Summary
Influenza vaccination during pregnancy decreased the incidence of acute lower respiratory tract infection hospitalizations in infants born to vaccinated mothers. The benefits of protecting against influenza virus infection during early infancy might extend beyond protecting only against influenza-confirmed illness.
Keywords: influenza vaccine, efficacy, phase 3 trial, lower respiratory tract infections, hospitalizations
Abstract
Background
Influenza immunization of pregnant women protects their young infants against laboratory-confirmed influenza infection. Influenza infection might predispose to subsequent bacterial infections that cause severe pneumonia. In a secondary analysis of a randomized clinical trial (RCT), we evaluated the effect of maternal vaccination on infant hospitalizations for all-cause acute lower respiratory tract infection (ALRI).
Methods
Infants born to women who participated in a double-blind placebo-controlled RCT in 2011 and 2012 on the efficacy of trivalent inactivated influenza vaccine (IIV) during pregnancy were followed during the first 6 months of life.
Results
The study included 1026 infants born to IIV recipients and 1023 born to placebo recipients. There were 52 ALRI hospitalizations (median age, 72 days). The incidence (per 1000 infant-months) of ALRI hospitalizations was lower in infants born to IIV recipients (3.4 [95% confidence interval {CI}, 2.2–5.4]; 19 cases) compared with placebo recipients (6.0 [95% CI, 4.3–8.5]; 33 cases) with a vaccine efficacy of 43.1% (P = .050). Thirty of the ALRI hospitalizations occurred during the first 90 days of life, 9 in the IIV group (3.0 [95% CI, 1.6–5.9]) and 21 in the placebo group (7.2 [95% CI, 4.7–11.0]) (incidence rate ratio, 0.43 [95% CI, .19–.93]) for a vaccine efficacy of 57.5% (P = .032). The incidence of ALRI hospitalizations was similar in the IIV and placebo group for infants >3 months of age. Forty-four of the hospitalized infants were tested for influenza virus infection and 1 tested positive.
Conclusions
Using an RCT as a vaccine probe, influenza vaccination during pregnancy decreased all-cause ALRI hospitalization during the first 3 months of life, suggesting possible protection against subsequent bacterial infections that influenza infection might predispose to.
Clinical Trial Registration
Acute lower respiratory infections (ALRIs), such as pneumonia and bronchiolitis, are important causes of morbidity and mortality in children. Although the incidence of childhood ALRI mortality has declined in the past decade, an estimated 0.9 million children younger than 5 years died from pneumonia in 2015, including 0.5 million child pneumonia deaths in sub-Saharan Africa [1]. Furthermore, there were approximately 15 million episodes of under-5 childhood pneumonia hospitalizations in 2010, with the incidence of hospitalization being >3 times higher in newborns and almost 1.3 times higher in the 0- to 11-month age group compared with the overall rate in children 0–59 months of age [2].
Temporal associations between influenza virus circulation and pneumonia hospitalization, which might be due to primary viral or secondary bacterial pneumonia, have been reported [3–5]. A large study in the United States compared the rates of hospitalization for acute cardiopulmonary conditions during influenza seasons to noninfluenza winter time periods and estimated the average annual hospitalization rate attributable to influenza to be highest for infants <6 months of age (104 hospitalizations/10000 children), compared with children 6–12 months and 1–3 years of age (50/10000 and 19/10000, respectively) [6]. Several lines of evidence support a pathogenic synergism between influenza virus and respiratory bacteria. Influenza infections in children increases the risk of new Streptococcus pneumoniae serotype acquisition; and because nasopharyngeal acquisition of a new serotype increases the risk of pneumococcal diseases, influenza infections may therefore predispose to the development of bacterial diseases [7, 8].
While active influenza vaccination is the most efficient way to prevent influenza virus infection, current vaccines are poorly immunogenic and not licensed for use in infants <6 months of age. An alternative strategy to prevent influenza illness in young infants is vaccination of pregnant women to achieve passive protection through transplacental transfer of antibodies [9–11]. Vaccination of pregnant women with inactivated influenza vaccine (IIV) is immunogenic and protects the women and their infants against influenza illness [9–11]. Recently we also reported that efficacy of IIV vaccination during pregnancy on preventing polymerase chain reaction (PCR)–confirmed influenza infection in the infants was 86% during the first 8 weeks of life, which decreased to 49% if considering the overall 6-month follow-up period, corresponding to the reduction in maternally acquired influenza antibodies in the infants [12].
