Viral metagenomic surveillance of 187 patients with indeterminate acute liver failure (ALF) from 1998 to 2010 suggests that these patients should be screened for the presence of uncommon viruses and coinfections, although novel viruses associated with ALF were not identified.
Keywords: pathogen discovery, metagenomic next-generation sequencing, indeterminate ALF, viral hepatitis, SURPI computational pipeline
Abstract
Background
Twelve percent of all acute liver failure (ALF) cases are of unknown origin, often termed indeterminate. A previously unrecognized hepatotropic virus has been suspected as a potential etiologic agent.
Methods
We compared the performance of metagenomic next-generation sequencing (mNGS) with confirmatory nucleic acid testing (NAT) to routine clinical diagnostic testing in detection of known or novel viruses associated with ALF. Serum samples from 204 adult ALF patients collected from 1998 to 2010 as part of a nationwide registry were analyzed. One hundred eighty-seven patients (92%) were classified as indeterminate, while the remaining 17 patients (8%) served as controls, with infections by either hepatitis A virus or hepatitis B virus (HBV), or a noninfectious cause for their ALF.
Results
Eight cases of infection from previously unrecognized viral pathogens were detected by mNGS (4 cases of herpes simplex virus type 1, including 1 case of coinfection with HBV, and 1 case each of HBV, parvovirus B19, cytomegalovirus, and human herpesvirus 7). Several missed dual or triple infections were also identified, and assembled viral genomes provided additional information on genotyping and drug resistance mutations. Importantly, no sequences corresponding to novel viruses were detected.
Conclusions
These results suggest that ALF patients should be screened for the presence of uncommon viruses and coinfections, and that most cases of indeterminate ALF in the United States do not appear to be caused by novel viral pathogens. In the future, mNGS testing may be useful for comprehensive diagnosis of viruses associated with ALF, or to exclude infectious etiologies.
(See the Editorial Commentary by Fredricks on pages 1486–8.)
Acute liver failure (ALF) is a complex syndrome characterized by rapid deterioration of liver function with hepatic encephalopathy in the absence of a known history of previous liver disease. ALF may progress to multiorgan failure and results in death or transplantation in 57% of cases. Acetaminophen toxicity is the most common documented cause of ALF in the United States, comprising 50% of cases, while infections from hepatitis A virus (HAV) and hepatitis B virus (HBV) combined account for 12% [1]. After standard clinical testing, a clear etiology is not found in approximately 12% of US ALF cases, thus considered indeterminate; an even higher percentage of indeterminate cases (~20%) is seen in developing countries [2]. Infection by a previously unrecognized hepatotropic virus has been posited as a cause for indeterminate ALF [3]. Beyond HAV and HBV, other potential viral etiologies of ALF include hepatitis C virus (HCV), hepatitis E virus (HEV), adenovirus, herpes simplex virus type 1 (HSV-1), Epstein-Barr virus (EBV), varicella zoster virus, and parvovirus B19, all of which are rare causes of ALF in the United States [4–7]. Appropriate management of ALF depends on accurate diagnosis, as specific antiviral treatments are sometimes available, and other therapies may be indicated for noninfectious etiologies [7]. Thus, it is imperative to determine the limitations of conventional diagnostic testing for ALF, and whether novel or previously unrecognized viruses may be associated with this condition.
Metagenomic next-generation sequencing (mNGS) is a comprehensive approach for sequence-based identification of pathogenic microbes, especially viruses, in clinical samples [8, 9]. Previous studies have shown that mNGS is useful for viral surveillance [10, 11] and identification of novel viruses circulating in blood [12, 13]. The US Acute Liver Failure Study Group (ALFSG) is a nationwide study established in 1998 to enroll patients with ALF and collect detailed clinical and laboratory information and biosamples for analysis. We hypothesized that mNGS screening of serum samples from 187 indeterminate ALFSG cases from 1998 to 2010 would allow broader identification of infectious causes of indeterminate ALF.
METHODS
Study Patients
A total of 204 patients with ALF (187 patients with indeterminate ALF and 17 as controls) were selected from the ALFSG cohort (Supplementary Methods) to undergo further mNGS testing for viral infection. Clinical and laboratory data for the 204 ALF patients, including available liver biopsy data and history of injection drug and alcohol use, are provided in Supplementary Tables 1–4. The 187 indeterminate ALF cases were consecutive (collected during enrollment years 1998–2010), provided a broad geographic representation across all study sites in the United States, and comprised 61% of the overall cohort to date (n = 307). Notably, 11 of the indeterminate samples tested positive for viruses at the study site, including human immunodeficiency virus (HIV) (n = 2), chronic HCV (n = 3), mixed HBV, HCV, and hepatitis D virus (HDV) (n = 1), combined EBV and cytomegalovirus (CMV) (n = 1), and HBV (n = 4). The 17 control patient samples included 8 samples from ALF-associated hepatitis A or B cases (n = 4 each) and 9 samples corresponding to noninfectious negative control groups: acetaminophen (N-acetyl-para-aminophenol [APAP]) toxicity (n = 3), autoimmune hepatitis (n = 2), drug-induced liver injury (n = 3), and hepatic ischemia (n = 1).
