age | 1.99 (.74–5.33) | 8.81 (3.44–22.6) | |
Interaction contrast ratio (ICR), –6.38 (95% confidence interval, –23.5 to –1.15). When interpreting the ICR, deviation from 0 indicates evidence of interaction on the additive scale (see Supplementary Methods).
Abbreviations: CI, confidence interval; RR, relative risk.
aRRs adjusted for study hospital.
Table 2.
Total and Direct Effect of Selected Characteristics on Risk of H30 Infection Versus Infection With Other Escherichia coli Types Using Log-Binomial Regression Models Stratified by Extended-Spectrum Cephalosporin Resistance Status
| Characteristic | ESC-R | ESC-S | ||||
|---|---|---|---|---|---|---|
| Total Effect RR (95% CI) | Direct Effect RR (95% CI) | Total Effect RR (95% CI) | Direct Effect RR (95% CI) | |||
| Crude | Adjusteda | Adjusteda | Crude | Adjusteda | Adjusteda | |
| Age 0–5 y | 1.98 (1.30–3.01)* | 1.83 (1.19–2.83)*,b | 1.91 (1.24–2.96)*,c | 0.48 (.27–.87)* | … | 0.52 (.29–.94)*,d |
| Antibiotics in last 30 d | … | … | … | 2.18 (1.22–3.91)* | 1.18 (.64–2.20)e | … |
| Underlying medical condition | … | … | … | 4.46 (2.42–8.21)* | 4.49 (2.43–8.31)*,f | 3.53 (1.74–7.17)*,g |
| Hospitalization in past 6 mo | … | … | … | 2.08 (1.12–3.84)* | 1.22 (.65–2.30)h | 1.01 (.51–2.00)i |
| Presence of indwelling device | … | … | … | 3.33 (1.87–5.92)* | 1.54 (.78–3.04)j | 1.53 (.77–3.01)d |
Abbreviations: CI, confidence interval; ESC-R, extended-spectrum cephalosporin-resistant; ESC-S, extended-spectrum cephalosporin-susceptible; RR, relative risk.
aAll models adjusted for study hospital.
bAdditional covariates: Asian race (yes/no).
cAdditional covariates: Asian race (yes/no), underlying medical condition (yes/no), antibiotics in the last 30 days (yes/no), hospitalization in the past 6 months (yes/no).
dAdditional covariates: underlying medical condition (yes/no), antibiotics in the last 30 days (yes/no), hospitalization in the past 6 months (yes/no).
eAdditional covariates: age (0–5 or 6–21), hospitalization in the past 6 months (yes/no), underlying medical condition (yes/no), indwelling device (yes/no).
fAdditional covariates: age (0–5 or 6–21).
gAdditional covariates: age (0–5 or 6–21), hospitalization in the past 6 months (yes/no), antibiotics in the last 30 days (yes/no), indwelling device (yes/no).
hAdditional covariates: age (0–5 or 6–21), underlying medical condition (yes/no).
iAdditional covariates: age (0–5 or 6–21), underlying medical condition (yes/no), antibiotics in the last 30 days, indwelling device (yes/no).
jAdditional covariates: underlying medical condition (yes/no), hospitalization in the past 6 months (yes/no).
*Confidence interval does not include 1.
A total of 1008 patients had one ESC-S isolate collected during the study period. Among these patients, patient age and several factors associated with underlying illness were associated with H30 infection (Table 1). Each of these variables was examined as a predictor of interest except for (i) history of transplantation, due to small numbers, and (ii) type of infection acquisition, as previous hospitalization and underlying medical conditions were examined independently. Underlying medical condition and indwelling device categories were collapsed into any vs none. Patient age ≤5 years was negatively associated with H30 infection (RR, 0.48 [95% CI, .27–.87]). Of the variables related to underlying illness, after adjusting for potential confounders, only presence of an underlying medical condition (RR, 4.49 [95% CI, 2.43–8.31]) remained as an independent predictor of H30 infection; results were very similar when analyzing presence of an underlying urologic condition only (Supplementary Table 4). When including potential mediators in the models, the magnitude of the associations between age ≤5 years and presence of an underlying medical condition with H30 infection decreased, but the associations remained statistically significant (Table 2). Evidence of interaction between age and underlying medical condition was observed; when examining joint effects, underlying medical condition was only significantly associated with H30 infection in combination with older age, and older age was only significantly associated with H30 infection in combination with presence of an underlying medical condition (Table 3).