Population-based and case-control studies on the effect of influenza vaccination during pregnancy on more severe outcomes in infants, including hospitalizations, have reported conflicting results [13–19]. The objective of this post hoc analysis of a randomized, placebo-controlled trial was to probe the association of influenza vaccination of human immunodeficiency virus (HIV)–uninfected pregnant women in preventing ALRI hospitalization in their infants <6 months of age [10]. The present analysis was restricted to infants born to women enrolled in the HIV-uninfected cohort.
SUBJECTS AND METHODS
Study Design
Details of the study have been published [10]. In brief, we conducted a randomized, double-blind, placebo-controlled trial of IIV in HIV-uninfected pregnant women in Soweto, South Africa. This involved 2 cohorts of HIV-uninfected pregnant women in their second/third trimester who were enrolled from 3 March to 4 August 2011 (n = 1060) and 6 March to 2 July 2012 (n = 1056). Women and their infants were followed up to 6 months postpartum. The present report describes results from the infants born to the mothers enrolled in the study. The study used the trivalent IIV recommended by the World Health Organization for the Southern Hemisphere for both the 2011 and 2012 influenza seasons (A/California/7/2009, A/Victoria/210/2009 and B/Brisbane/60/2008-like virus; VAXIGRIP; Sanofi-Pasteur, Lyon, France) [20, 21], and sterile 0.9% normal saline solution as placebo (1:1).
Surveillance of Participants
Active surveillance for acute respiratory illness was done by weekly contact of the study participants throughout the study period. Nasopharyngeal aspirates were collected when infants (i) attended the study center for any unsolicited respiratory illness; (ii) were hospitalized for acute cardiopulmonary illness at the single public hospital serving the study population; or (iii) were identified through weekly contacts as having signs or symptoms of respiratory illness. Nasopharyngeal aspirates were collected in universal transport medium (Copan, Brescia, Italy) and transported to the study laboratory where samples were immediately tested by qualitative real-time reverse transcription PCR assay for influenza; the remaining specimens were stored and tested at a later stage for Bordetella pertussis, respiratory syncytial virus (RSV), and rhinovirus by PCR.
All-cause hospital admissions were documented and for the current analysis, hospitalizations for ALRI were defined as a discharge with a principal or secondary diagnosis with an International Classification of Diseases, Tenth Revision (ICD-10) codes of 1 or more of the following: pneumonia (J12–J18), bronchiolitis (J21), or an unspecified acute lower respiratory tract infection (J22). To account for misclassification of congenital pneumonia and other causes of respiratory distress during the newborn period, only hospitalized infants older than 7 days were included in the analyses. The diagnosis of neonatal jaundice, unspecified (P59.9) was analyzed as an outcome that should be unaffected by vaccine exposure. Bacterial cultures from a normally sterile site (eg, cerebrospinal fluid [CSF], blood, or pleural fluid) were performed at the discretion of the attending physician and results were available to the study team.
Statistical Analysis
All analyses of study outcomes were performed under the principle of intention-to-treat, defined as infants born any time after their mothers’ vaccination. A respiratory pathogen was associated with a hospitalization if it was detected by PCR on a respiratory specimen collected within 2 weeks of the date of hospital admission.
Incidence rates of ALRI hospitalizations were calculated as incidence density using Poisson regression and person-time as denominator; incidence rate ratios were estimated between the IIV group and placebo group. Time-to-event data were censored after the first ALRI hospitalization or at study termination (maximum 175 days of age). Between-group differences in the time to the ALRI episode were compared in survival analyses by means of the log-rank test. The South African influenza seasons were defined using the National Institute for Communicable Diseases surveillance data; the influenza epidemic periods were from 16 May to 6 November 2011 and from 21 May to 14 October 2012 [10, 22]. An exploratory analysis was done restricted to the hospitalizations that occurred within the influenza seasons ±2 weeks (extended influenza season period) to account for possible events that lag in time to influenza virus infection. Proportions were compared by χ2 or Fisher exact test and demographic continuous variables by Student t test or Mann-Whitney test. Logistic regression was performed to adjust the analysis for sex and low birth weight. P values ≤ .05 were considered significant. Study data were collected and managed using Research Electronic Data Capture (REDCap). Analyses were performed using Stata version 13.1 (StataCorp, College Station, Texas).