Metagenomic Next-Generation Sequencing and Analysis
Serum samples from each patient were processed in a blinded fashion for metagenomic sequencing individually or in pools of 2–6 (Supplementary Table 5), using an approach demonstrating high sensitivity for unbiased virus detection [14]. Individual samples or pools were first treated with a cocktail of Turbo DNase (Ambion) and Baseline-ZERO DNase (Epicentre) prior to nucleic acid extraction. Pretreatment with DNase biases mNGS testing toward viral detection as microbial and human host DNA is depleted whereas encapsidated (“protected”) viral nucleic acid is preserved [15]. With analysis of serum, there is also a minor limitation with respect to potential decreased sensitivity of detection of integrated proviruses (eg, HIV type 1 [HIV-1]), episomal viruses (eg, herpesviruses), or strongly cell-associated viruses (eg, human T-cell lymphotropic virus 1) [16]. Nucleic acid extraction was performed on the Qiagen EZ1 Advanced XL automated system using the EZ1 Virus Mini Kit version 2.0 (Qiagen). Extracted nucleic acid was amplified with random hexamers to generate a complementary DNA (cDNA) library as previously described [17]. In brief, 23 pools and 12 individual samples were initially processed using a modified TruSeq protocol (Illumina) [18], and 116 individually prepared samples were later processed using the NexteraXT protocol (Illumina). Samples were sequenced on 10 Illumina HiSeq lanes, and sequencing reads were analyzed using sequence-based ultra-rapid pathogen identification (SURPI), a computational pipeline for comprehensive pathogen identification from mNGS data by comparison to microbial sequences in the National Center for Biotechnology Information (NCBI) nucleotide (NT) database [9]. SURPI+, the clinical version of SURPI used for analysis [18, 19], employs taxonomic classification according to a lowest common ancestor algorithm for more accurate read assignments. As the first set of TruSeq libraries used only single-end barcode indexes (vs paired-end barcodes for the NexteraXT libraries), a predetermined threshold of >25 reads and >2% genome coverage was used for assessment of positive viral signatures by mNGS in these single-end barcoded samples given the potential for cross-contamination. Detected reads from viruses of unknown pathogenicity that constitute part of the normal viral flora circulating in blood, such as anelloviruses and human pegivirus 1/GB virus C [20, 21], were not considered as causes of ALF (Supplementary Table 6). Manual analyses for bona fide reads corresponding to nonviral pathogens (bacteria, fungi, and parasites) revealed only the presence of known laboratory/environmental contaminants or false-positive identifications due to misannotations in the NCBI NT database.
Details regarding genome assembly, genotyping, and identification of resistance mutations, as well as sequencing data deposition into the NCBI Sequence Read Archive, are provided in the Supplementary Methods.
Confirmation by Research Nucleic Acid Testing
Individual samples or all samples comprising a pool were selected for research nucleic acid testing (NAT) confirmation if reads from a human viral pathogen (HAV, HBV, HCV, HDV, HEV, CMV, HIV, parvovirus B19, and human papillomavirus virus type 159 [HPV-159]) were detected by mNGS. Total nucleic acid was extracted using the Qiagen Ultrasens Virus Kit (Qiagen), followed by construction of cDNA libraries using random hexamers and Superscript III Reverse Transcriptase (Invitrogen). Libraries were screened by polymerase chain reaction (PCR) (Qiagen OneStep RT-PCR Kit) using previously published primer sets and conditions for detection of individual viruses (Supplementary Table 7).
Confirmation by Clinical Nucleic Acid Testing
In parallel, NAT was independently performed on 0.5 mL serum from samples testing positive by mNGS and with sufficient volume available using the Procleix Ultrio assay on the Tigris platform (Grifols Diagnostic Solutions and Hologic). This US Food and Drug Administration–approved clinical assay is employed in the United States and internationally for screening blood donors for simultaneous detection of HIV types 1/2 and HBV and HCV nucleic acids [22, 23]. The analytic sensitivities of Ultrio for detection of HIV, HCV, and HBV are 10–15 copies or IU/mL at a 95% lower limit of detection.
RESULTS
Clinical Testing
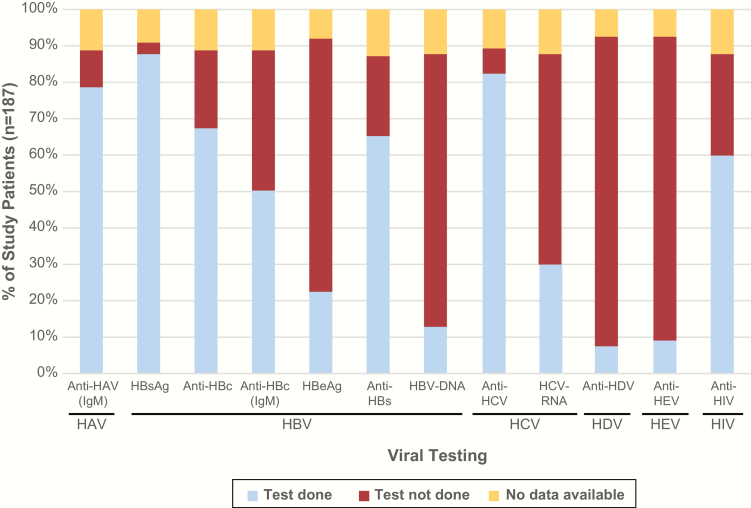
Serum samples from 204 patients were investigated in a blinded fashion using mNGS, of which 187 (92%) were classified as indeterminate, 8 (4%) were positive controls, and 9 (4%) were negative controls (Table 1). The extent of primary clinical testing for hepatitis viruses at the study sites was variable, with HCV serology and HBV antigen testing being performed >80% of the time, and HDV or HEV PCR testing <10% of the time (Figure 1).
Table 1.
Cause of Acute Liver Failure at Time of Hospital Discharge for Patients in the Study (N = 204)
| Cause of Acute Liver Failurea | Samples, No. (%) |
|---|---|
| Indeterminate | 187 (91.7) |
| Hepatitis A virus infectionb | 4 (2.0) |
| Hepatitis B virus infectionb | 4 (2.0) |
| Acetaminophen toxicityc | 3 (1.5) |
| Autoimmune hepatitisc | 2 (1.0) |
| Drug-induced hepatitisc | 3 (1.5) |
| Hepatic ischemiac | 1 (0.5) |
aAn “indeterminate” diagnosis is given to patients when the cause of acute liver failure is unclear at time of hospital discharge.
bViral positive control.
cNoninfectious negative control.
