Summary
Successful contact with patients lost to follow-up from HIV care has a strong, short-term effect on return to care, but is of limited overall efficiency because many patients have died, are not found in person, or are in care elsewhere.
Keywords: antiretroviral therapy, Africa, retention, loss to follow-up.
Abstract
Background.
The effect of tracing human immunodeficiency virus (HIV)–infected patients who are lost to follow-up (LTFU) on reengagement has not been rigorously assessed. We carried out an ex post analysis of a surveillance study in which LTFU patients were randomly selected for tracing to identify the effect of tracing on reengagement.
Methods.
We evaluated HIV-infected adults on antiretroviral therapy who were LTFU (>90 days late for last visit) at 14 clinics in Uganda, Kenya, and Tanzania. A random sample of LTFU patients was selected for tracing by peer health workers. We assessed the effect of selection for tracing using Kaplan-Meier estimates of reengagement among all patients as well as the subset of LTFU patients who were alive, contacted in person by the tracer, and out of care.
Results.
Of 5781 eligible patients, 991 (17%) were randomly selected for tracing. One year after selection for tracing, 13.3% (95% confidence interval [CI], 11.1%–15.3%) of those selected for tracing returned compared with 10.0% (95% CI, 9.1%–10.8%) of those not randomly selected, an adjusted risk difference of 3.0% (95% CI, .7%–5.3%). Among patients found to be alive, personally contacted, and out of care, tracing increased the absolute probability of return at 1 year by 22% (95% CI, 7.1%–36.2%). The effect of tracing on rate of return to clinic decayed with a half-life of 7.0 days after tracing (95% CI, 2.6 %–12.9%).
Conclusions.
Tracing interventions increase reengagement, but developing methods for targeting LTFU patients most likely to benefit can make this practice more efficient.
(See the Editorial Commentary by Armstrong and del Rio on pages 1555–6.)
Identifying public health strategies to maximize retention in care for human immunodeficiency virus (HIV)–infected persons in low- and middle-income countries (LMICs) is a priority in implementation science, but the effect of many strategies is unknown. This includes practices that are widely used in day-to-day operations. For example, requiring a treatment supporter for antiretroviral therapy (ART) initiation was widely adopted even before a randomized trial showed no benefit on HIV RNA outcomes [1]. Although counseling on adherence before treatment is supported by a randomized trial [2], standard practice in diverse settings has included a requirement for patients to attend 3 pre–ART initiation counseling sessions. Yet when investigators examined the effect of the number of counseling sessions on subsequent adherence, no link could be identified between the number of counseling sessions on adherence nor retention among new ART initiators [3]. To optimize efficiency and effectiveness of ART delivery, rigorous assessments of practices to enhance the HIV cascade are needed to identify useful interventions to maintain and scale as well those which should be discontinued.
Although tracing patients on ART who are lost to follow-up in order to return them to care is widely practiced in LMICs [4], the causal effect of tracing has not been definitively established. A number of studies, summarized in a meta-analysis, found that programs that carry out tracing have a higher retention in care [4]. While the ecological association in this assessment is intriguing, it is also possible that common causes of retention and tracing (such as clinic resources or overall quality of care) drive the observed association, rather than tracing per se. In many program reports, a high fraction of patients who are traced indeed return to care. But without a counterfactual, it is unknown how many patients who miss a visit would return to care without further intervention, and therefore the effect of tracing cannot be inferred from these studies. Qualitative studies suggest that lost patients feel encouraged by tracers to return to care [5]. Some patients, however, simply cannot afford transportation or time off work and therefore are unlikely to benefit from typical counseling, education, and reminders offered through tracing [6–8].
In previous work, our group has used a sampling-based approach to correct for the effects of loss to follow-up on estimates of retention [9]. The sampling-based approach identifies a numerically small but randomly selected sample of patients lost to follow-up and conducts intensive tracing in the field to contact the patients selected. Patient outcomes (such as vital status and current care status) that are identified through tracing are used through probability weights to correct estimates of mortality and retention in entire populations [9]. Although designed as an epidemiologic surveillance methodology, patients who are contacted are offered standard of care counseling and encouragement to return to the clinic. We use the random selection for tracing in this setting as a quasi-natural experiment to estimate the causal effect of tracing on return to clinic.
METHODS
Patients
As reported elsewhere, the tracing study was carried out at 14 clinic sites in Eastern Africa located in 5 geographical settings: Mbarara, Uganda; Eldoret, Kenya; Kisumu, Kenya; Kampala, Uganda; and Morogoro, Tanzania [9]. Clinics in each of these settings participate in the East African International Epidemiologic Databases to Evaluate AIDS (IeDEA-EA) consortium. At these sites we identified patients who had visited one of the 14 participating clinics in the 2.5 years preceding the date of sampling and who were lost to follow-up, defined as being >90 days late for their last appointment on the day of sampling. A random subset of lost patients was selected for tracing. These lost patients, whether randomly selected or not selected for tracing, form the cohort of interest in this analysis.