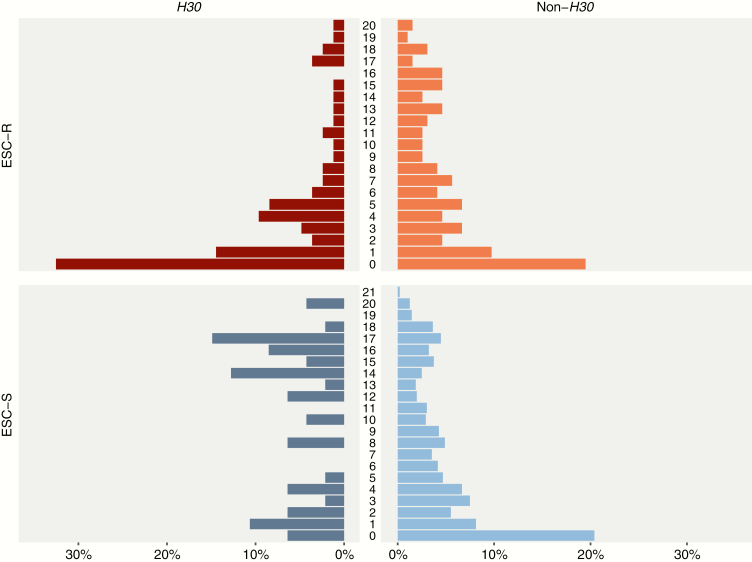
Since patient age was important in the analyses of both ESC-R and ESC-S isolates, we also visually inspected the distributional differences of age measured continuously. While the non-H30 age distributions are very similar, the H30 age distributions display marked differences between ESC-R and ESC-S isolates (Figure 2).
Figure 2.
Distributions of age (in years) by ST131-H30 and non-ST131-H30 status and extended-spectrum cephalosporin resistance status. Abbreviations: ESC-R, extended-spectrum cephalosporin-resistant; ESC-S, extended-spectrum cephalosporin-susceptible.
Antimicrobial Resistance Characteristics By Extended-Spectrum Cephalosporin Resistance and H30Rx Status
A total of 278 ESC-R isolates were examined (the first isolate collected per individual). Among these isolates, nearly all H30Rx and H30-non-Rx isolates were nonsusceptible to fluoroquinolones, compared to less than half of non-H30 isolates (Table 4). Similarly, all ESC-R H30Rx and the vast majority of H30-non-Rx isolates were ESBL-producing, while non-H30 isolates were more evenly split between ESBL producers and AmpC producers. H30 was the most common subclone identified among the ESC-R isolates in the study (Supplementary Table 1); it made up 29.9% (83/278) of ESC-R isolates, and when restricting to ESBL-producing isolates only, it made up 43.3% (81/187) of the total. The vast majority of ESBL-producing H30Rx isolates had a CTX-M-15 β-lactamase, while ESBL-producing H30-non-Rx isolates were dominated by the CTX-M-27 β-lactamase; ESBL-producing non-H30 isolates were more evenly split between CTX-M-15 and CTX-M-14 β-lactamases (Table 4). Systematic differences in the types of ESC-R resistance determinants by study hospital or year were not observed (Supplementary Figure 5).
Table 4.