Figure 1.
Clinical diagnostic testing for viral infections in study patients with indeterminate acute liver failure. The percentage of patients in the study positive for a given viral infection (y-axis) is plotted against the specific laboratory test that was ordered by the clinical site (x-axis). Abbreviations: anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; HAV, hepatitis A virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; HIV, human immunodeficiency virus; IgM, immunoglobulin M.
Metagenomic Next-Generation Sequencing Results
Serum samples collected at the earliest available time point after clinical presentation of ALF were analyzed (median, 7 days [range, 2–38 days] from symptom onset to sample collection; Table 2). A total of 151 individual or pooled sample libraries from 204 serum samples were sequenced across 10 Illumina HiSeq lanes, yielding 1.5 billion raw sequence reads. After preprocessing to remove low-complexity and low-quality sequences, 84% of the remaining preprocessed reads were classified as human and 1.0% as viral by SURPI+ (Supplementary Table 8). The 12 million viral reads included canonical hepatitis viruses (HAV, HBV, HCV, and HDV) and additional viruses rarely associated with hepatitis, such as HSV-1 and parvovirus B19 (Table 2). Reads from anelloviruses and/or human pegivirus 1, considered nonpathogenic flora [20, 21], were identified in 135 of 151 (89.4%) sequencing libraries.
Table 2.
Acute Liver Failure Patients With Serum Positive for a Virus By Metagenomic Next-Generation Sequencing and/or Confirmatory Nucleic Acid Testing
| Patient ID | Initial Site Diagnosis | Clinical Site Testing | Illness Onset to Sample Collection for mNGS, d | mNGS Result | UCSF Lab Research NAT | Ultrio NAT | ALF Etiologyb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAV IgM | HBsAg | Anti-HBc | Anti-HBc IgM | HBeAg | Anti- HBs | HBV DNA | Anti- HDV | Anti- HCV | HCV RNA | Anti- HEV | Anti- HIV | |||||||
| 10–2006c | HAV | + | – | ND | – | ND | ND | ND | ND | – | ND | ND | ND | 9 | HAV + | HAV + | ND | HAV |
| 13–2207c | HAV | – | – | ND | – | ND | – | ND | ND | + | + | ND | – | 11 | HAV + | HAV + | ND | HAV |
| 15–2455a,c | HAV | + | – | – | – | – | – | – | – | – | ND | ND | – | 7 | HAV + | HAV + | ND | HAV |
| 23–2911c | HAV | + | – | – | ND | ND | – | ND | ND | – | ND | ND | – | 9 | HAV + | HAV + | ND | HAV |
| 14–2440c,d | Hepatitis B | – | + | + | + | + | – | + | ND | – | ND | ND | ND | 5 | HBV + | HBV + | ND | HBV |
| 18–2652c | Hepatitis B | – | + | + | + | + | – | + | – | – | – | ND | – | 5 | HBV – | HBV + | ND | HBV |
| 18–2653a,c | Hepatitis B | – | + | + | – | + | + | + | – | – | ND | + | ND | 21 | HBV + | HBV + | ND | HBV |
| 33–3251c | Hepatitis B | – | + | + | + | + | + | + | ND | – | ND | ND | – | 2 | HBV +, HSV-1 + | HBV +, HSV-1 + | ND | HBV and HSV-1 coinfection |
| 10–2053e,f | Indeterminateg | – | + | – | – | ND | – | ND | – | – | – | ND | – | 4 | HBV + | HBV + | + | HBV |
| 13–2383e,h | Indeterminateg | ND | + | + | – | ND | + | + | – | – | ND | ND | – | 7 | HBV + | HBV + | + | HBV |
| 21–2833e,i | Indeterminatej | – | – | ND | ND | ND | + | ND | ND | – | ND | ND | ND | 6 | HBV – | HBV + | ND | HBV |
| 35–3354e | Indeterminatek | – | + | – | + | ND | – | ND | ND | – | – | ND | ND | 5 | HBV + | HBV + | + | HBV |
| 11–2071e,l | Indeterminate | – | + | – | – | – | – | ND | + | + | + | ND | ND | 7 | HBV –, HCV +, HDV + | HBV +, HCV +, HDV + | + | HBV, HCV, HDV coinfection |
| 10–3875e,m | Indeterminate | – | – | ND | – | ND | – | ND | ND | + | + | – | – | 2 | HCV + | HCV + | + | Indeterminate |
| 38–3540e,n | Indeterminate | – | – | + | ND | – | ND | ND | ND | + | + | ND | – | 6 | HCV + | HCV + | ND | Indeterminate |
| 15–2479e,o | Indeterminate | ND | ND | – | – | ND | ND | ND | ND | + | ND | ND | ND | 4 | HCV + | HCV + | ND | Acetaminophen toxicity |
| 10–2010a,p | Indeterminate | – | – | ND | – | ND | – | ND | ND | – | – | ND | + | 38 | HIV – | HIV – | – | Indeterminate |
| 13–2273a,e,q | Indeterminate | – | – | ND | ND | ND | + | ND | ND | + | – | ND | + | 4 | HIV + | HIV + | ND | Indeterminate |
| 13–3941e,r | Indeterminate | – | – | – | ND | ND | – | ND | ND | – | ND | – | – | 7 | CMV –, EBV + | CMV –, EBV + | ND | Multifactorial: CMV, EBV, lymphoma |
| 37–4067 | Indeterminate | – | – | ND | – | ND | + | ND | ND | – | ND | ND | ND | 4 | HPV type 159 + | HPV type 159 + | – | Indeterminate |
| 13-2236s | Indeterminate | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 38 | CMV + | CMV + | ND | CMV |
| 20-2779t | Indeterminate | ND | – | – | – | ND | ND | ND | ND | – | – | ND | – | 18 | HHV-7 + | HHV-7 – | ND | Indeterminate |
| 21–2824 | Indeterminate | – | – | + | – | – | – | – | ND | – | ND | ND | – | 16 | HSV-1 + | HSV-1 + | ND | HSV-1 |
| 43–3569u | Indeterminate | – | – | ND | – | ND | ND | ND | ND | – | ND | ND | – | 3 | HSV-1 + | HSV-1 + | ND | HSV-1 |
| 43–3578u | Indeterminate | – | – | ND | ND | – | – | – | ND | – | – | ND | – | 10 | HSV-1 + | HSV-1 + | ND | HSV-1 |
| 18–2649 | Indeterminate | – | – | – | – | ND | ND | ND | ND | – | ND | ND | ND | 31 | B19V + | B19V + | ND | B19V |
Abbreviations: –, negative; +, positive; ALF, acute liver failure; anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; B19V, human parvovirus B19; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; HHV, human herpesvirus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSV, herpes simplex virus; IgM, immunoglobulin M; mNGS, metagenomic next-generation sequencing; NAT, nucleic acid testing; ND, not done; UCSF, University of California, San Francisco; Ultrio, Procleix Ultrio assay for simultaneous detection of HIV types 1/2 and HBV and HCV nucleic acids (Grifols Diagnostic Solutions and Hologic).