Procedures
Patient tracing was carried out by cadres of community health workers at each of these sites. Although their exact job titles varied, tracers were drawn from existing health workers who were affiliated with each of these facilities and who were mostly persons living with HIV. All tracers who were employed by the study had experience seeking patients in the community as a part of routine programmatic work. The tracers were given lists of patients who were lost to follow-up. Tracers used public transportation, walked, or rode motorcycles as appropriate and available. If contact with a patient was made, the tracer solicited information about their updated care status and asked about reasons for nonreturn. The interaction was semistructured, and used 3–5 questions to ascertain current care status as well as reasons for stopping care or transferring care. The interaction took a total of on average 10–15 minutes. The tracers also offered routine encouragement and counseling, as per standard practices in routine tracing in each of their programs, to encourage patients to return. No monetary incentive or other inducement to return to clinic was provided.
Measurements
Sociodemographic (eg, sex, age at enrollment) and clinical (eg, CD4 values, World Health Organization stage, visit dates) data were taken from electronic databases at each of the clinics. Date of tracing and contact with the patient was taken from a standardized form on which tracer recorded their activities, described in greater detail in previous publications [10, 11]. Return to the original clinic was taken from existing medical records of visits at each of the clinic sites. Loss to follow-up was defined as being 3 months late to a scheduled appointment or failing to be seen for 4 months if no follow-up was scheduled. Those patients known by the clinic to be dead or having transferred out of the clinic were not considered to be lost to follow-up.
Analysis
Effectiveness of Tracing Among All Patients Lost to Follow-up Using a Quasi-natural Experiment
The primary analysis included all patients who were lost to follow-up using information from the respective clinics. This diverse group includes patients who are later found to be dead, alive and in care, or alive and out of care. Inclusion of all patients can be considered an intent-to-treat analysis. Kaplan-Meier estimates for the time from sampling of patients for tracing until to return to clinic were compared between those randomly selected for tracing and not selected for tracing. In this analysis, time zero is the date of random selection of patients, even though contact with the tracer occurred after the date of randomization, to preserve exchangeability between the groups afforded by random selection. The event is the date of first return to clinics in the program. All other observations are censored at database closure. A log-rank test was used to evaluate statistical significance.
Efficacy of Contact With the Patients Not in Care on Return to Clinic Using an Instrumental Variable Approach
The benefit of tracing on reengagement in care is expected to depend on the status of the patient and the results of tracing. For example, patients who are dead or sought but not found are unable to benefit from the intervention of tracing. We therefore used an instrumental variable approach where we considered random selection for tracing the instrument, contact with patients alive and out of care to be the “treatment” and return to clinic as the outcome of interest. We believed a priori that that random selection for tracing had a strong effect on contact between a tracer and the patient: it is virtually axiomatic that if a patient was not selected for tracing, no contact between the tracer and the patient would occur. Because the instrument was randomly assigned, we assume it shared no common causes with the outcome. We also assumed that random selection for tracing had no effect on return to clinic other than through contact in the field between the tracer and the patient. Finally, we also assume contact never leads to failure to return in a patient who would have otherwise returned and that contact with one patient does not influence the return of another. These assumptions yield a local average treatment effect among patients who are lost, not in care, and contactable in person, even if unmeasured common causes of successful contact and the return exist (ie, unmeasured confounders). We estimated the effect of contact on return by 1 year after random sampling under these assumptions using a maximum likelihood estimation [12].
Estimating the Rate of Return Before and After Tracing
In additional analyses, we examined changes in the rate of return to clinic among the random sample of patients selected both before tracing and in the first 14 days, 15–90 days, 91–180 days, and >180 days after the first tracing attempt. We examine these rates of return separately among all patients sampled; those sampled, found alive and in person and who reported not being in care; those alive and contacted who reported being in care elsewhere; and those found to be alive by report of an informant only (and not contacted in person). We then estimated the association between tracing and reengagement in each of these populations through using the first date of tracing as a time-varying covariate in both an unadjusted Cox proportional hazards model as well as one adjusted for sex, pretherapy CD4 level, age, setting, and calendar date of enrollment into care. Although change in the rate of return before and after tracing attempt among those patients found in person may be confounded by unmeasured common causes of tracing (ie, timing or outcome) and return, this analysis also provides a “negative control” through which we can confirm the absence of an effect among the strata of individuals for whom we assume selection for tracing could have no effect (eg, those sought but not contacted, those who died, and those contacted but already in care elsewhere). In the sample of patients who were found alive and not in care, we estimated the half-life of the effect of tracing. by fitting the daily rate of return, in units of number of people returning per person-day susceptible to return, to a function composed of a baseline rate of return , a fold increase in return rate on the day of tracing , and a half-life for decay of the return rate back to the baseline rate:
.
