We report substantial declines in all-cause pneumonia hospitalizations and related costs after introduction of pneumococcal conjugate vaccines in Ontario, Canada. We observed reduced hospitalizations in both vaccinated and unvaccinated age groups, supporting the presence of indirect effects of vaccination.
Keywords: Streptococcus pneumoniae, pneumonia, pneumococcal conjugate vaccine, hospitalization, cost
Abstract
Background
In Ontario, Canada, pneumococcal conjugate vaccine (PCV) was approved for infants in 2001 and became part of the publicly funded routine immunization schedule in 2005. We assessed the population-level impact of PCV on pneumonia hospitalizations and related costs.
Methods
We used the difference-in-differences approach to evaluate the impact of pneumococcal vaccination on pneumonia hospitalizations and related costs, using nonpneumonia hospitalization as the control condition. We extracted monthly hospitalization costs, stratified by age group, from population-based health administrative data between April 1992 and March 2014. The study period was divided into 5 intervals: prevaccine period, availability of 7-valent PCV (PCV7) for private purchase, public funding for PCV7, replacement of PCV7 with 10-valent PCV (PCV10), and replacement of PCV10 with 13-valent PCV (PCV13).
Results
A total of 1063700 pneumonia hospitalizations were recorded during the study period. In the vaccine-eligible age group, pneumonia hospitalizations declined by 34% (95% confidence interval, 32%–37%), 38% (32%–43%), and 45% (40%–51%) and hospitalization-related costs declined by 38% (25%–51%), 39% (33%–45%), and 46% (41%–52%) after public funding for PCV7, PCV10, and PCV13, respectively. Pneumonia hospitalizations and related costs also declined substantially for PCV-ineligible older children and elderly persons (aged >65 years).
Conclusions
Our results suggest that the publicly funded PCV immunization program is responsible for substantial reductions in pneumonia hospitalizations and related healthcare costs, among both young children eligible for publicly funded vaccination and other age groups not included in the publicly funded program.
Pneumonia is a substantial cause of disease and death in high-income settings, particularly among infants and the elderly. In Ontario, Canada, pneumonia is a leading cause of death from infectious disease, with significant healthcare costs due to hospitalizations and physician visits [1]. Streptococcus pneumoniae is one of the most common bacterial causes of community-acquired pneumonia [2, 3].
In June 2001, a 7-valent pneumococcal conjugate vaccine (PCV7) was approved for infants and became available for out-of-pocket private purchase in Canada [4]. This approval was based on prelicensure clinical trials that demonstrated the efficacy of PCV7 against invasive pneumococcal disease (IPD) and pneumonia [5, 6]. Ontario began publicly funding PCV7 for children <2 years of age in January 2005. The 10-valent PCV (PCV10) replaced PCV7 in October 2009, and the 13-valent PCV (PCV13) replaced PCV10 in November 2010. As of 2017, Ontario’s immunization program recommends 3 doses of conjugate pneumococcal vaccine, at 2, 4, and 12 months of age, and a catch-up program, consisting of 1 dose at the first visit for unvaccinated children aged ≤4 years with a second dose 2 months later for those aged ≤23 months at the first visit. For adults aged ≥65 years, Ontario has had publicly funded 23-valent pneumococcal polysaccharide vaccine since 1996. Although PCV13 was authorized for sale in Canada for adults aged ≥50 years in 2012 and younger adults in 2014, public funding in Ontario has been available only for immunocompromised adults aged ≥50 years and only since December 2014.
Recent work highlights the significant impact of routine PCV vaccination in Ontario on the incidence and case fatality rate of IPDs among both vaccinated and unvaccinated age groups [4, 7]. However, the effect of PCV on pneumonia in Ontario remains unknown. This is an important area of interest, as pneumonia causes a greater burden of pneumococcal disease than IPD [8].
To determine PCV’s impact on pneumonia, we examined the effects of private availability and subsequent public funding of PCV7, PCV10, and PCV13 on pneumonia hospitalization rates and related costs among vaccine-eligible children in Ontario (ie, birth cohorts eligible for publicly funded PCV) and potential indirect effects on other age groups.