aImmunosuppressed patients, either HIV-positive (10–2010 and 13–2273) or currently being treated with steroids (15–2455) or steroids and mycophenolate mofetil (18–2653).
bAfter adjudication by Acute Liver Failure Study Group Causality Committee.
cPositive control samples.
dHSV-1 immunoglobulin M (IgM)/immunoglobulin G (IgG) positive, HSV-2 IgM positive, HSV-2 IgG negative, CMV IgG positive, EBV IgG positive.
eTested positive for a viral infection by clinical site testing, although classified as indeterminate.
fHSV-1/HSV-2 IgG negative.
gKnown to be chronically infected with HBV; unclear whether HBV infection was the cause of ALF and death, and thus was classified as indeterminate.
hAPAP (N-acetyl-p-aminophenol/acetaminophen) adduct negative.
iAPAP adduct negative.
jNegative for HBsAg and acetaminophen toxicity was thought to be a likely cause for patient’s ALF (although adducts not measured); thus, was classified as indeterminate.
kPositive for HBsAg, although HBV DNA was not done; clinical site may have misadjudicated this case as indeterminate.
lHSV-1/HSV-2 IgG negative.
mKnown chronic HCV positive.
nKnown chronic HCV positive; HSV-1/HSV-2 IgG positive; HSV-1/HSV-2 IgM negative; anti-EBV negative; anti-CMV negative.
oAPAP-adducts positive.
pPossible lymphoma, on HIV medications, EBV PCR negative; HSV-1/HSV-2 PCR negative.
qAPAP adduct negative.
rKnown lymphoma; CMV DNA quantitative polymerase chain reaction (PCR) 2680 copies/mL, EBV quantitative PCR 194000 copies/mL, EBV anti-Epstein Barr nuclear antigen (EBNA) IgG positive, EBV anti-viral capsid antigen (VCA) IgM negative.
sEvidence of CMV and EBV exposure; heterophile agglutinin negative, CMV antibody positive, CMV antigen positive, EBV anti-EBNA IgG positive, VCA IgM negative, anti-HSV-1 positive.
tRe-extraction and repeat mNGS testing confirmed presence of HHV-7.
uSamples 43-3569 and 43-3578 were pooled together for mNGS analysis.
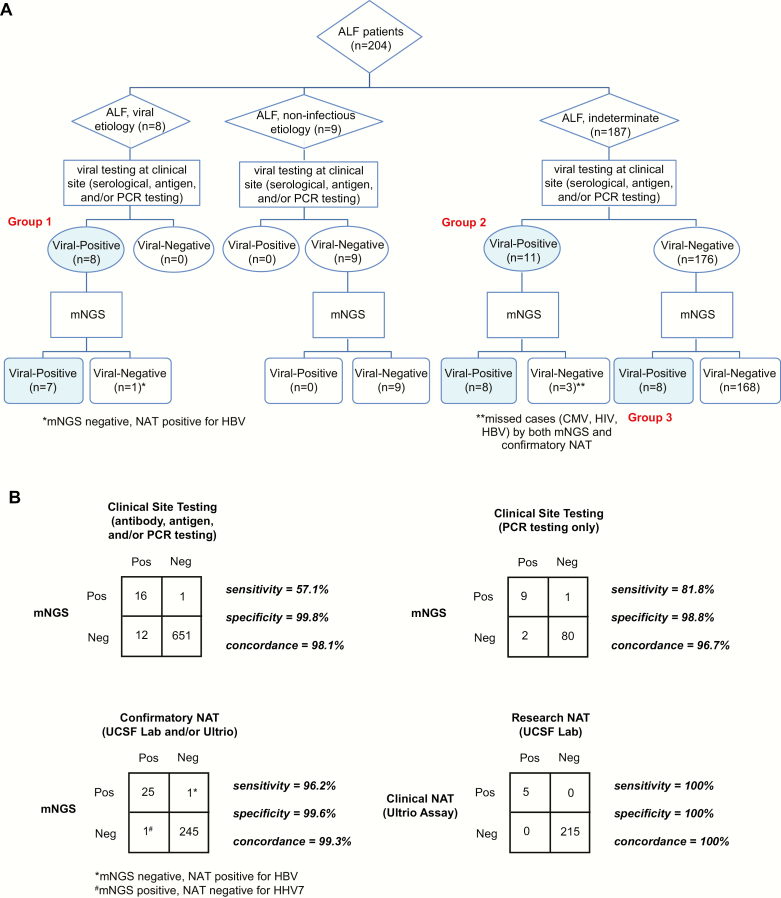
Blinded mNGS analysis yielded 27 serum samples positive for a pathogenic virus by NGS, classified into 3 groups (Figure 2A; Table 2). First, 7 of 8 (87.5%) positive controls containing HAV or HBV were identified correctly. The single missed positive control had only 2 HBV sequences and was not called positive by mNGS at the predetermined thresholds, although it was subsequently positive for HBV by NAT. Another HBV-positive control was found to be coinfected by HSV-1. The second group comprised 11 cases that tested positive for viral infection by serology, PCR, or both, but were considered indeterminate by the site investigator due to residual uncertainty regarding the true etiology. Metagenomic sequencing confirmed 8 of these 11 cases (72.7%), failing to detect a case of HIV, a case of CMV in the context of CMV/EBV coinfection, and a case of HBV in mixed HBV, HCV, and HDV coinfection. However, in each of these 3 discrepant cases, the serum was also NAT negative for the missed virus, indicating that the viral nucleic acid may have been degraded or that the initial positive detection had been made using a method other than nucleic acid detection by mNGS or NAT (eg, serology). Finally, previously unrecognized viral infections were found in 8 cases: HSV-1 alone in 3 cases, and 1 case each of HBV, parvovirus B19, HHV-7, CMV, and HPV-159, a cutaneous betapapillomavirus [24]. The clinical relevance of betapapillomavirus detection by NGS is unknown, as these cutaneous HPV types are part of the normal skin flora [24], so likely represent contamination introduced during venipuncture. Importantly, 9 negative control samples with noninfectious etiologies for ALF were all virus negative by mNGS and clinical site testing. On a per-test, per-virus basis, mNGS results were 98.1% and 99.3% concordant with clinical site and confirmatory NAT testing, respectively (Figure 2B).