The study was approved by relevant institutional review boards at each of the participating sites, none of which required consent for tracing as this practice was already present at all sites.
RESULTS
A total of 5781 patients were classified as lost to follow-up on the date of randomization, and 991 (17.1%) were randomly selected for tracing (Supplementary Figure 1: flowchart). Overall, 38.0% of sampled patients were male; the median age was 34 years and the median CD4 count at ART initiation was 138 cells/µL. Differences were similar across facilities (Table 1). At the time of random selection for tracing, 37.5% of patients had been lost to follow-up for <1 year, 40.4% had been lost for 1–2 years, and 22.1% had been lost for >2 years. At the last clinic visit, 21.2% had initiated ART within the prior month, 33.9% had initiated ART between 1 month and 1 year prior, and 45.0% been on ART for >1 year. Of the 991 traced patients, 233 (23.5%) had died, 212 (21.4%) were interviewed and reported being in care elsewhere, 148 (14.9%) were interviewed and reported being out of care, 267 (26.9%) were alive according to an informant but could not be reached directly, and 131 (13.2%) had unknown vital status because neither the patient nor an informant could be contacted.
Table 1.
Patient Characteristics
| Characteristic | All Patients Who Were Lost to Follow-up at Time of Random Selection for Tracing | Lost Patients Randomly Sampled for Tracing | Lost Patients Not Randomly Sampled for Tracing | Traced Patient Found to Be Alive | Traced Patients Found Alive and Contacted in Person | Traced Patients Found Alive, Contacted in Person, and Who Reported Being in Care Elsewhere | Traced Patients Found Alive, Contacted in Person, and Who Reported Being out of Care |
|---|---|---|---|---|---|---|---|
| No. | 5781 | 991 | 4790 | 758 | 360 | 212 | 148 |
| Age, y, median (IQR)a | 34 (28–41) | 34 (28–41) | 34 (28–41) | 33 (28–40) | 33 (28–39) | 33 (28–39) | 33 (28–39) |
| Sex, No. (%) | |||||||
| Male | 2033 (35.2) | 377 (38.0) | 1656 (34.6) | 269 (35.5) | 121 (33.6) | 63 (29.7) | 58 (39.2) |
| Nonpregnant female | 3333 (57.7) | 533 (53.8) | 2800 (58.5) | 413 (54.5) | 194 (53.9) | 130 (61.3) | 64 (43.2) |
| Pregnant female | 415 (7.2) | 81 (8.2) | 334 (7.0) | 76 (10.0) | 45 (12.5) | 19 (9.0) | 26 (17.6) |
| Treatment program | |||||||
| Eldoret, Kenya | 2220 (38.4) | 441 (44.5) | 1779 (37.1) | 355 (46.8) | 164 (45.6) | 79 (37.3) | 85 (57.4) |
| Morogoro, Tanzania | 1317 (22.8) | 175 (17.7) | 1142 (23.8) | 129 (17.0) | 62 (17.2) | 35 (16.5) | 27 (18.2) |
| Kampala, Uganda | 698 (12.1) | 148 (14.9) | 550 (11.5) | 104 (13.7) | 40 (11.1) | 26 (12.3) | 14 (9.5) |
| Kisumu, Kenya | 639 (11.1) | 114 (11.5) | 525 (11.0) | 80 (10.6) | 57 (15.8) | 43 (20.3) | 14 (9.5) |
| Mbarara, Uganda | 907 (15.7) | 113 (11.4) | 794 (16.6) | 90 (11.9) | 37 (10.3) | 29 (13.7) | 8 (5.4) |
| CD4 level at ART initiation, cells/µLb, median (IQR)b | 138 (57–222) | 136 (60–225) | 138 (57–222) | 154 (74–238) | 160 (79–256) | 150 (79–252) | 174 (83–261) |
| WHO stage, No. (%)c | |||||||
| I | 975 (18.5) | 178 (19.6) | 797 (18.2) | 156 (22.8) | 83 (25.2) | 39 (20.0) | 44 (32.8) |