METHODS
Study Population, Setting, and Design
We included all Ontario residents eligible for the province’s universal health insurance plan (Ontario Health Insurance Plan [OHIP]) between April 1992 and March 2014. Ontario is Canada’s most populous province, with a population of 13.7 million in 2014. We applied the difference-in-differences (DD) approach to examine the impact of pneumococcal vaccines on pneumonia hospitalizations and related costs. We used nonpneumonia hospitalizations as the control condition because pneumococcal vaccination is not expected to impact this outcome. As an average of all other illnesses excluding pneumonia, it captures secular trends in Ontario’s healthcare system. We obtained ethics approval for this study from the Research Ethics Board of Sunnybrook Health Sciences Centre, Toronto, Canada.
Data Sources
We obtained data on pneumonia and nonpneumonia hospitalizations and related costs from the Discharge Abstract Database of the Canadian Institute for Health Information (CIHI), which contains information on all acute care hospitalizations in Ontario. Up to 25 discharge diagnoses are coded for each hospitalization using diagnostic codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada [9] and the International Classification of Diseases, Ninth Revision, Clinical Modification [10]. As physicians may bill OHIP separately for inpatient services, we also used the OHIP database to capture costs from physician billing claims during hospital admissions. The OHIP database contains information on all fee-for-service reimbursement claims paid to physicians eligible to claim under OHIP. Birth dates of hospitalized cases were obtained from Ontario’s Registered Persons Database, which contains basic demographic information for every person who has been eligible for OHIP since 1990. Annual population estimates are from Statistics Canada. We linked and analyzed all data sets using unique, encoded identifiers at the Institute for Clinical Evaluative Sciences.
Outcomes
Our primary outcomes were pneumonia hospitalization rates and related costs. Hospitalization for pneumonia was defined similarly as by Griffin et al [11]: any hospitalization with pneumonia listed as either the primary diagnosis on the discharge record, or as a secondary diagnosis in conjunction with meningitis, septicemia, or empyema as the primary diagnosis (Supplementary Appendix). For the control condition, we included all hospitalizations that did not have pneumonia among any of the listed diagnoses, in order to capture secular trends in hospitalizations and healthcare costs.
Hospitalization costs were determined using the Ontario hospital cost distribution methodology, calculated as the resource intensity weight associated with each hospitalization multiplied by the yearly cost per weighted case [12]. The resource intensity weight is the ratio of the cost of a patient’s hospitalization to the average cost of a typical acute care patient. Costs per weighted case were not available before 2002 and were thus extrapolated using a linear regression equation based on later data. Overall costs were adjusted to the 2013 Ontario Consumer Price Index [12].
Statistical Analysis
The study period was divided into 5 intervals: (1) the prevaccine period (April 1992 to May 2001), (2) PCV7 availability for private purchase (June 2001 to December 2004), (3) public funding for PCV7 (January 2005 to September 2009), (4) replacement of PCV7 with PCV10 (October 2009 to October 2010), and (5) replacement of PCV10 with PCV13 (November 2010 to March 2014).
We used DD analysis to evaluate the impact of each change in pneumococcal vaccine availability on pneumonia hospitalizations and related costs, using nonpneumonia hospitalizations as the control condition. DD is a well-established statistical technique used frequently in economics and other social sciences, and more recently in health services research, to generate evidence to suggest causality between an intervention and the observed outcome when a randomized controlled study design is not feasible [13]. DD estimation consists of identifying a specific intervention or treatment—in this case the introduction of various pneumococcal vaccination regimes—and using regression models to compare the before-and-after-intervention difference in outcomes for groups affected by the intervention with the difference for unaffected groups. The appeal of DD estimation lies in its simplicity and potential to circumvent many endogeneity problems that arise when making comparisons among heterogeneous groups [13].