Ethical Considerations
The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (101106), was registered at ClinicalTrials.gov (NCT01306669), and was conducted in accordance with Good Clinical Practice guidelines. Mothers provided written informed consent for themselves and their infants.
RESULTS
A total of 2049 live births were recorded from pregnant women enrolled in the study, including 1026 to IIV recipients and 1023 to placebo recipients (Table 1). Infants were born a mean of 81 days (range, 1–175 days) after maternal vaccination and followed up for a median of 172 days (interquartile range [IQR], 168–175). The baseline demographics and follow-up period were similar between infants in the IIV and placebo groups (Table 1). Due to the primary objective of the study and when enrollment occurred, most of the follow-up time of the infants on the study was during the periods of extended influenza circulation, with an overall 11201 months of follow-up, of which 6514 months were during the extended influenza seasons. Infants were particularly exposed to influenza virus during the first 90 days of life (5933 months of total follow-up, of which 4743 [80.4%] months were during the extended influenza seasons) compared to during 91–175 days of life (5268 months of total follow-up, of which 1771 [33.6%] months were during the extended influenza seasons).
Table 1.
Infant Characteristics
| Characteristic | IIV (n = 1026) | Placebo (n = 1023) |
|---|---|---|
| Girls, No. (%)a | 484 (47.2) | 484 (47.3) |
| Births <37 wk gestational age, No. (%) | 108 (10.5) | 96 (9.4) |
| Mean birth weight, kg (SD)b | 3.0 (0.5) | 3.1 (0.5) |
| Birth weight <2500 g, No. (%)b | 133 (13.0) | 122 (12.0) |
| Mean days between maternal vaccination and birth (SD) | 81.5 (35.3) | 80.6 (35.4) |
| Median days of follow-up (IQR) | 172 (168–175) | 172 (168–175) |
| Deaths, No. (%) | 15 (1.5) | 21 (2.1) |
Abbreviations: IIV, inactivated influenza vaccine; IQR, interquartile range; SD, standard deviation.
aInformation on sex missing in 1 infant in the IIV group.
bBirth weight information missing in 2 infants in the IIV group and 2 infants in the placebo group.
Three hundred fourteen infants had at least 1 hospital admission during the study follow-up period; of these, 151 were born to IIV recipients and 163 to placebo recipients (risk ratio, 0.92 [95% confidence interval {CI}, .75–1.1]; P = .42). Fifty-two infants at a median age of 72 days (IQR, 32–131) were hospitalized for physician-diagnosed ALRI during the follow-up period. Only 2 of the hospitalized infants with ALRI were born <28 days after their mothers were vaccinated (18 and 25 days, both to IIV recipients). Of the 52 hospitalized infants, 11.5% were born at <37 weeks of gestational age compared with 9.4% of the 1997 nonhospitalized infants (P = .67), 21.2% (11/52) had a birth weight of <2500 g compared with 12.2% (244/1993) of nonhospitalized infants (P = .055), and boys were more frequently hospitalized for ALRI (67.3% [35/52]) than girls (P = .033).