| II | 1265 (24.0) | 211 (23.3) | 1054 (24.1) | 166 (24.3) | 79 (24.0) | 52 (26.7) | 27 (20.1) |
| III | 2320 (44.0) | 407 (44.9) | 1913 (43.8) | 296 (43.3) | 138 (41.9) | 88 (45.1) | 50 (37.3) |
| IV | 715 (13.6) | 111 (12.2) | 604 (13.8) | 66 (9.6) | 29 (8.8) | 16 (8.2) | 13 (9.7) |
| NNRTI in first regimend | |||||||
| NVP | 3400 (73.4) | 545 (72.5) | 2855 (73.6) | 420 (73.3) | 198 (71.2) | 115 (70.1) | 83 (72.8) |
| EFV | 1233 (26.6) | 207 (27.5) | 1026 (26.4) | 153 (26.7) | 80 (28.8) | 49 (29.9) | 31 (27.2) |
| NRTI in first regimene | |||||||
| ZDV | 2693 (56.0) | 439 (56.0) | 2254 (56.0) | 338 (56.0) | 165 (55.0) | 93 (53.8) | 72 (56.7) |
| d4T | 1955 (40.7) | 326 (41.6) | 1629 (40.5) | 253 (41.9) | 127 (42.3) | 74 (42.8) | 53 (41.7) |
| TDF | 161 (3.3) | 19 (2.4) | 142 (3.5) | 13 (2.2) | 8 (2.7) | 6 (3.5) | 2 (1.6) |
| Time between last clinic visit and randomization, mean (IQR) | |||||||
| <1 y | 2170 (37.5) | 383 (38.6) | 1787 (37.3) | 307 (40.5) | 163 (45.3) | 88 (41.5) | 75 (50.7) |
| 1–2 y | 2337 (40.4) | 424 (42.8) | 1913 (39.9) | 318 (42.0) | 139 (38.6) | 94 (44.3) | 45 (30.4) |
| >2 y | 1274 (22.1) | 184 (18.6) | 1090 (22.8) | 133 (17.5) | 58 (16.1) | 30 (14.2) | 28 (18.9) |
| Time on ART at last clinic visit, mean (IQR) | |||||||
| <1 mo | 1225 (21.2) | 231 (23.3) | 994 (20.8) | 160 (21.1) | 62 (17.2) | 34 (16.0) | 28 (18.9) |
| 1 mo to 1 y | 1957 (33.9) | 313 (31.6) | 1644 (34.3) | 227 (29.9) | 107 (29.7) | 65 (30.7) | 42 (28.4) |
| >1 y | 2599 (45.0) | 447 (45.1) | 2152 (44.9) | 371 (48.9) | 191 (53.1) | 113 (53.3) | 78 (52.7) |
| Year of ART initiation | |||||||
| Before 2008 | 1452 (25.1) | 236 (23.8) | 1216 (25.4) | 198 (26.1) | 100 (27.8) | 56 (26.4) | 44 (29.7) |
| 2008 | 1248 (21.6) | 206 (20.8) | 1042 (21.8) | 160 (21.1) | 85 (23.6) | 55 (25.9) | 30 (20.3) |
| 2009 | 1716 (29.7) | 300 (30.3) | 1416 (29.6) | 229 (30.2) | 89 (24.7) | 55 (25.9) | 34 (23.0) |
| 2010 or later | 1365 (23.6) | 249 (25.1) | 1116 (23.3) | 171 (22.6) | 86 (23.9) | 46 (21.7) | 40 (27.0) |
Abbreviations: ART, antiretroviral therapy; d4T, stavudine; EFV, efavirenz; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization; ZDV, zidovudine.
aMissing in 90 (1.6%).
bMissing in 1077 (18.6%).
cMissing in 506 (8.8%).
dMissing in 1148 (19.9%).
eMissing in 972 (16.8%).
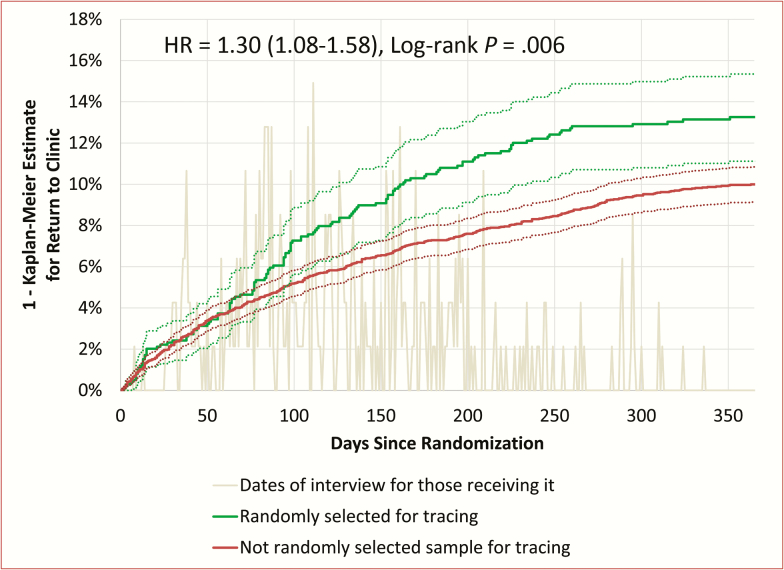
One year after sampling, in the entire patient population (Figure 1), 13.3% (95% confidence interval [CI], 11.1%–15.3%) of patients selected for tracing had returned, whereas 10.0% (95% CI, 9.1%–10.8%) not selected for tracing had returned (log-rank test, P = .006). The adjusted hazard ratio (HR) for return to clinic was 1.30 (95% CI, 1.08–1.58). The adjusted risk difference for return at 1 year was 3.0% (95% CI, .7%–5.3%), yielding a number needed to treat (NNT) of 33. The instrumental variable approach suggested that actual contact between a tracer and a patient alive and not in care elsewhere increased the probability of return from 15.1% to 37.1%, representing a risk ratio of 2.47 (95% CI, 1.05–5.78), an absolute risk difference of 22.1% (95% CI, 7.1%–36.2%), and an NNT between 4 and 5.