Selected Antimicrobial Resistance Characteristics of H30Rx, H30-Non-Rx, and Non-H30 Isolates Stratified by Extended-Spectrum Cephalosporin Resistance Status
| Characteristic | ESC-R (n = 278) | ESC-S (n = 1008) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H30 (n = 83) | P Value vs Non-H30a | H30 (n = 47) | P Value vs Non-H30a | |||||||
| Rx (n = 64) | Non-Rx (n = 19) | Non-H30 (n = 195) | Rx | Non-Rx | Rx (n = 5) | Non-Rx (n = 42) | Non-H30 (n = 961) | Rx | Non-Rx | |
| Co-resistance | ||||||||||
| Ciprofloxacin | 62 (96.9) | 18 (94.7) | 76 (39.0) | <.001* | <.001* | 5 (100) | 36 (85.7) | 25 (2.6) | <.001* | <.001* |
| Gentamicin | 28 (43.8) | 6 (31.6) | 73 (37.4) | .453 | .798 | 0 (…) | 13 (31.0) | 34 (3.5) | 1.00 | <.001* |
| TMP/SMX | 43 (67.2) | 15 (78.9) | 121 (62.1) | .555 | .226 | 1 (20.0) | 26 (61.9) | 240 (25.0) | 1.00 | <.001* |
| TMP/SMX & ciprofloxacin | 41 (64.1) | 15 (78.9) | 64 (32.8) | <.001* | <.001* | 1 (20.0) | 23 (54.8) | 15 (1.6) | .080 | <.001* |
| All 3 | 19 (29.7) | 5 (26.3) | 36 (18.5) | .084 | .374 | 0 (…) | 8 (19.0) | 2 (0.2) | 1.00 | <.001* |
| ESC-R type | <.001* | .007* | ||||||||
| ESBL only | 64 (100)c | 17 (89.5) | 102 (52.6) | … | … | … | … | … | ||
| AmpC only | 0 (…) | 2 (5.4) | 88 (45.4) | … | … | … | … | … | ||
| ESBL & AmpC | 0 (…) | 0 (…) | 4 (2.06) | … | … | … | … | … | ||
| Undetermined | 0 (…) | 0 (…) | 1 (0.5) | … | … | … | … | … | ||
| ESBL determinantsc | n = 64 | n = 17 | n = 106 | |||||||
| CTX-M-15 | 60 (93.8)b | 3 (17.6) | 48 (45.3) | <.001* | .060 | … | … | … | … | … |
| CTX-M-14 | 0 (…) | 2 (11.8) | 44 (41.5) | <.001* | .037* | … | … | … | … | … |
| CTX-M-27 | 1 (1.6) | 10 (58.8) | 1 (0.9) | 1.000 | <.001* | … | … | … | … | … |
| CTX-M others | 0 (…) | 1 (5.3) | 7 (6.6) | .046* | 1.000 | … | … | … | … | … |
| ESBL SHV | 0 (…) | 0 (…) | 3 (2.8) | .292 | 1.000 | … | … | … | … | … |
| ESBL TEM | 0 (…) | 0 (…) | 0 (…) | … | … | … | … | … | … | … |
| None identified | 3 (4.7) | 1 (5.3) | 4 (3.8) | 1.000 | .531 | … | … | … | … | … |
| AmpC determinantsc | n = 0 | n = 2 | n = 92 | |||||||
| CMY-2 | … | 1 (50.0) | 79 (96.3) | … | .277 | … | … | … | … | … |
| DHA | … | 0 (…) | 2 (2.2) | … | 1.000 | … | … | … | … | … |
| FOX | … | 0 (…) | 2 (2.2) | … | 1.000 | … | … | … | … | … |
| None identified | … | 1 (50.0) | 10 (10.9) | … | 1.000 | … | … | … | … | … |
Abbreviations: AmpC, AmpC-type-beta-lactamase; ESBL, extended-spectrum β-lactamase; ESC-R, extended-spectrum cephalosporin-resistant; ESC-S, extended-spectrum cephalosporin-susceptible; TMP/SMX, trimethoprim/sufamethoxazole.
a P values generated via χ2 test; Fisher exact test was used when expected frequencies were <5.
bOne of these isolates had both a CTX-M-15 gene identified as well as a KPC-3 carbapenemase gene, and was resistant to meropenem.
cTotal exceeds 100% as isolates could have >1 determinant identified.
*P value <.05.
Among the 1008 ESC-S isolates examined, fluoroquinolone nonsusceptibility was dominant among H30 isolates, while only a small fraction of non-H30 ESC-S isolates were nonsusceptible to fluoroquinolones (Table 4).
DISCUSSION
We utilized a multiyear, multicenter case-control study of extraintestinal E. coli infections in children’s hospitals to address a critical knowledge gap about the epidemiology of the globally important ST131-H30 subclone among US children. Our results can be summarized into 3 main findings. First, the estimated prevalence of H30 among pediatric extraintestinal E. coli isolates of 5.3% was lower than the 10%–20% that has been observed in US adults [6, 14, 15]. However, H30 was nearly as dominant among ESBL-producing isolates in children (43.3%) as has been reported in adults (about 50%) [16, 17]. Second, patient age was associated with infection due to H30, and the nature of this association contrasted sharply between ESC-R and ESC-S infections. Among ESC-R infections, H30 was associated with young age (≤5 years), whereas among ESC-S infections, H30 was associated with older age (6–21 years), as well as with the presence of an underlying medical condition. Third, the antimicrobial resistance characteristics of H30 and H30Rx collected from children were consistent with what has been previously reported [12, 16–18, 24]. ESC-R H30 isolates were almost always fluoroquinolone-resistant and ESBL-producing, and ESBL-producing H30Rx isolates were associated with the CTX-M-15 β-lactamase, while ESBL-producing H30-non-Rx isolates were associated with the CTX-M-27 β-lactamase.