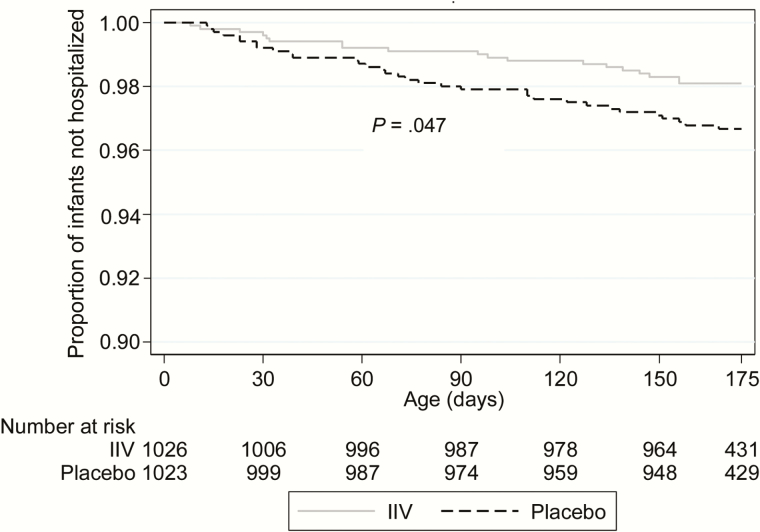
Of the 52 hospitalized infants with ALRI, 19 were born to IIV recipients and 33 to placebo recipients for a vaccine efficacy of 43.1% (incidence rate ratio [IRR], 0.57 [95% CI, .32–1.0]; P = .050; Table 2 and Figure 1). Time to ALRI hospitalization was longer in the IIV group compared with the placebo group (P = .047; Figure 1). Thirty (57.7%) ALRI hospitalizations occurred during the first 90 days of life, 9 in the IIV group (47.4%) and 21 in the placebo group (63.6%). The incidence (per 1000 infant-months) of ALRI hospitalization was lower in infants born to IIV recipients (3.0 [95% CI, 1.6–5.9]) compared to placebo recipients (7.2 [95% CI, 4.7–11.0]) (IRR, 0.43 [95% CI, .19–.93]; P = .032) for a vaccine efficacy of 57.5% during the first 90 days of life. In infants >90 days of age, the incidence (per 1000 infant-months) of ALRI hospitalization was similar between the IIV group (3.8 [95% CI, 2.0–7.1]) and placebo group (4.6 [95% CI, 2.6–8.1]) (IRR, 0.83 [95% CI, .36–1.9]; P = .66; Table 2). Similar estimates were obtained when restricting the ALRI hospitalizations to those that occurred during the extended influenza seasons (Table 2). Outside the influenza season period, although a similar trend was observed, numbers were too small for further stratification by age group.
Table 2.
Incidence Rates of Lower Respiratory Infection–Associated Hospitalizations by Study Group
| Outcome and Infant Age | IIV | Placebo | Incidence Rate Ratio (95% CI) | P Value | Adjusted Incidence Rate Ratiob (95% CI) | Adjusted P Valueb | ||
|---|---|---|---|---|---|---|---|---|
| No. | Rate (95% CI)a | No. | Rate (95% CI)a | |||||
| Overall study follow-up | ||||||||
| Infants ≤90 d | 9 | 3.0 (1.6–5.9) | 21 | 7.2 (4.7–11.0) | 0.43 (.19–.93) | .032 | 0.42 (.19–.92) | .030 |
| Infants 91–175 d | 10 | 3.8 (2.0–7.1) | 12 | 4.6 (2.6–8.1) | 0.83 (.36–1.9) | .66 | 0.82 (.36–1.9) | .65 |
| Infants ≤175 d | 19 | 3.4 (2.2–5.4) | 33 | 6.0 (4.3–8.5) | 0.57 (.32–1.0) | .050 | 0.56 (.32–.99) | .046 |
| During extended influenza seasonc | ||||||||
| Infants ≤90 d | 8 | 3.4 (1.7–6.9) | 15 | 6.3 (3.8–10.4) | 0.55 (.23–1.3) | .17 | 0.54 (.23–1.3) | .16 |
| Infants 91–175 d | 6 | 6.7 (3.0–14.9) | 6 | 6.9 (3.1–15.4) | 0.97 (.31–3.0) | .95 | 0.98 (.32–3.0) | .97 |
| Infants ≤175 d | 14 | 4.4 (2.6–7.4) | 21 | 6.5 (4.2–10.0) | 0.67 (.34–1.3) | .25 | 0.67 (.34–1.3) | .24 |
| Outside extended influenza season | ||||||||
| Infants ≤175 d | 5 | 2.1 (.88–5.1) | 12 | 5.2 (3.0–9.2) | 0.40 (.14–1.1) | .09 | 0.40 (.14–1.1) | .09 |
Abbreviations: CI, confidence interval; IIV, inactivated influenza vaccine.
aRates calculated as number of cases per 1000 infant-months, using person time between birth and event or end of study.
bAdjusted for birth weight <2500 g and sex.
cExtended influenza season was defined as the period between 2 weeks prior to the start of and 2 weeks after end of the epidemic influenza period each year.
Figure 1.