Figure 1.
Inverted Kaplan-Meier estimate for returning to clinic over time, where time zero is the date of random sampling of lost patients to identify the sample to be traced. The gray line shows the distribution of dates that the tracers contacted patients, relative to the date of randomization. The solid green line shows the Kaplan-Meier estimate for return to clinic among all those randomized to tracing (including patients who were found to have died prior to tracing). The solid red line shows the Kaplan-Meier estimate for return to clinic among those not randomly assigned to tracing. Dashed lines show 95% confidence intervals (CIs). The hazard ratio (HR) for returning to clinic was 1.30 (95% CI, 1.08–1.58), which was statistically significant (log-rank test, P = .006).
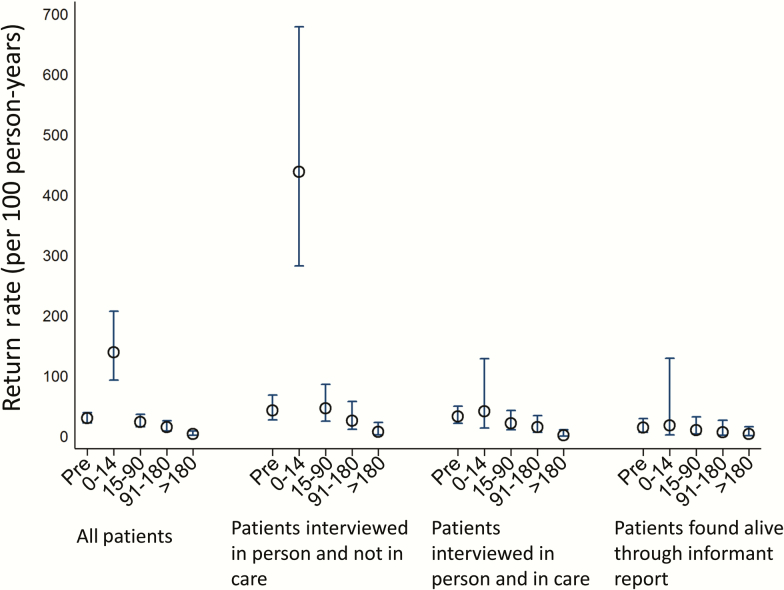
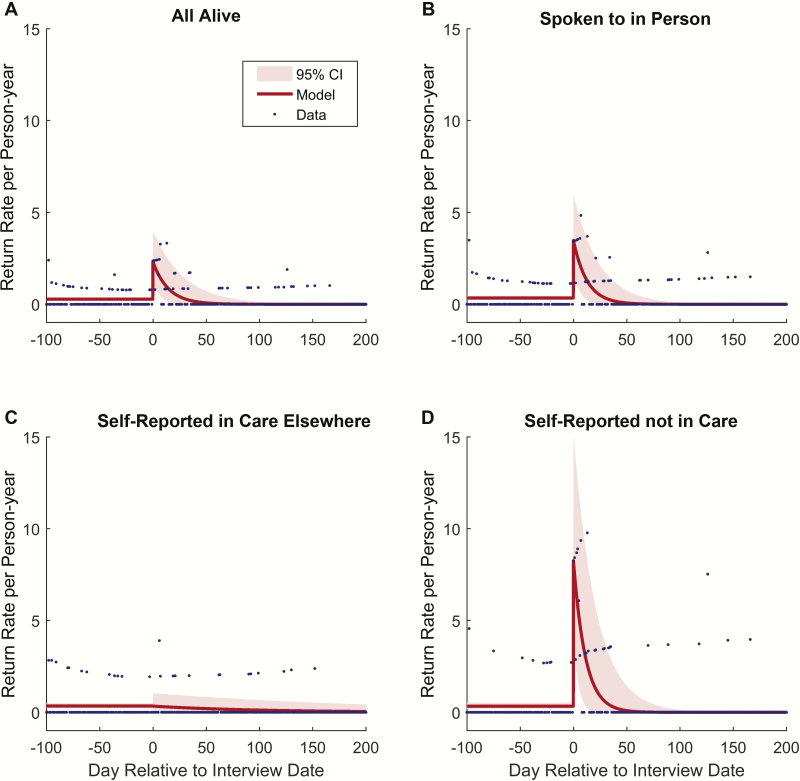
The before and after analysis, in which the first date of tracing is considered time zero, found that after adjustment for age, sex, and CD4 count at ART initiation, return to clinic rose sharply in the first 14 days after tracing started, and fell 15–90 days, 91–180 days, and >180 days after tracing, (Figure 2; Table 2). When all those who were traced were included in the analysis, the HR for return to clinic was significantly elevated in the first 2 weeks after tracing (HR, 5.9; 95% CI, 3.4–10.2) relative to the pretracing rate of return, but showed no significant elevation after 2 weeks had elapsed. Restriction to only those interviewed in person and reporting to be out of care sharpened the change in rate of return: relative to time before tracing commencement, the rate of return increased markedly in the first 2 weeks after tracing (HR, 8.1; 95% CI, 3.9–16.6). The exponential decay model to identify the “half-life” of the effect of tracing displayed the best fit (Figure 3; Table 3) compared with other specifications (Supplementary Figures 2–6 and Supplementary Tables 1 and 2). This analysis suggested that the effect of tracing among patients who are alive and out of care has a half-life of 7.0 days (95% CI, 2.6–12.9 days). A simple cumulative distribution function of the proportion returned relative to tracing date in each group (eg, those ascertained as alive and out of care) (Supplementary Figure 7) reflects the results of each analysis above.
Figure 2.
Rates of return before and after tracing stratified by time interval, where time zero is the date of tracing. Rates are adjusted for age, sex, and CD4 count at antiretroviral therapy initiation using a Cox model. Among all those traced, the hazard ratio (HR) for return to clinic was significantly elevated in the first 2 weeks after tracing (HR, 5.9; 95% confidence interval [CI], 3.4–10.2). When the traced population is stratified into those interviewed in person and out of care, those interviewed in person and in care elsewhere, and those not directly interviewed (ie, interview conducted with an informant), the increase in return rate was only significant in those interviewed in person and out of care (HR, 8.1; 95% CI, 3.9–16.6). After 2 weeks, the return rate was no longer significantly different than the pretracing return rate, suggesting that tracing has a large but transient effect on return to clinic.