Other studies have suggested that H30 is less prevalent among children than adults; however, very few pediatric isolates were included in these studies [15, 16]. Interestingly, we observed that H30 was nearly as dominant among ESBL-producing E. coli infections in children as has been reported in adults [16, 17]. These findings are consistent with a recent study from a pediatric setting conducted in the Midwestern United States [25]. However, in the context of all clinical extraintestinal E. coli infections, ESBL-producing organisms are still relatively rare in both adults and children. The bulk of the H30 isolates circulating in the population are non-ESBL-producing but fluoroquinolone-resistant, and these isolates were much less common in our study than has been observed in adult populations [15, 16]. This observation may be explained by differential antibiotic use in these populations. Fluoroquinolones are infrequently prescribed to children due to concerns about toxicity [26]; in our study, about 5% of patients received fluoroquinolones in the year before collection of their first isolate, while 46% of patients received any antibiotic in that same time period (Supplementary Figure 6). Lower rates of fluoroquinolone use likely translate to less selective pressure on fluoroquinolone-resistant organisms such as H30. Interestingly, a recent study conducted in adults in Australia and New Zealand, a population that also has low rates of fluoroquinolone use, reported an overall prevalence of H30 of 3.5%, but a prevalence of H30 among ESC-R E. coli of 39%, which is similar to our findings [27].
The association we identified between H30 and young age among ESC-R isolates is consistent with the findings of a recent longitudinal study showing that among children, the prevalence of ESBL-producing Enterobacteriaceae was highest and increasing most rapidly in children aged 1–5 years [28]. Why H30/H30Rx is more frequently found among young children with ESC-R infections compared to older children with ESC-R infections, as well as where young children are acquiring this pathogen, deserves further investigation. Previous studies have portrayed H30 as an opportunistic pathogen that favors compromised hosts including the elderly [14], and young children’s developing immune systems could be associated with H30 infection. Maternal infection or colonization may also play a role; a recent study found H30 colonization during the first several years of life of healthy twins was associated with the mother also being colonized; however, none of these H30 isolates were ESBL-producing [29]. Finally, while transmission of H30 between children within healthcare facilities has not been documented, there are reports of transmission of, and persistent colonization with, H30/H30Rx among healthy children within daycares and households [29–32]. Future studies might focus on systematic sampling in the community setting to better elucidate the reservoirs and transmission dynamics of H30/H30Rx among young children.
The association we observed between ESC-S H30 infections and older children is not consistent with the limited existing data [15, 33]. Our post hoc interaction analyses suggest that age and underlying illness interact, with the strongest risk of an infection being H30 observed in older children with underlying medical conditions. We hypothesize that these observed associations could be driven by different selective pressures in older, less healthy children: specifically, fluoroquinolones are likely prescribed more frequently to older children than younger children due to less concern about toxicity. This prescribing pattern was borne out in our data; the median age was 12.6 years among patients who received fluoroquinolones in the year prior to their infection, whereas the median age among those that received any antibiotic was 6 years (Supplementary Figure 6). A more refined examination of the role of antibiotic exposure, specifically focusing on fluoroquinolones, is warranted.
Notably, previous studies conducted in adult populations have described H30 as being associated with healthcare contact and compromised hosts [14, 15]; however, we found those associations only among ESC-S H30 infections. The fact that we observed these patterns among ESC-S isolates is not surprising; compromised hosts and healthcare contact are consistently associated with antimicrobial resistant infections [34], and as is shown in Table 4, H30 isolates are more antimicrobial-resistant than other ESC-S isolates. However, we observed that when compared to other ESC-R organisms, there is no evidence of an association between H30 and underlying illness. This observation raises the question of whether some host correlates observed in previous studies are specific to the H30 subclone, or just reflect risk factors for MDR extraintestinal E. coli in general. Future studies should consider comparing H30 to other MDR E. coli where possible.