Figure 2.
Testing of serum samples from 204 patients with acute liver failure (ALF) by metagenomic next-generation sequencing (mNGS). A, Flowchart of ALF cases subdivided into viral, noninfectious, and indeterminate etiologies. All cases positive for a pathogenic virus or discrepant between mNGS and clinical site testing were confirmed by virus-specific nucleic acid testing (NAT). B, Contingency tables in a 2 × 2 format comparing the relative performance of mNGS, clinical site testing, and confirmatory NAT (clinical Ultrio and research UCSF lab NAT) in the detection of viral pathogens. Abbreviations: ALF, acute liver failure; CMV, cytomegalovirus; HBV, hepatitis B virus; HIV, human immunodeficiency virus; mNGS, metagenomic next-generation sequencing; NAT, nucleic acid testing; Neg, negative; PCR, polymerase chain reaction; Pos, positive; UCSF, University of California, San Francisco; Ultrio, Procleix Ultrio assay for simultaneous detection of HIV types 1/2 and HBV and HCV nucleic acids (Grifols Diagnostic Solutions and Hologic).
Identification of Resistance Mutations
Consensus genomes for each of the viruses identified by mNGS were obtained by mapping the reads to the closest matched viral genome in the NCBI NT database and assembling a complete or partial consensus genome (Table 3). Ten HBV, HCV, and HSV-1 strains with complete sequence coverage of the relevant viral genes (eg, thymidine kinase and polymerase genes for HSV-1 [25]) were analyzed for resistance mutations. The analysis revealed an insertion in codon 145 of the thymidine kinase gene in 1 HSV-1 strain resulting in a frameshift mutation, suggesting possible resistance to acyclovir [25, 26], and an NS3(174S) polymorphism in 1 HCV strain, suggesting possible resistance to telaprevir [27, 28]. No previously described resistance mutations were detected in the remaining 8 strains.
Table 3.
Metagenomic Assembly, Genotyping, and Mutation Analyses of Acute Liver Failure Virus-Positive Samples
| Sample ID | Metagenomic Next-Generation Sequencing Result | No. of Reads | % Coverage | % Pairwise Identity | Resistance Mutationsb |
|---|---|---|---|---|---|
| 10–2006 | Hepatitis A virus (HAV) | 69368 | 99.6 | 97.7 | – |
| 13–2207 | Hepatitis A virus (HAV) | 11805 | 72.1 | 98.3 | – |
| 15–2455 | Hepatitis A virus (HAV) | 7778 | 95.8 | 97.2 | – |
| 23–2911 | Hepatitis A virus (HAV) | 57557 | 95.5 | 97.6 | – |
| 14–2440 | Hepatitis B virus genotype A (HBV-A) | 1395 | 58.2 | 99.1 | Not detected |
| 18–2653 | Hepatitis B virus genotype B (HBV-B) | 244005 | 100.0 | 97.2 | Not detected |
| 33–3251 | Hepatitis B virus genotype D (HBV-D) | 50420 | 100.0 | 95.2 | Not detected |
| 33–3251 | Human herpesvirus 1 (herpes simplex virus type 1 [HSV-1]) | 104341 | 83.3 | 96.4 | TK(145ins)c |
| 10–2053 | Hepatitis B virus genotype A (HBV-A) | 2375 | 90.4 | 94.4 | Not detected |
| 21-2833 | Hepatitis B virus genotype B (HBV-B) | 100 | 34.9 | 96.3 | |
| 13–2383 | Hepatitis B virus genotype C (HBV-C) | 20947 | 97.4 | 96.7 | Not detected |
| 35–3354 | Hepatitis B virus genotype D (HBV-D) | 17901 | 100.0 | 96.6 | Not detected |
| 17–2605 | Hepatitis B virus genotype A (HBV-A) | 520 | 47.6 | 97.2 | |
| 11–2071 | Hepatitis C virus subtype 1a (HCV-1a) | 77 | 3.4 | 97.6 | – |
| 10–3875 | Hepatitis C virus subtype 2b (HCV-2b) | 8173 | 93.9 | 96.6 | Not detected |
| 38–3540 | Hepatitis C virus subtype 3a (HCV-3a) | 17413 | 91.0 | 97.8 | Not detected |
| 15–2479 | Hepatitis C virus subtype 1a (HCV-1a) | 14322 | 98.2 | 95.9 | NS3 (174S)d |
| 11–2071 | Hepatitis D virus (HDV) | 366900 | 93.2 | 94.6 | – |
| 13–2273 | Human immunodeficiency virus 1 (HIV-1) | 29 | 10.0 | 98.2 | – |
| 13–3941 | Human herpesvirus 4 (Epstein-Barr virus) | 162 | 4.3 | 94.9 | – |
| 37–4067 | Human papillomavirus type 159 | 22 | 3.9 | 97.6 | – |
| 13–2236 | Human herpesvirus 5 (cytomegalovirus) | 837 | 15.3 | 96.8 | – |
| 20–2779 | Human herpesvirus 7 (HHV-7) | 6 | 0.2 | 97.2 | – |
| 21–2824 | Human herpesvirus 1 (HSV-1) | 1688 | 37.7 | 88.3 | – |
| 43–3569a | Human herpesvirus 1 (HSV-1) | 27346 | – | – | – |
| 43–3578a | Human herpesvirus 1 (HSV-1) | 27346 | – | – | – |
| 18–2649 | Human parvovirus B19 | 41969 | 94.9 | 96.2 | – |
Abbreviation: –, not reported.