We first examined the impact of pneumococcal vaccination on children <2 years of age by using year of birth as a proxy for exposure to pneumococcal vaccine. Cohorts born between 2002 and 2004 were eligible for private purchase of PCV7. Cohorts eligible for public funding of PCV7 were defined as children born in 2005 to 2009, whereas those born in 2010 were defined as eligible for PCV10. Finally, cohorts born in 2011 and 2012 were eligible for PCV13. The unexposed, or vaccine-ineligible, group consisted of children born in 1992 to 2001. We estimated the impact of the vaccine regime by comparing the mean of pneumonia-related outcomes of exposed cohorts to the mean of unexposed cohorts (difference 1), less the difference in non–pneumonia-related outcomes between the exposed and unexposed cohorts (difference 2).
The second part of the analysis investigated indirect effects (also known as herd immunity or spillover effects) on age groups that were ineligible for publicly funded vaccine. For each age group, the impact of the vaccine regime was estimated using the DD approach by comparing the mean of the pneumonia-related outcomes under the different vaccine regimes with the mean during the prevaccine period (difference 1), less the difference in non–pneumonia-related outcomes in the same periods (difference 2).
The data were analyzed at the disease-month level from April 1992 to March 2014 with the number of hospitalizations and healthcare costs for pneumonia or nonpneumonia stratified by age group. Because hospitalizations are a count variable, we used a Poisson model to estimate the effect on hospitalizations, with population as the offset. When examining hospitalization costs, we used its logarithmic form. As such, regression estimates can be interpreted as percentage changes.
To capture the influence of aggregate time trends (for example, rising overall healthcare costs), we incorporated year fixed effects, and to account for potential seasonality of pneumonia- and non–pneumonia-related outcomes, we incorporated season fixed effects in our regression models, represented by separate dichotomous variables for each season. We also accounted for public funding of a 23-valent pneumococcal polysaccharide vaccine for adults aged ≥65 years after January 1996 and approval of PCV13 for adults aged ≥50 years beginning in January 2012 (even though public funding only began in December 2014 and only for immunocompromised individuals). We also refitted our models for years (2006–2014) and age groups for which reliable data on influenza vaccine coverage and effectiveness are available [14, 15], to determine whether changes in influenza vaccination coverage might have confounded the analysis. All analyses were conducted using SAS 9.3 (SAS Institute) and Stata 14 (StataCorp) software.
RESULTS
Overall, 1063700 pneumonia hospitalizations occurred during the study period, with a mean annual hospitalization rate of 693.3 per 100000 and annual pneumonia-related hospitalization costs of Can$25.7 million (Table 1). Hospitalization rates were highest among adults >75 years of age, followed by children <2 years of age. Pneumonia-related hospitalization costs were much higher among the older age groups. Hospitalization rates and related costs due to nonpneumonia causes followed a similar distribution, with higher frequencies and costs among older adults and young children.
Table 1.
Mean Age-Specific Annual Pneumonia and Nonpneumonia Hospitalization Rates and Related Healthcare Costs in Ontario, Canada, 1992–2014
| Age Group, y | Hospitalization Rates (per 100000 Population) | Hospitalization Costs, × 1 Million Can$a | ||
|---|---|---|---|---|
| Pneumonia | Nonpneumonia | Pneumonia | Nonpneumonia | |
| Overall | 202.5 | 9096.9 | 205.8 | 1648.1 |
| <2 | 645.9 | 58915.7 | 7.4 | 496.3 |
| 2–4 | 320.3 | 3229.6 | 5.7 | 51.9 |
| 5–17 | 59.4 | 2228.8 | 5.5 | 223.1 |
| 18–39 | 36.3 | 6828.7 | 10.1 | 1237.3 |
| 40–64 | 108.7 | 6828.6 | 41.3 | 2020.2 |
| 65–74 | 450.4 | 16755.5 | 36.8 | 1350.7 |
| 75–84 | 1167.0 | 26682.3 | 56.3 | 1391.9 |
| ≥85 | 2758.3 | 35624.7 | 42.8 | 644.9 |
aCosts were adjusted to the 2013 Ontario Consumer Price Index [12].