A number of limitations need to be considered in the interpretation of these data. First, because of the case-control design of the parent study, the prevalence of H30 and H30Rx among clinical E. coli isolates could not be calculated directly. However, we believe the assumptions employed in our prevalence estimates are reasonable and that these data provide the best estimate of the prevalence of H30 in children to date. The design of the parent study was also a strength, as it allowed us to enrich the collection with the less common MDR isolates and examine risk factors for infection with H30 among those with ESC-R E. coli isolates specifically. Second, because this study was an exploratory investigation of an existing dataset, all findings should be interpreted cautiously; there could be residual confounding due to unmeasured or incompletely measured variables, spurious associations identified due to multiple testing, or missed associations due to lack of power. To mitigate this, we attempted to make thoughtful model building decisions and interpretations by using conceptual models rather than taking a purely data-driven approach. Third, the isolates did not undergo multilocus sequence typing (MLST) or other molecular characterization relevant to H30 such as typing of the gyrA and parC alleles. However, the H30 isolates in this study have since undergone whole-genome sequencing, and in silico MLST analyses have confirmed that isolates classified as H30 are ST131 (data not shown). Finally, although this was a multicenter study, our data were collected from freestanding children’s hospitals between 2009 and 2013, so the results may not be generalizable to other settings, and epidemiologic patterns may have shifted during the subsequent several years. Despite these limitations, this study significantly improves our understanding of the impact of H30 in children, and is one of the most robust examinations of the clinical burden of, and risk factors for, H30 infections to date.
CONCLUSIONS
Although E. coli ST131-H30 is not as prevalent among children as has been reported in adults, perhaps as a result of low rates of fluoroquinolone use in pediatrics, this clone is dominant among ESC-R extraintestinal E. coli infections in children. In particular, ESBL-producing H30 appears to disproportionately affect young children relative to other ESC-R E. coli, even when accounting for other underlying host factors. More densely sampled studies are needed to elucidate the reservoirs and transmission dynamics of this difficult-to-treat pathogen in a pediatric population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Carey-Ann Burnham, Alexis Elward, Jason Newland, Rangaraj Selvarangan, Kaede Sullivan, Theoklis Zaoutis, and Xuan Qin for their provision of bacterial isolates and associated clinical data; and Jeff Myers and Huxley Smart for their assistance with molecular typing of isolates.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH via the National Institute of Allergy and Infectious Diseases (grant number R01AI083413), and the National Center for Advancing Translational Sciences (grant number TL1TR000422).
Potential conflicts of interest. E. V. S and V. T. have patent applications to detect E. coli strains. E. V. S. is a major shareholder in IDGenomics, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Antimicrobial resistance: global report on surveillance. Available at: http://www.who.int/drugresistance/documents/surveillancereport/en/. Accessed 15 April 2015.
- 2. Johnson JR, Nicolas-Chanoine MH, Deb Roy C et al. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967–2009. Emerg Infect Dis 2012; 18:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 2008; 61:273–81. [DOI] [PubMed] [Google Scholar]
- 4. Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 2010; 51:286–94. [DOI] [PubMed] [Google Scholar]
- 5. Mathers AJ, Peirano G, Pitout JD. Escherichia coli ST131: the quintessential example of an international multiresistant high-risk clone. Adv Appl Microbiol 2015; 90:109–54. [DOI] [PubMed] [Google Scholar]
- 6. Johnson JR, Tchesnokova V, Johnston B et al. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J Infect Dis 2013; 207:919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Price LB, Johnson JR, Aziz M et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. Mbio 2013; 4:e00377–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petty NK, Ben Zakour NL, Stanton-Cook M et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A 2014; 111:5694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson JR, Porter S, Thuras P, Castanheira M. Epidemic emergence in the United States of Escherichia coli sequence type 131-H30, 2000–2009. Antimicrob Agents Chemother 2017. doi:10.1128/AAC.00732-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stoesser N, Sheppard AE, Pankhurst L et al. Modernizing Medical Microbiology Informatics Group (MMMIG) Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. MBio 2016; 7:e02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben Zakour NL, Alsheikh-Hussain AS, Ashcroft MM et al. Sequential acquisition of virulence and fluoroquinolone resistance has shaped the evolution of Escherichia coli ST131. Mbio 2016; 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumura Y, Pitout JD, Gomi R et al. Global Escherichia coli sequence type 131 clade with blactx-M-27 gene. Emerg Infect Dis 2016; 22:1900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baquero F, Tedim AP, Coque TM. Antibiotic resistance shaping multi-level population biology of bacteria. Front Microbiol 2013; 4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson JR, Thuras P, Johnston BD et al. The pandemic H30 subclone of Escherichia coli sequence type 131 is associated with persistent infections and adverse outcomes independent from its multidrug resistance and associations with compromised hosts. Clin Infect Dis 2016; 62:1529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banerjee R, Johnston B, Lohse C et al. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrob Agents Chemother 2013; 57:5912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drawz SM, Porter S, Kuskowski MA et al. Variation in resistance traits, phylogenetic backgrounds, and virulence genotypes among Escherichia coli clinical isolates from adjacent hospital campuses serving distinct patient populations. Antimicrob Agents Chemother 2015; 59:5331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banerjee R, Robicsek A, Kuskowski MA et al. Molecular epidemiology of Escherichia coli sequence type 131 and its H30 and H30-Rx subclones among extended-spectrum-β -lactamase-positive and -negative E. coli clinical isolates from the Chicago region, 2007 to 2010. Antimicrob Agents Chemother 2013; 57:6385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peirano G, Pitout JD. Fluoroquinolone-resistant Escherichia coli sequence type 131 isolates causing bloodstream infections in a Canadian region with a centralized laboratory system: rapid emergence of the H30-Rx sublineage. Antimicrob Agents Chemother 2014; 58:2699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 2010; 126:1084–91. [DOI] [PubMed] [Google Scholar]
- 20. Jacobson SH, Eklöf O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ 1989; 299:703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zerr DM, Miles-Jay A, Kronman MP et al. Previous antibiotic exposure increases risk of infection with extended-spectrum-β-lactamase- and ampc-producing Escherichia coli and Klebsiella pneumoniae in pediatric patients. Antimicrob Agents Chemother 2016; 60:4237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Das S, Adler AL, Miles-Jay A et al. Antibiotic prophylaxis is associated with subsequent resistant infections in children with an initial extended-spectrum cephalosporin-resistant Enterobacteriaceae infection. Antimicrob Agents Chemother 2017; 61:e02656–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weissman SJ, Johnson JR, Tchesnokova V et al. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl Environ Microbiol 2012; 78:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olesen B, Frimodt-Moller J, Leihof RF et al. Temporal trends in antimicrobial resistance and virulence-associated traits within the Escherichia coli sequence type 131 clonal group and its H30 and H30-Rx subclones, 1968 to 2012. Antimicrob Agents Chemother 2014; 58:6886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Logan LK, Hujer AM, Marshall SH et al. Analysis of β-lactamase resistance determinants in Enterobacteriaceae from Chicago children: a multicenter survey. Antimicrob Agents Chemother 2016; 60:3462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradley JS, Jackson MA; Committee on Infectious Diseases; American Academy of Pediatrics The use of systemic and topical fluoroquinolones. Pediatrics 2011; 128:e1034–45. [DOI] [PubMed] [Google Scholar]
- 27. Rogers BA, Ingram PR, Runnegar N et al. ASID CRN Sequence type 131 fimH30 and fimH41 subclones amongst Escherichia coli isolates in Australia and New Zealand. Int J Antimicrob Agents 2015; 45:351–8. [DOI] [PubMed] [Google Scholar]
- 28. Logan LK, Braykov NP, Weinstein RA, Laxminarayan R. Extended-spectrum β-lactamase-producing and third-generation cephalosporin-resistant Enterobacteriaceae in children: trends in the United States, 1999–2011. J Pediatric Infect Dis Soc 2014; 3:320–8. [DOI] [PubMed] [Google Scholar]
- 29. Gurnee EA, Ndao IM, Johnson JR et al. Gut colonization of healthy children and their mothers with pathogenic ciprofloxacin-resistant Escherichia coli. J Infect Dis 2015; 212:1862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madigan T, Johnson JR, Clabots C et al. Extensive household outbreak of urinary tract infection and intestinal colonization due to extended-spectrum β-lactamase-producing Escherichia coli sequence type 131. Clin Infect Dis 2015; 61:e5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blanc V, Leflon-Guibout V, Blanco J et al. Prevalence of day-care centre children (France) with faecal CTX-M-producing Escherichia coli comprising o25b:H4 and O16:H5 ST131 strains. J Antimicrob Chemother 2014; 69:1231–7. [DOI] [PubMed] [Google Scholar]
- 32. Johnson JR, Davis G, Clabots C et al. Household clustering of Escherichia coli sequence type 131 clinical and fecal isolates according to whole genome sequence analysis. Open Forum Infect Dis 2016; 3:ofw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 2013; 34:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.