Table 2.
Hazard Ratios From Cox Proportional Hazards Models and Rates of Return to Clinic Care During the 2-Year Study Observation Period Among Clinic Patients Who Were Lost to Clinic Care at the Start of the Study
| Patient Group | Study Time Interval | Rate (95% CI) (Unadjusted Rate per 100 PY) |
Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| All alive (irrespective of whether spoken to in person or through proxy) | Before tracing attempts initiated | 29.7 (22.3–39.5) | Reference | Reference |
| From 0 to 14 d after tracing started | 138.8 (93.0–207.0) | 5.9 (3.4–10.2) | 9.6 (5.1–18.1) | |
| From 15 to 90 d after tracing | 23.9 (15.6–36.6) | 1.2 (.7–2.2) | 2.7 (1.3–5.6) | |
| From 91 to 180 d after tracing | 15.1 (8.9–25.4) | 1.3 (.6–2.9) | 3.5 (1.3–9.7) | |
| All alive and spoken to in person | Before tracing attempts initiated | 36.9 (27.0–50.3) | Reference | Reference |
| From 0 to 14 d after tracing started | 194.7 (129.4–293.0) | 6.2 (3.5–11.0) | 10.5 (5.4–20.1) | |
| From 15 to 90 d after tracing | 30.4 (19.2–48.3) | 1.1 (.6–2.1) | 2.4 (1.1–5.1) | |
| From 91 to 180 d after tracing | 19.2 (10.9–33.8) | 1.1 (.5–2.6) | 2.8 (1.0–8.1) | |
| All alive and spoken to in person and reported not in care | Before tracing attempts initiated | 43.0 (27.1–68.3) | Reference | Reference |
| From 0 to 14 d after tracing started | 437.9 (282.5–678.8) | 8.1 (3.9–16.6) | 13.9 (5.8–33.2) | |
| From 15 to 90 d after tracing | 46.4 (24.9–86.1) | 1.0 (.4–2.4) | 1.8 (.6–5.4) | |
| From 91 to 180 d after tracing | 25.7 (11.6–57.2) | 0.6 (.2–2.0) | 1.6 (.4–7.3) | |
| All alive and spoken to in person and reported in care elsewhere | Before tracing attempts initiated | 33.0 (21.7–50.1) | Reference | Reference |
| From 0 to 14 d after tracing started | 41.4 (13.4–128.4) | 2.1 (.6–7.4) | 2.6 (.5–12.4) | |
| From 15 to 90 d after tracing | 21.3 (10.6–42.5) | 1.3 (.5–3.5) | 3.6 (1.2–11.1) | |
| From 91 to 180 d after tracing | 15.3 (6.9–34.1) | 2.2 (.6–8.8) | 6.5 (1.0–40.6) | |
| All dieda | Before tracing attempts initiated | 0 | ||
| From 0 to 14 d after tracing started | 0 | |||
| From 15 to 90 d after tracing | 0 | |||
| From 91 to 180 d after tracing | 0 |
Patients were sampled from 14 clinics across 5 program sites in East Africa (Kenya, Uganda, and Tanzania). Hazard ratios were adjusted for the following covariates: age, sex, pre–antiretroviral therapy (ART) CD4 T-cell count, program site, time on ART before becoming disengaged from care, and time lost at sampling.