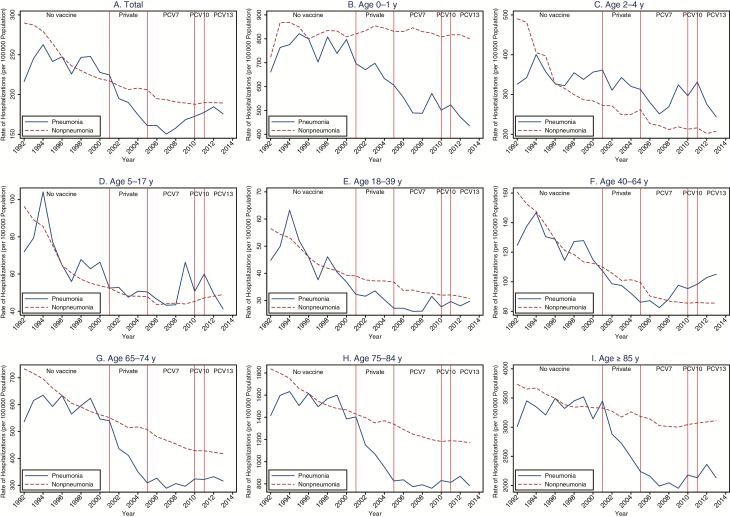
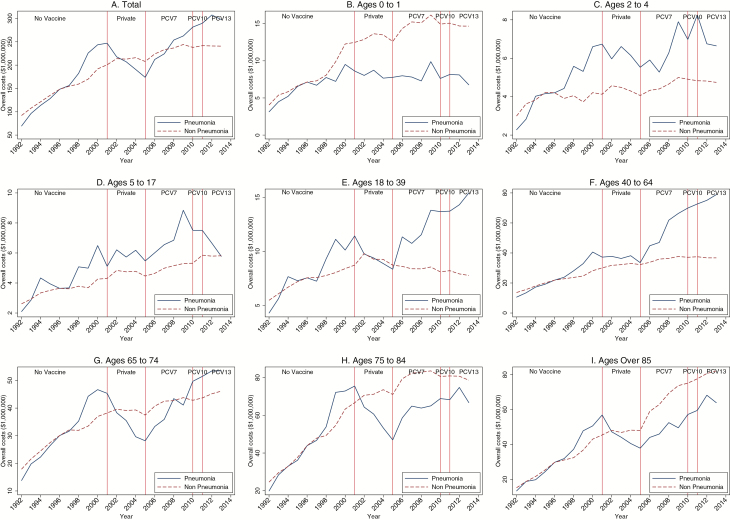
When we normalized nonpneumonia outcomes to the same level as pneumonia outcomes at the middle of the prevaccine period (ie, 1996), hospitalization rates and costs for pneumonia and nonpneumonia causes trended similarly in the prevaccine period for all age groups (Figures 1 and 2) but began diverging in the postvaccine periods, with nonpneumonia above pneumonia for most age groups. The divergence was especially evident for the youngest (<1 year) and oldest age groups (≥65 years).
Figure 1.
Annual hospitalization rates due to pneumonia and nonpneumonia illnesses, overall and for each age group, 1992–2014. PCV7 (7-valent), PCV10 (10-valent), and PCV13 (13-valent) pneumococcal conjugate vaccine, respectively. Source: Canadian Institute for Health Information’s Discharge Abstract Database. Abbreviation: PCV, pneumococcal conjugate vaccine.
Figure 2.
Annual healthcare costs due to pneumonia and nonpneumonia hospitalizations, overall and for each age group, 1992–2014. PCV7 (7-valent), PCV10 (10-valent), and PCV13 (13-valent) pneumococcal conjugate vaccine, respectively. Source: Canadian Institute for Health Information’s Discharge Abstract Database. Abbreviation: PCV, pneumococcal conjugate vaccine.
Impact on Children <2 Years of Age
Relative to cohorts ineligible for pneumococcal vaccine, cohorts of children eligible for private and public funding of PCV7, PCV10, and PCV13 had reductions in pneumonia-related hospitalizations by 17% (95% confidence interval, 14%–20%), 34% (32%–37%), 38% (32%–43%), and 45% (40%-51%), respectively, and reductions in pneumonia-related hospitalization costs by 18% (10%-26%), 38% (25%–51%), 39% (33%–45%), and 46% (41%–52%) (Table 2). The reductions associated with private availability were smaller in magnitude than those associated with publicly funded vaccination. The largest observed reduction occurred for cohorts who were eligible for PCV13, and the reductions were comparable between hospitalization costs and rates.