aSamples 43-3569 and 43-3578 were pooled and sequenced together, so the read count, percentage coverage, percentage pairwise identity, and resistance mutations for each individual sample cannot be determined. The total HSV-1 read count for the pool is reported.
bResistance information is reported when there is >90% coverage of the target viral gene.
cPossible resistance to acyclovir.
dPossible resistance to telaprevir.
DISCUSSION
In the current study, we used a metagenomic sequencing approach to identify potential viral pathogens in 204 adult patients with ALF collected by the United States ALFSG. A total of 187 patients (92%) were clinically defined as indeterminate, while the remaining patients represented blinded controls for either hepatitis A or B infection, or established noninfectious causes of ALF. The overall concordance of mNGS relative to clinical site and confirmatory NAT testing was high at 98.1% and 99.3%, respectively (Figure 2B). Previously unrecognized viruses of likely or potential clinical significance, including HBV, HSV-1, parvovirus B19, CMV, and HHV-7, were identified in serum samples from 8 patients (4.3%). Importantly, no sequences corresponding to novel viruses were detected.
Notably, the mNGS analyses identified several cases of dual or triple infections with multiple viruses. These included 1 case of HBV/HSV-1 coinfection, 1 case of CMV/EBV coinfection, and 1 triple infection with HBV, HCV, and HDV. The patient with HBV/HSV-1 coinfection had a markedly elevated alanine aminotransferase (ALT) level of 7280 units per liter (U/L) [normal range 8–48 U/L] and died within 21 days of study admission; coinfection with 2 hepatotropic viruses may have resulted in a rapidly progressive, more fulminant disease course. The case of coinfection with CMV and EBV occurred in the setting of a patient with known lymphoma, and reactivation by these 2 herpesviruses may have precipitated ALF. Interestingly, the case of triple coinfection was associated with hepatitis B surface antigen antigenemia but undetectable HBV viral loads (PCR and mNGS negativity). However, triple coinfection with HBV, HCV, and HDV has been reported to be associated with increased liver damage and severe chronic disease [29].
HSV-1 infection was identified in 4 of 187 (2.1%) indeterminate ALF patients by mNGS, subsequently confirmed by PCR. All HSV-1 cases had not been previously recognized by clinical site testing, as HSV testing is not routinely ordered in the initial workup of ALF. These results contrast with those from a prior smaller study, also from the ALFSG cohort, that found no new cases of HSV in 51 indeterminate patients by PCR [5]. The 4 additional cases of HSV-1 detected in the current study may have been unmasked by screening of a greater number of patients, as differences in rates of detection were not significant (P = .58 by 2-tailed Fisher exact test).
Although HSV-1 has been described as an extremely rare cause of ALF, the prevalence of mNGS- and PCR-positive cases in patients with indeterminate ALF in the current study (2.1%) and generally poor clinical outcomes [30] suggest that HSV-1 should be considered as part of the early workup, even if the potential of inadvertent contamination or reactivation (unrelated to ALF) from HSV-1 cannot be ruled out. The 4 immunocompetent patients who were positive for systemic HSV by mNGS consisted of 2 men and 2 women between 18 years and 62 years of age, none of whom had a history of liver disease. The 2 HSV-1 infected patients with extremely high aspartate aminotransferase (AST)/ALT enzyme levels (7000–16000 U/L) died during the initial hospitalization. The 2 remaining patients had milder elevations (400–2000 U/L) and either survived or were of unknown clinical status at the 21-day follow-up. Three of the 4 patients had normal white blood cell (WBC) counts, except for 1 patient who was coinfected with HBV and had elevated WBC counts of 29.4 × 109 cells/L (normal, 3.5–10 × 109 cells/L).
We also found a case positive for parvovirus B19 infection among 187 cases of indeterminate ALF, versus none in a previous study [4]. Acute parvovirus B19 infections are largely asymptomatic or cause flu-like symptoms [31], but in rare instances can cause acute hepatitis [32], including a reported case of parvovirus-associated hepatic failure requiring liver transplantation [33]. Our patient with parvovirus B19 infection was a 75-year-old man on no outpatient medications who presented with ALF of unknown etiology (AST, 1194 U/L [normal range 7–55 U/L]; ALT, 1172 U/L [normal range 8–48 U/L]; alkaline phosphatase, 124 U/L [normal range 45–115 U/L]; bilirubin, 20.5 mg/dL [normal range 0.1–1.2 mg/dL]; international normalized ratio, 2.0 [normal range 0.8–1.1]; and albumin, 2 g/dL [normal range 3.5–5.0 g/dL]). The patient tested negative for HAV, HBV, and HCV serology, and mNGS did not reveal any viral infections apart from parvovirus B19. Bacterial and fungal blood cultures were negative, as was a toxicology screen. The patient eventually developed multiorgan failure and died 21 days postadmission. In the absence of an alternative diagnosis, we believe that parvovirus B19 was the likely cause of ALF in this patient.