Abbreviations: CI, confidence interval; HR, hazard ratio; PY, person-years.
aNo one who died had a return date. Most died before sampling; 2 died after sampling (and were included in estimates above—they did not die during study observation and were censored at database closure). Another 4 patients died during study observation and were dropped from this analysis but none had returned before death, so the rate of return was 0 among those 4 patients. The 4 patients with any tracing interview who died during the study observation period were not included in the proportional hazards model.
Figure 3.
Half-life of the effect of tracing. Date of return to care on each day of observation, where day zero is the date of tracing, was fit to an exponential decay model using Levenberg-Marquardt nonlinear least squares optimization, with confidence intervals (CIs) generated by bootstrap resampling of traced patients. Model parameters including the half-life of the impact of tracing were estimated for all traced patients (half-life of 7.8 days; 95% CI, 3.4–13.0 days), only those patients interviewed in person (half-life of 7.3 days; 95% CI, 3.4–12.2 days), and the subset of all interviewed patients, including both those who reported being in care or out of care (half-life of 7.0 days; 95% CI, 2.6–12.9 days). No significant trend in return date could be identified for those self-reporting to be in care elsewhere. The effect of tracing appeared to be concentrated in patients self-reporting to be out of care, and had a half-life of approximately 1 week until.
Table 3.
Half-life Model Parameters With Bootstrap Confidence Intervals
| Parameter | All Alive (Irrespective of Whether Spoken to in Person or Through Proxy) | Alive and Spoken to in Person | Alive and Spoken to in Person and Reported Not in Care | Alive and Spoken to in Person and Reported in Care Elsewhere |
|---|---|---|---|---|
| Model selection among 6 alternative model specifications | ||||
| Best model based on AICc | Box-decay | Box-decay | Half-life | Interval |
| Best model based on BIC | Half-life | Box-decay | Half-life | No decay |
| Best model based on CAIC | Half-life | Half-life | Half-life | No decay |
| Half-life model parameter estimates (bootstrapped 95% CIs) | ||||
| Return rate per PY before tracing | 0.28 (.20–.36) | 0.34 (.24–.46) | 0.34 (.18–.53) | 0.35 (.22–.50) |
| Decay half-life (days) | 7.82 (3.47–13.02) | 7.29 (3.36–12.23) | 7.03 (2.58–12.91) | 0.80 (.77–1830000000) |
| Return rate per PY on day of tracinga | 2.32 (1.26–4.00) | 3.48 (1.89–6.01) | 8.26 (4.38–15.18) | 0.34 (–.00 to 1.04) |
| Fold increase in rate at time of tracinga | 16.45 (9.24–28.76) | 19.96 (11.07–35.43) | 40.15 (20.99–79.25) | –0.30 (–.49 to 7.67) |
Abbreviations: AICc, Akaike information criterion with correction for finite sample size; BIC, Bayesian information criterion; CAIC, consistent Akaike information criterion; CI, confidence interval; PY, person-year.
aRate after tracing and fold increase in rate are redundant with each other, and so cannot be estimated simultaneously. Two different model specifications were run using the maximum likelihood and bootstrap method to separately estimate these parameters. This is why the estimate of fold increase is close, but not exactly the ratio of the posttracing to the pretracing rate.
DISCUSSION
Selecting lost patients at random to be intensively traced led to a 3% absolute rise in the fraction of lost patients returning to clinic, or an NNT of 33. The small magnitude of effect, however, is largely due to the heterogeneity of true patient outcomes among those lost, which include many states that cannot be influenced by tracing (such as death or those not contacted) and states that perhaps should not be influenced by tracing (such as patients who are transferred to another facility). Among the subset of lost patients who are most plausibly influenced by the intervention—those who are alive, able to be contacted in person, and not enrolled in care elsewhere—tracing had a much larger effect, leading to a 22% rise in the absolute probability of return, or an NNT between 4 and 5. This effect was transient and had a half-life of approximately 1 week. The use of random selection for tracing as an instrument for the “treatment” of finding an out-of-care patient in person enables a causal interpretation even in the presence of potential unmeasured common causes of contacting an out-of-care patient in person and return (ie, unmeasured confounding).