Table 2.
Effect of Pneumococcal Vaccination on Pneumonia Hospitalizations and Related Costs Among Children Aged 0–2 Years, 1992–2014
| PCV Type | Change in Outcome Variable (95% CI), % | |
|---|---|---|
| No. of Hospitalizationsa | Hospitalization Costs (95% CI)b | |
| Privately purchased PCV7 | −16.8 (−19.8 to −13.8) | −18.1 (−26.2 to −10.0) |
| Publicly funded PCV7 | −34.2 (−36.8 to −31.5) | −37.9 (−50.7 to −25.0) |
| Publicly funded PCV10 | −37.8 (−43.0 to −32.4) | −38.9 (−44.5 to −33.4) |
| Publicly funded PCV13 | −45.3 (−50.7 to −39.8) | −46.1 (−51.6 to −40.5) |
Abbreviations: CI, confidence interval; PCV, pneumococcal conjugate vaccine; PCV7, PCV10, and PCV13: 7-valent, 10-valent, and 13-valent PCV, respectively.
aHospitalizations in children aged 0–2 y during the study period. Poisson regression was used, with the exposure variable being the population size of the age group. All results shown displayed are statistically significant. (P < 0.05).
bNatural logarithm of overall pneumonia-related healthcare utilization costs among children aged 0–2 y during the study period. The regression model is estimated using ordinary least squares. All regressions include fixed effects for disease and each vaccine interval. All results displayed are statistically significant. (P < 0.05).
Impact on Age Groups Ineligible for Publicly Funded Pneumococcal Vaccination
When nonpneumonia illnesses were used as the control condition to evaluate the indirect effects of pneumococcal vaccination on age groups ineligible to receive pneumococcal vaccination, substantial effects were observed, with large and statistically significant reductions in both hospitalizations and related costs across almost all age groups (Table 3). The largest indirect effects were observed among older children and older adults. These effects started with private funding for PCV7 and became more pronounced with the introduction of public funding of PCV7 and subsequent pneumococcal vaccines.
Table 3.
Effect of Pneumococcal Vaccination on Pneumonia Hospitalizations and Related Costs Among Older Age Groups During the Study Period
| Age Group, y | Change (95% CI), % | |||
|---|---|---|---|---|
| Privately Purchased PCV7 | Publicly funded PCV7 | Publicly funded PCV10 | Publicly funded PCV13 | |
| Hospitalization Rate Due to Pneumoniaa | ||||
| 5–17 | −3.6 (−7.3 to 0.2) | −12.3 (−16.0 to −8.6)b | −25.6 (−33.7 to −17.4)b | −80.7 (−87.2 to −74.1)b |
| 18–39 | −16.6 (−20.0 to −13.2)b | −21.9 (−25.1 to −18.7)b | −3.1 (−8.8 to 2.6) | −3.1 (−6.5 to 0.4) |
| 40–64 | −5.7 (−7.7 to −3.7)b | −2.2 (−4.0 to −0.4)b | 15.0 (12.0–18.0)b | 19.7 (17.8–21.5)b |
| 65–74 | −21.8 (−23.9 to −19.6)b | −43.1 (−45.2 to −41.0)b | −36.7 (−40.4 to −33.0)b | −27.7 (−31.1 to −24.3)b |
| 75–84 | −23.3 (−25.0 to −21.6)b | −46.2 (−47.9 to −44.6)b | −47.7 (−50.7 to −44.7)b | −39.6 (−42.4 to −36.9)b |
| ≥85 | −16.3 (−18.4 to −14.3)b | −40.3 (−42.2 to −38.4)b | −46.8 (−49.9 to −43.7)b | −38.2 (−41.0 to −35.4)b |
| Hospitalization Costs Due to Pneumoniac | ||||
| 5–17 | 3.8 (−9.8 to 17.5) | −5.2 (−17.6 to 7.2) | −12.9 (−35.1 to 9.4) | −81.1 (−95.0 to −67.2)b |
| 18–39 | −10.0 (−81.1 to 61.0) | −7.1 (−71.6 to 57.3) | 13.2 (−103.6 to 129.1) | 19.5 (−52.8 to 91.8) |
| 40–64 | −8.2 (−61.2 to 45.3) | −2.3 (−50.8 to 46.3) | 3.6 (−83.6 to 90.8) | 16.1 (−68.3 to 100.5) |
| 65–74 | −25.7 (−73.8 to 22.3) | −40.4 (−84.7 to 3.9) | −40.9 (−115.5 to 33.7) | −21.3 (−93.6 to 51.1) |
| 75–84 | −24.9 (−64.3 to 14.4) | −48.2 (−84.4 to −11.9)b | −60.8 (−121.9 to 0.4) | −47.4 (−106.7 to 11.9) |
| ≥85 | −15.4 (−47.7 to 17.0) | −47.5 (−77.3 to −17.6)b | −66.0 (−116.3 to −15.6)b | −51.9 (−100.6 to −3.1)b |
Abbreviations: CI, confidence interval; PCV7, PCV10, and PCV13, the 7-valent, 10-valent, and 13-valent pneumococcal conjugate vaccine, respectively.