HHV-7 was also detected by mNGS in a single case. This finding is of unclear clinical significance, as acute HHV-7 infection is often asymptomatic and >90% of individuals are seropositive by adulthood [34]. Nevertheless, acute hepatitis from primary HHV-7 infection has been previously described in an infant, with viral DNA detected in the liver and subsequent seroconversion [35]. For the 2 identified cases of HIV-1, neither patient was coinfected with HBV or HCV, which can accelerate cirrhosis and liver decompensation, or, for HBV, precipitate ALF by viral reactivation in the setting of medication withdrawal [36, 37]. Thus, the etiology of ALF in the 2 HIV-1–infected patients remains to be established, although 1 patient may have developed ALF secondary to HIV-associated lymphoma.
The sparse detection rate of infections from a pathogenic virus (7 of 187 [3.2%]) in indeterminate ALF cases by mNGS is unlikely due to decreased sensitivity, as the sensitivity between mNGS and “gold standard” confirmatory NAT was 96.2% (Figure 2B). Rather, the low yield of viruses in the current study suggests that either most cases of ALF are due to noninfectious causes, such as acetaminophen toxicity [38], or that analysis of invasively acquired samples, such as liver biopsy (not available in the current study), may be needed to boost diagnostic sensitivity.
Clinical mNGS testing is likely to become part of a routine diagnostic workup for acute infectious diseases such as hepatitis [9, 19, 39, 40]. Unlike multiplex PCR, which targets a predefined panel of microorganisms, mNGS can interrogate clinical samples for any and all pathogens simultaneously. Thus, limited material is not expended by following the traditional diagnostic paradigm of serial testing for a priori targeted infectious agents, which can be expensive, time-consuming, and low-yield. However, rigorous assessment of the performance characteristics of mNGS testing in the clinical laboratory can be challenging, although validation efforts are now under way at multiple sites, including ours [41]. Given its high specificity, an mNGS clinical assay may be useful in the near future not only for direct diagnosis but also as a test to exclude (“rule out”) infection.
In conclusion, the indeterminate ALF patient group represents a heterogeneous mix, comprised of patients for whom diagnostic testing may be incomplete leading to unclear or “no apparent” diagnosis, as well as those with dual/triple diagnoses or ambiguous results. Testing for rare and unexpected viral infections is not performed in routine clinical practice, as specific antiviral treatments are currently limited. Nevertheless, HSV testing does appear to be of value, as 2.1% (4 of 187) of indeterminate ALF cases were found to be positive, disease progression may be unusually rapid with HSV infection, and prompt treatment with antiviral medications such acyclovir can be life-saving. It is also reassuring that the use of mNGS for comprehensive screening of 187 indeterminate ALF patients yielded only 7 additional cases of infection from viral pathogens and no novel viruses. These results suggest that standard clinical testing for the hepatitis A–E viruses, supplemented by additional targeting of uncommon viruses such as HSV and parvovirus B19, is sufficient to screen for blood-borne viral infections associated with ALF, at least until more comprehensive diagnostic technologies such as mNGS become clinically available.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) (grant number R01 HL105704 to C. Y. C.); the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant number U01-DK-58369 to W. M. L. for ALFSG); and an Abbott Viral Discovery Award (to C. Y. C.). The NIDDK Central Repositories supplied the samples from the United States Acute Liver Failure Study reported here.
Potential conflicts of interest. C. Y. C. is the director of the UCSF-Abbott Viral Diagnostics and Discovery Center and receives research funding from Abbott Laboratories. W. M. L. receives research support from Merck and Conatus for pharmaceutical studies unrelated to acute liver failure. C. Y. C. and S. N. N. are inventors of patent PCT/US156/52912 titled “Pathogen Detection Using Next-Generation Sequencing” pending with regards to the SURPI+ computational pipeline. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Reuben A, Tillman H, Fontana RJ et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med 2016; 164:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013; 369:2525–34. [DOI] [PubMed] [Google Scholar]
- 3. Fagan EA. Acute liver failure of unknown pathogenesis: the hidden agenda. Hepatology 1994; 19:1307–12. [PubMed] [Google Scholar]
- 4. Lee WM, Brown KE, Young NS et al. ; Acute Liver Failure Study Group Brief report: no evidence for parvovirus B19 or hepatitis E virus as a cause of acute liver failure. Dig Dis Sci 2006; 51:1712–5. [DOI] [PubMed] [Google Scholar]
- 5. Levitsky J, Duddempudi AT, Lakeman FD et al. ; US Acute Liver Failure Study Group Detection and diagnosis of herpes simplex virus infection in adults with acute liver failure. Liver Transpl 2008; 14:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mellinger JL, Rossaro L, Naugler WE et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci 2014; 59:1630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol 2009; 6:542–53. [DOI] [PubMed] [Google Scholar]
- 8. Chiu CY, Miller S. Next-generation sequencing. In: Persing DH, Tenover FC, Hayden RT, Ieven G, MIller MB, Nolte FS, eds. Molecular microbiology, diagnostic principles and practice, 3rd ed Washington, DC: ASM Press, 2016:68–79. [Google Scholar]