Although this study demonstrates that tracing is effective at returning a subset of patients to care, the large NNT suggests that targeting all lost patients is unlikely to be efficient and strategies that reduce loss to follow-up in the first place should be prioritized. In many settings, patients who miss a visit face challenges to continuation of care such as required “defaulter classes.” Qualitative studies from Uganda suggest that missing a visit triggers guilt and fear of reprimand, which block reengagement [5]. Quantitative work from Uganda, Kenya, and Tanzania recently suggest that a leading clinic-based driver of stopping or switching the site of care is fear of scolding for missing an appointment [13].
Although small in aggregate, the impact of tracing in a specific subpopulation (ie, those alive, able to be contacted in person, and out of care) implies that tracing lost patients could be optimized through targeting the right patients. The relatively strong effect of contact between a tracer and a patient who is out of care on return—identified by an instrumental variable approach—is consistent with research from other fields that face-to-face contact has a unique effect on influencing behavior. Strategies to increase voter turnout, for example, suggest that direct door-to-door and face-to-face conversations are the most consistent strategy that demonstrates change [14]. In addition, a long tradition of diffusion research has focused on social networks and interpersonal communication as the basis of behavioral changes [15]. With regard to loss to follow-up from an HIV program, our results confirm that a conversation offering knowledge, encouragement, or problem solving can be a sufficient cause of return to care. A challenge for targeting patients is that their vital and care status are not known a priori. Correlates of a lost patient’s ultimate status that are known to the clinic could be used to prioritize for tracing those patients who are most likely to benefit, perhaps through the development of clinical prediction rules.
This study has several limitations. It is a post hoc analysis of a randomized sampling strategy originally deployed to help clinics correct underreported statistics for patient mortality. Post hoc analyses must be treated with caution due to positive publication bias and the lack of a prespecified endpoint. Furthermore, unlike routine tracing, where a steady stream of patients is identified as lost over time, our study generated a single roster of patients on the date of randomization. As a result, the study had a longer time lag between identification of lost patients and initiation of tracing efforts, as compared to routine tracing. It is possible that this leads to an underestimate of the impact of tracing because more patients will fall into groups that cannot be improved by the intervention (eg, died). On the other hand, it is possible that effects of tracing soon after a missed visit may be even smaller, as the rate of spontaneous return early after a missed visit is higher. In either case, this limits external generalizability. We do not have a cost-effectiveness analysis, which would have been useful. Additionally, the study only assessed return to the original clinic. The effect of tracing on entry into care at another site was not captured.
In short, we present high-quality evidence based on random assignment of tracing, through a quasi-natural experiment, that the practice of tracing patients who are lost to follow-up from HIV treatment programs in Africa strongly but transiently increases rates of return to care for those patients who are alive, can be contacted, and are not already in care elsewhere—a relatively small proportion of all lost patients. Tracing should be a part of the retention toolbox, but can be more efficient and effective if targeted, perhaps through prediction rules, to the subset of lost patients who are most able to benefit.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers U01AI069918 and P30 AI027763).
Potential conflicts of interest. All authors: No potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nachega JB, Chaisson RE, Goliath R et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS 2010; 24:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chung MH, Richardson BA, Tapia K et al. A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. PLoS Med 2011; 8:e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siedner MJ, Lankowski A, Haberer JE et al. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PLoS One 2012; 7:e39894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMahon JH, Elliott JH, Hong SY, Bertagnolio S, Jordan MR. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One 2013; 8:e56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ware NC, Wyatt MA, Geng EH et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med 2013; 10:e1001369; discussion e1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav 2010; 14:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav 2014; 18:1199–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siedner MJ, Lankowski A, Tsai AC et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS 2013; 27:1503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geng EH, Odeny TA, Lyamuya RE et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2015; 2:e107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geng EH, Bwana MB, Muyindike W et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr 2013; 63:e64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geng EH, Odeny TA, Lyamuya RE et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2015; 2:e107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swanson SA, Hernán MA. Commentary: how to report instrumental variable analyses (suggestions welcome). Epidemiology 2013; 24(3):370–374. [DOI] [PubMed] [Google Scholar]
- 13. Geng EH, Odeny TA, Lyamuya R et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis 2016;62:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerber AS, Green DP. The effects of canvassing, telephone calls, and direct mail on voter turnout: a field experiment. Am Polit Sci Rev 2000; 94:653–63. [Google Scholar]
- 15. Rogers EM. Diffusion of innovations. New York, NY: Simon and Schuster, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.