aThis outcome variable is the number of hospitalizations due to pneumonia in the respective age groups during the period of study. Poisson regression was used, with the exposure variable being the population size of the age group.
bStatistically significant results. (P < 0.05).
cThis outcome variable is the natural logarithm of pneumonia-related hospitalization costs in the respective age groups during the period of study. The regression model is estimated using ordinary least squares. All regressions include fixed effects for disease and vaccine interval.
Impact of Influenza Vaccination Programs
When we accounted for influenza vaccine coverage and effectiveness, we found no qualitative differences in our estimates of the effects of pneumococcal vaccination (data not shown).
DISCUSSION
Our study suggests that publicly funded immunization programs for PCV7, PCV10, and PCV13 were associated with substantial reductions in pneumonia-related hospitalizations and related costs among young children. Substantial reductions also occurred among older children and older adults who were ineligible for publicly funded conjugate vaccine. Our results suggest that, like the indirect effects previously observed for IPD in Ontario and elsewhere, the benefits of pneumococcal vaccination against pneumonia extend beyond vaccine recipients [4, 16]. We also observed significant reductions in pneumonia-related hospitalization costs, reductions that tended to be larger in magnitude than the reductions in hospitalization rates. A possible explanation is that pneumococcal vaccination decreases not only the likelihood but also the severity of pneumonia (and therefore corresponding lengths of hospital stay [17]).
This study represents the longest observation period of any analysis examining the impact of PCV on pneumonia hospitalizations to date. The sustained reduction in pneumonia hospitalization rates nearly 15 years after PCV introduction emphasizes that serotype replacement has not eroded the vaccine’s health benefits. The decline in pneumonia incidence mirrors the patterns of IPD observed in Ontario, where the serotype replacement that has occurred has been limited and the greatest relative reductions in incidence have occurred in the age groups with the greatest disease burden [4].
The substantial reductions in pneumonia hospitalizations after the introduction of PCV10 and PCV13 strongly suggest that serotypes unique to these vaccines caused a significant proportion of the residual pneumococcal burden in the post-PCV7 period. The observed decline in the proportion of IPD caused by serotypes unique to PCV10 and PCV13 over the same period in both vaccinated and unvaccinated Ontario populations further supports this conclusion [18, 19]. In addition, based on the duration of the decline in pneumonia hospitalizations observed after PCV7 implementation, the impact of PCV13 may be underestimated in our analysis because we could include only 3 years of data after introduction of the PCV13 program.
Our findings are consistent with reported reductions in pneumonia incidence and related healthcare use in other populations after the introduction of PCVs. A 2008 study conducted in the neighboring province of Quebec reported a 13% reduction in pneumonia hospitalizations among children <5 years of age after public funding of PCV7 [20]. This is lower than the 34% reduction observed among children <2 years of age in our study population, probably owing to differences in the age groups studied. Several US studies also reported significant reductions in pneumonia hospitalizations in both vaccinated and unvaccinated populations after the introduction of PCV [11, 21–24].