- 9. Naccache SN, Federman S, Veeraraghavan N et al. A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome Res 2014; 24:1180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheval J, Sauvage V, Frangeul L et al. Evaluation of high-throughput sequencing for identifying known and unknown viruses in biological samples. J Clin Microbiol 2011; 49:3268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wylie KM, Mihindukulasuriya KA, Sodergren E, Weinstock GM, Storch GA. Sequence analysis of the human virome in febrile and afebrile children. PLoS One 2012; 7:e27735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berg MG, Lee D, Coller K et al. Discovery of a novel human pegivirus in blood associated with hepatitis C virus co-infection. PLoS Pathog 2015; 11:e1005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu CY. Viral pathogen discovery. Curr Opin Microbiol 2013; 16:468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mee ET, Preston MD, Minor PD, Schepelmann S; CS533 Study Participants Development of a candidate reference material for adventitious virus detection in vaccine and biologicals manufacturing by deep sequencing. Vaccine 2016; 34:2035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allander T, Emerson SU, Engle RE, Purcell RH, Bukh J. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc Natl Acad Sci U S A 2001; 98:11609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 2007; 7:270–80. [DOI] [PubMed] [Google Scholar]
- 17. Lee D, Das Gupta J, Gaughan C et al. In-depth investigation of archival and prospectively collected samples reveals no evidence for XMRV infection in prostate cancer. PLoS One 2012; 7:e44954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greninger AL, Messacar K, Dunnebacke T et al. Clinical metagenomic identification of Balamuthia mandrillaris encephalitis and assembly of the draft genome: the continuing case for reference genome sequencing. Genome Med 2015; 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monkolrattanothai K, Naccache SN, Bender JM et al. Neurobrucellosis: unexpected answer from metagenomics next-generation sequencing. J Pediatric Infect Dis Soc 2016. doi:10.1093/jpids/piw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chams V, Fournier-Wirth C, Chabanel A, Hervé P, Trépo C. Is GB virus C alias “hepatitis” G virus involved in human pathology? [in French]. Transfus Clin Biol 2003; 10:292–306. [DOI] [PubMed] [Google Scholar]
- 21. Okamoto H. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol 2009; 331:1–20. [DOI] [PubMed] [Google Scholar]
- 22. Assal A, Barlet V, Deschaseaux M et al. Sensitivity of two hepatitis B virus, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) nucleic acid test systems relative to hepatitis B surface antigen, anti-HCV, anti-HIV, and p24/anti-HIV combination assays in seroconversion panels. Transfusion 2009; 49:301–10. [DOI] [PubMed] [Google Scholar]
- 23. Roth WK, Busch MP, Schuller A et al. International survey on NAT testing of blood donations: expanding implementation and yield from 1999 to 2009. Vox Sang 2012; 102:82–90. [DOI] [PubMed] [Google Scholar]
- 24. Kocjan BJ, Hosnjak L, Seme K, Poljak M. Complete genome sequence of a novel human betapapillomavirus, HPV-159. Genome Announc 2013; 1:e00298–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sauerbrei A, Bohn-Wippert K, Kaspar M, Krumbholz A, Karrasch M, Zell R. Database on natural polymorphisms and resistance-related non-synonymous mutations in thymidine kinase and DNA polymerase genes of herpes simplex virus types 1 and 2. J Antimicrob Chemother 2016; 71:6–16. [DOI] [PubMed] [Google Scholar]
- 26. Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Herpes simplex virus thymidine kinase mutations associated with resistance to acyclovir: a site-directed mutagenesis study. Antimicrob Agents Chemother 2005; 49:1055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishiya AS, de Almeida-Neto C, Ferreira SC et al. HCV genotypes, characterization of mutations conferring drug resistance to protease inhibitors, and risk factors among blood donors in São Paulo, Brazil. PLoS One 2014; 9:e86413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welsch C, Domingues FS, Susser S et al. Molecular basis of telaprevir resistance due to V36 and T54 mutations in the NS3-4A protease of the hepatitis C virus. Genome Biol 2008; 9:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riaz M, Idrees M, Kanwal H, Kabir F. An overview of triple infection with hepatitis B, C and D viruses. Virol J 2011; 8:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ichai P, Roque Afonso AM, Sebagh M et al. Herpes simplex virus-associated acute liver failure: a difficult diagnosis with a poor prognosis. Liver Transpl 2005; 11:1550–5. [DOI] [PubMed] [Google Scholar]
- 31. Woolf AD, Campion GV, Chishick A et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med 1989; 149:1153–6. [PubMed] [Google Scholar]
- 32. Bihari C, Rastogi A, Saxena P et al. Parvovirus B19 associated hepatitis. Hepat Res Treat 2013; 2013:472027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krygier DS, Steinbrecher UP, Petric M et al. Parvovirus B19 induced hepatic failure in an adult requiring liver transplantation. World J Gastroenterol 2009; 15:4067–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark DA. Human herpesvirus 6 and human herpesvirus 7: emerging pathogens in transplant patients. Int J Hematol 2002; 76(suppl 2):246–52. [DOI] [PubMed] [Google Scholar]
- 35. Hashida T, Komura E, Yoshida M et al. Hepatitis in association with human herpesvirus-7 infection. Pediatrics 1995; 96:783–5. [PubMed] [Google Scholar]
- 36. Bloquel B, Jeulin H, Burty C, Letranchant L, Rabaud C, Venard V. Occult hepatitis B infection in patients infected with HIV: report of two cases of hepatitis B reactivation and prevalence in a hospital cohort. J Med Virol 2010; 82:206–12. [DOI] [PubMed] [Google Scholar]
- 37. Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol 2010; 8:1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larson AM, Polson J, Fontana RJ et al. ; Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–72. [DOI] [PubMed] [Google Scholar]
- 39. Naccache SN, Peggs KS, Mattes FM et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clin Infect Dis 2015; 60:919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson MR, Naccache SN, Samayoa E et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med 2014; 370:2408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schlaberg R, Chiu CY, Miller S et al. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch Pathol Lab Med 2017; 141:776–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.