Kaplan-Meier survival curve showing cumulative proportion of infants without lower respiratory infection–associated hospitalizations during the follow-up period by study group. P values calculated by log-rank test. Abbreviation: IIV, inactivated influenza vaccine.
The median length of ALRI hospitalization was similar in the IIV group (4 [IQR, 2–8] days) and the placebo group (6 [IQR, 3–8] days; P = .64). Forty-four of the ALRI hospitalized infants (84.6%) had a respiratory sample collected during (n = 35) or within 5 days of hospitalization (n = 9) that were tested by PCR for influenza virus, of whom only 1 infant (71 days old in the placebo group) tested positive. Of these respiratory samples, 41 (93.2%) were available and further tested by PCR for Bordetella pertussis and RSV and 36 (81.8%) were tested for rhinovirus. Bordetella pertussis was identified in 3 infants (2 [11.8%] in the IIV group and 1 [4.2%] in the placebo group), RSV in 12 (4 [23.5%] in the IIV group and 8 [33.3%] in the placebo group; P = .73), and rhinovirus in 11 infants (3 [21.4%] in the IIV group and 8 [36.4%] in the placebo group; P = .47).
Of the 52 hospitalized ALRI cases, blood cultures were performed in 26 (50%) infants (57.9% of 19 cases in the IIV group and 45.5% of 33 in the placebo group) and CSF cultures in 17 (32.7%) infants (42.1% of the IIV group and 27.3% of the placebo group). No pathogenic bacteria were isolated from the blood or CSF specimens.
One infant in the IIV group who was diagnosed with suspected pertussis associated with pneumonia died in hospital at 61 days of age. Two infants in the placebo group died at home 11 days postdischarge at 84 and 173 days of age. During the first 90 days of life, 93 infants were hospitalized with jaundice; 47 born to IIV recipients and 46 to placebo recipients (IRR, 1.0 [95% CI, .68–1.5]; P = .92).
DISCUSSION
Using our randomized, placebo-controlled clinical trial as a vaccine probe, we demonstrate that vaccination with IIV during pregnancy reduced the risk for all-cause ALRI hospitalization by 57.5% during the first 90 days of life. Notably, this observation was independent of identifying influenza virus among the ALRI cases. This suggests that the benefits of protecting against influenza virus infection during early infancy might extend beyond protecting only against influenza-confirmed illness. While the paucity of laboratory-confirmed influenza hospitalizations may be partly explained by inadequate sample or imperfect test sensitivity, it is also likely that the influenza virus may initiate a causal chain of events and no longer be present or detectable at the time of hospitalization. For example, a primary influenza virus infection, including possibly subclinical or mild infection, may increase susceptibility to new bacterial nasopharyngeal acquisition, as well as increase density of present colonizing bacteria, with disease from these bacteria only manifesting a few weeks later and beyond when influenza virus shedding has cased [3, 23]. This hypothesis is corroborated by a previous report from a randomized clinical trial (RCT) of pneumococcal conjugate vaccine, in which was shown that by protecting against pneumococcal disease, there was also a decrease in hospitalizations for influenza, RSV, human metapneumovirus, and polyomavirus-associated ALRI [24–26]. The current data suggest that protecting against these virus-associated infections could conversely protect against bacterial infections. The failure to identify bacterial etiology among our cases is not surprising, considering the lack of a sensitive diagnostic tool to diagnose bacterial pneumonia, including the sensitivity of blood culture only being <5% for diagnosing bacterial pneumonia in children [27].
Epidemiological evidence from influenza epidemics and animal challenge models have demonstrated that influenza virus infection can enhance the susceptibility to infection with bacteria [28, 29], including Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus [23, 30–32]. In a South African study among patients hospitalized with ALRI, infection with influenza virus was associated with S. pneumoniae colonization and patients with a respiratory virus coinfection had significantly higher colonization densities and, consecutively, increased risk of invasive pneumococcal pneumonia [33]. The co-pathogenesis between influenza and superinfecting bacteria is complex and multifactorial, with sequential infections being challenging to correctly diagnose if the first pathogen has cleared by the time the patient presents with secondary bacterial complications [3]. In the case of S. pneumoniae, a new serotype acquisition is associated with increased susceptibility to developing disease up until 2 months later, by which time influenza shedding may no longer be present [8]. Likewise, it is biologically plausible that a preceding influenza infection could lead to airway changes that predispose to more severe disease with subsequent infections as well.