Three studies using the same pneumonia case definition as our analysis reported reductions of 39%, 43%, and 52% among children <2 years of age after PCV7 introduction, which are similar to our estimates [11, 23, 24]. Moreover, significant reductions in pneumonia hospitalizations were observed in nonvaccinated age groups in all the studies [11, 23]. One of these studies also assessed the impact of PCV on costs related to pneumonia hospitalizations among children <2 years of age; Zhou and colleagues [24] reported a 45% reduction in costs 4 years after the introduction of PCV7 into the routine immunization schedule, similar to the 38% reduction in costs our study reports.
Our study has several strengths. We were able to examine the impact of several versions of PCV; our study is the first to formally compare pneumonia-related outcomes during 5 distinct periods of vaccine implementation and represents the longest observation period of any analysis examining the impact of PCV on pneumonia hospitalizations to date. Using the DD approach with nonpneumonia hospitalizations as a comparator allowed us to control for secular changes in hospitalization rates or health trends in the population. The key identifying assumption of a DD analysis is that, absent the intervention, pneumonia-related and non–pneumonia-related outcomes would generally exhibit parallel trends.
Although this assumption cannot be formally tested, we believe this argument is reasonable for 2 reasons. First, pneumonia and nonpneumonia hospitalization rates and costs trended closely during the prevaccine period across most age groups. Second, using the average across all nonpneumonia illnesses should capture in aggregate secular trends in the Ontario healthcare system during the study period. These reasons bolster the argument that nonpneumonia hospitalizations capture the counterfactual trends in pneumonia-related outcomes. However, we recognize that there may be other factors that affected pneumonia-related outcomes that are not fully captured in the comparator condition. For example, if there were factors that led to an additional decline in pneumonia-related hospitalizations and costs (but not in nonpneumonia diseases), then our estimates would be upward biased.
This study’s main limitation is the lack of individual-level data for pneumococcal vaccination status, so the analysis essentially estimates the intention-to-treat effect. Absence of these vaccination data also makes identifying trends in vaccine uptake difficult, although our DD approach should help circumvent the issue of omitted variable bias. Another limitation is that nonpneumonia hospitalizations may not capture counterfactual trends in hospitalization rates accurately. For example, nonpneumonia hospitalizations may have included outcomes that could have been prevented by PCVs, such as IPD without pneumonia, although such hospitalizations would account for a negligible percentage of the universe of nonpneumonia hospitalizations. Furthermore, we did not capture pneumonia cases managed entirely in ambulatory settings or other infections caused by S. pneumoniae, such as IPD or otitis media; therefore we probably underestimated the impact of pneumococcal vaccination.
We did not account for private market sales of PCV, which may have contributed to the observed reductions in pneumonia-related hospitalizations among older age groups during the period of private availability of PCV7 [25]. For simplicity, our estimates assume that the full effect of each program occurred at the onset of the program and was replaced by the effect of the next program; however, because the population effect takes time, this may underestimate the effect of each program, particularly that of private use of PCV7 in children between 2001 and 2005. We tested for and found no evidence that changes in vaccine coverage or effectiveness affected our results, although data were not available to perform the analysis for all age groups and all years.
In conclusion, the introduction of publicly funded pneumococcal vaccination with increasing serotype coverage was associated with large reductions in pneumonia-related hospitalizations and costs in Ontario. The benefits of these programs extended beyond those who were eligible to receive the vaccine, which may have additional implications for parent, grandparent, and caregiver productivity. These results highlight the need to evaluate and incorporate such broader benefits of vaccination in economic analyses of immunization.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimers. This article’s opinions, results, and conclusions are those of the authors and are independent from the funding sources. The study sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial support. This work was supported by the Harvard T.H. Chan School of Public Health and the National Institute on Aging of the National Institutes of Health (award P30AG024409), the Canadian Institutes for Health Research (New Investigator award to J. C. K.), the University of Toronto Department of Family and Community Medicine (Clinical Scientist award to J. C. K.), and Public Health Ontario and the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care.