Using a vaccine-probe approach, we estimated that for every 1000 pregnant women vaccinated with influenza vaccine, 4 ALRI hospitalizations could be prevented in infants <3 months of age. Two-thirds of the ALRI hospitalizations in the placebo group occurred during the first 3 months of life, corroborating that very young infants are at increased risk of severe pneumonia [6, 34]. The observation from our trial that even in older infants, who had less exposure to the influenza season, >50% of the hospitalizations occurred during the periods of influenza circulation supports the temporal association of influenza virus infections and ALRI hospitalizations.
This is the first RCT, to our knowledge, which has measured the efficacy of influenza vaccination during pregnancy on severe respiratory outcomes in infants. Two earlier large retrospective cohort studies from the United States did not find an association of influenza vaccination during pregnancy and the rates of acute respiratory illness among their infants [14, 17]. These studies were, however, limited either by the study design or the absolute rates of hospitalization in the infants being very low, limiting the statistical power [14, 17]. In another prospective cohort study in the White Mountain and Navajo reservations in the United States, Eick et al reported a nonsignificant association between maternal influenza vaccination and influenza-like illness in the infants; however, when the outcome was restricted to influenza-like illness requiring hospitalization, a 39% reduction in risk (risk ratio, 0.61 [95% CI, .45–.84]) was found for infants born to IIV-vaccinated compared with unvaccinated mothers [16]. In accordance, although we demonstrate here the efficacy of IIV vaccination during pregnancy against infant ALRI hospitalization, we did not observe a significant effect on medically attended outpatient visits at our study clinic [10]. Furthermore, a recent retrospective cohort study from Australia also described that influenza vaccination during pregnancy was associated with a 25% reduction (adjusted hazard ratio, 0.75 [95% CI, .56–.99]) in hospitalizations for any acute respiratory illness in infants <6 months of age during influenza season [19]. Additionally, 3 other observational studies found that influenza vaccination during pregnancy was associated with a 48%–91% reduced risk of laboratory-confirmed influenza hospitalizations in infants <6 months of age [13, 15, 18].
The lack of tools to adequately investigate for bacterial pneumonia in infants and the fact that chest radiograph information was not available for this study to use as a proxy for bacterial pneumonia, albeit it being a more specific than sensitive marker for pneumococcal pneumonia, are limitations of our study [27]. Furthermore, not all infants were investigated for evidence of influenza virus infection at the time of hospitalization.
As influenza virus infection can be asymptomatic and the virus might be undetectable by the time the patient presents for care, even if the virus was part of the causal pathway of infection, we used the randomized placebo-controlled design of our trial as a vaccine probe to delineate the effect of maternal influenza vaccination on all-cause ALRI hospitalization in infants [35]. This type of analysis helps to more comprehensively describe the disease burden than simple etiologic studies that may underestimate the real impact of influenza infection.
Notes
Acknowledgments. The authors thank all the study participants, the staff of the Departments of Obstetrics, Neonatology, and Paediatrics at Chris Hani Baragwanath Academic hospital, Soweto, South Africa, for their dedication to their patients, including our trial participants; and the study midwives, nurses, laboratory staff, counselors, and data capturers. We thank Niteen Wairagkar, program officer acting on behalf of the Bill & Melinda Gates Foundation, David Moore from Respiratory and Meningeal Pathogens Research Unit, and Jorge Vidal from Emory University.
Disclaimer. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of their institutions or organizations or of the sponsors. The funders did not participate in any aspect of the study, including study conduct, data collection, analyses of the data, or the write-up of the manuscript. J. R. O. is an employee of the World Health Organization.