Parts of this material are based on data and information compiled and provided by the Canadian Institute of Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of CIHI. No endorsement by the Institute for Clinical Evaluative Sciences, Public Health Ontario, the Ontario Ministry of Health and Long-Term Care, or CIHI is intended or should be inferred.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kwong JC, Ratnasingham S, Campitelli MA et al. The impact of infection on population health: results of the Ontario Burden of Infectious Diseases Study. PLoS One 2012; 7:e44103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain S, Self WH, Wunderink RG et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jain S, Williams DJ, Arnold SR et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudnick W, Liu Z, Shigayeva A et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine 2013; 31:5863–71. [DOI] [PubMed] [Google Scholar]
- 5. Black S, Shinefield H, Fireman B et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000; 19:187–95. [DOI] [PubMed] [Google Scholar]
- 6. Black SB, Shinefield HR, Ling S et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21:810–5. [DOI] [PubMed] [Google Scholar]
- 7. Lim GH, Wormsbecker AE, McGeer A et al. Have changing pneumococcal vaccination programmes impacted disease in Ontario?Vaccine 2013; 31:2680–5. [DOI] [PubMed] [Google Scholar]
- 8. Kwong JC, Ratnasingham S, Campitelli MA et al. Ontario Burden of Infectious Disease Study (ONBOIDS): an OAHPP/ICES report. Toronto, Ontario, Canada: Ontario Agency for Health Protection and Promotion, Institute for Clinical Evaluative Sciences, 2010. [Google Scholar]
- 9. International statistical classification of diseases and related health problems, tenth revision, Canada (ICD-10-CA). Ottawa, Ontario, Canada: Canadian Institute for Health Information, 2009. [Google Scholar]
- 10. Public Health Service, US Department of Health and Human Services. International classification of diseases, ninth revision, clinical modification. Washington, DC: US National Center for Health Statistics, 1988. [Google Scholar]
- 11. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wodchis WB, Wodchis K, Nikitovic M, McKillop I.. Guidelines on person-level costing using administrative databases in Ontario. Toronto, Ontario, Canada: Health System Performance Research Network, 2013. [Google Scholar]
- 13. Meyer BD. Natural and quasi-experiments in economics. J Bus Econ Stat 1995; 13:151–61. [Google Scholar]
- 14. Buchan SA, Kwong JC. Trends in influenza vaccine coverage and vaccine hesitancy in Canada, 2006/07 to 2013/14: results from cross-sectional survey data. CMAJ Open 2016; 4:E455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005–2016 Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed 27 April 2017.
- 16. Shiri T, Datta S, Madan J et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health 2017; 5:e51–9. [DOI] [PubMed] [Google Scholar]
- 17. Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis 2006; 42:1093–101. [DOI] [PubMed] [Google Scholar]
- 18. Desai S, Policarpio ME, Wong K, Gubbay J, Fediurek J, Deeks S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: a population-based study. CMAJ Open 2016; 4:E545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGeer A, Rudnick W, Green K, Li J, Pong-Porter S, Plevneshi A. Invasive pneumococcal disease (IPD) in children and adults following introduction of pneumococcal conjugate vaccines: data from population based surveillance 2001–2015. Open Forum Infect Dis 2016: 3(suppl 1):915. [Google Scholar]
- 20. De Wals P, Robin E, Fortin E, Thibeault R, Ouakki M, Douville-Fradet M. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J 2008; 27:963–8. [DOI] [PubMed] [Google Scholar]
- 21. Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio 2011; 2:e00309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med 2014; 2:387–94. [DOI] [PubMed] [Google Scholar]
- 23. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. [DOI] [PubMed] [Google Scholar]
- 24. Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med 2007; 161:1162–8. [DOI] [PubMed] [Google Scholar]
- 25. Kwong JC, Stukel TA, Lim J et al. The effect of universal influenza immunization on mortality and health care use. PLoS Medicine 2008; 5:1440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.