Financial support. This study was supported by the Bill & Melinda Gates Foundation (grant number OPP1002747). There was also partial support from the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation in Vaccine Preventable Diseases; and the Medical Research Council Respiratory and Meningeal Pathogens Research Unit.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Liu L, Oza S, Hogan D et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nair H, Simões EA, Rudan I et al. ; Severe Acute Lower Respiratory Infections Working Group Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013; 381:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis 2012; 205:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shrestha S, Foxman B, Berus J et al. The role of influenza in the epidemiology of pneumonia. Sci Rep 2015; 5:15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain S, Williams DJ, Arnold SR et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225–31. [DOI] [PubMed] [Google Scholar]
- 7. Grijalva CG, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect Dis 2014; 58:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray BM, Converse GM 3rd, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 1980; 142:923–33. [DOI] [PubMed] [Google Scholar]
- 9. Zaman K, Roy E, Arifeen SE et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–64. [DOI] [PubMed] [Google Scholar]
- 10. Madhi SA, Cutland CL, Kuwanda L et al. ; Maternal Flu Trial (Matflu) Team Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371:918–31. [DOI] [PubMed] [Google Scholar]
- 11. Tapia MD, Sow SO, Tamboura B et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016; 16:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nunes MC, Cutland CL, Jones S et al. ; Maternal Flu Trial Team Duration of infant protection against influenza illness conferred by maternal immunization: secondary analysis of a randomized clinical trial. JAMA Pediatr 2016; 170:840–7. [DOI] [PubMed] [Google Scholar]
- 13. Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 2010; 51:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D; Vaccine Safety Datalink Workgroup Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol 2004; 21:333–9. [DOI] [PubMed] [Google Scholar]
- 15. Dabrera G, Zhao H, Andrews N et al. Effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants, England, 2013/14. Euro Surveill 2014; 19:20959. [DOI] [PubMed] [Google Scholar]
- 16. Eick AA, Uyeki TM, Klimov A et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011; 165:104–11. [DOI] [PubMed] [Google Scholar]
- 17. France EK, Smith-Ray R, McClure D et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med 2006; 160:1277–83. [DOI] [PubMed] [Google Scholar]
- 18. Poehling KA, Szilagyi PG, Staat MA et al. ; New Vaccine Surveillance Network Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204:S141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regan AK, de Klerk N, Moore HC, Omer SB, Shellam G, Effler PV. Effect of maternal influenza vaccination on hospitalization for respiratory infections in newborns: a retrospective cohort study. Pediatr Infect Dis J 2016; 35:1097–103. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization. Recommended viruses for influenza vaccines for use in the 2011 Southern Hemisphere influenza season. Geneva, Switzerland: WHO, 2010. Available at: http://www.who.int/ influenza/vaccines/ virus/recommendations/201009_Recommendation.pdf?ua=1. Accessed 7 June 2016. [Google Scholar]
- 21. VAXIGRIP. Inactivated influenza vaccine (split virion) product information. Lyon, France: Sanofi Pasteur, 2011. Available at: http://www.sanofipasteur.com/en. Accessed 7 June 2016. [Google Scholar]
- 22. National Institute for Communicable Diseases, National Health Laboratory Service Influenza surveillance report—South Africa. Available at: http://www.nicd.ac.za/?page=seasonal_influenza&id=72. Accessed 7 June 2016. [Google Scholar]
- 23. McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 2002; 186:341–50. [DOI] [PubMed] [Google Scholar]
- 24. Nunes MC, Kuschner Z, Rabede Z et al. Polyomaviruses-associated respiratory infections in HIV-infected and HIV-uninfected children. J Clin Virol 2014; 61:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madhi SA, Klugman KP; Vaccine Trialist Group A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004; 10:811–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madhi SA, Ludewick H, Kuwanda L et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis 2006; 193:1236–43. [DOI] [PubMed] [Google Scholar]
- 27. Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis 2005; 40:1511–8. [DOI] [PubMed] [Google Scholar]
- 28. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mina MJ, Klugman KP. The role of influenza in the severity and transmission of respiratory bacterial disease. Lancet Respir Med 2014; 2:750–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 2008; 14:558–64. [DOI] [PubMed] [Google Scholar]
- 31. Lee LN, Dias P, Han D et al. A mouse model of lethal synergism between influenza virus and Haemophilus influenzae. Am J Pathol 2010; 176:800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis 2011; 203:880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolter N, Tempia S, Cohen C et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis 2014; 210:1649–57. [DOI] [PubMed] [Google Scholar]
- 34. Rudan I, O’Brien KL, Nair H et al. ; Child Health Epidemiology Reference Group (CHERG) Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013; 3:010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feikin DR, Scott JA, Gessner BD. Use of vaccines as probes to define disease burden. Lancet 2014; 383:1762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]