Diabetes mellitus independently poses an increased risk of adverse tuberculosis (TB) treatment outcomes. There was a significant association between metformin use and decreased mortality during TB treatment, suggesting a potential role for this agent as adjuvant therapy.
Keywords: diabetes mellitus, host-directed therapy, metformin, treatment outcomes, tuberculosis
Abstract
Background
The global type 2 diabetes mellitus (DM) epidemic threatens progress made in reducing tuberculosis (TB)–related mortality worldwide. Previous clinical studies have not fully evaluated potential confounding variables in addressing the impact of DM on TB treatment outcomes. The antidiabetic agent metformin regulates autophagy and may play a role as a host-directed therapeutic adjuvant to antitubercular treatment.
Methods
We conducted a retrospective cohort study comprising patients aged ≥13 years undergoing treatment for culture-confirmed, drug-susceptible pulmonary TB. We assessed the effect of DM on mortality during TB treatment and 2-month TB sputum-culture conversion. We also evaluated the effect of metformin use on survival during TB treatment.
Results
Among 2416 patients undergoing TB treatment, after adjusting for age, sex, chronic kidney disease, cancer, hepatitis C, tobacco use, cavitary disease, and treatment adherence, patients with DM had 1.91 times higher odds (95% confidence interval [CI], 1.51–2.40) of death during TB treatment than patients without DM, and 1.72 (95% CI, 1.25–2.38) times higher odds of remaining culture-positive at 2 months. Metformin use in patients with DM was significantly associated with decreased mortality during TB treatment (hazard ratio, 0.56 [95% CI, .39–.82]), and metformin users had similar mortality as patients without DM.
Conclusions
This study suggests that despite multiple potential confounding variables, DM poses an increased risk of adverse TB treatment outcomes. There was a significant association between metformin use and decreased mortality during TB treatment, suggesting a potential role for this agent as adjunctive, host-directed therapy.
Tuberculosis (TB) is the leading infectious cause of death worldwide, claiming approximately 1.8 million lives per year [1]. The number of new TB cases is decreasing by approximately 2% per year, narrowly achieving the Millennium Development Goal set in 2000 to reverse the spread of the disease by 2015 [2]. However, this progress is threatened by the global demographic transition resulting in a rapid rise in the burden of noncommunicable diseases among populations in TB-endemic countries [3], particularly type 2 diabetes mellitus (DM), which is expected to more than double in number in Africa and increase by nearly two-thirds in Asia over the next 20 years [4].
Patients with DM are not only at increased risk for developing active TB [5], but they also experience worse treatment outcomes [6]. However, the precise impact of DM on TB treatment outcomes remains unknown. Thus, although a meta-analysis found that DM was a risk factor for poor treatment outcomes, of the 33 studies reviewed, only 4 controlled for potential confounding variables, and only 2 controlled for factors other than age and sex, both of which were conducted in the United States [6]. These are important considerations as DM is often associated with older age, obesity, multiple comorbidities [7] including chronic kidney disease and cancer, and poor treatment adherence [8], all of which can influence TB risk and treatment outcomes [2, 9, 10].
Finally, in the past 50 years, there have been only 4 new US Food and Drug Administration–approved drugs for the treatment of TB, of which only 2 are from novel classes [11]. Recent attention has focused on host-directed therapy, which may have the potential to reduce TB related-inflammation and associated lung damage, as well as treatment duration and rates of resistance [12]. In particular, metformin, a first-line drug for DM, has received significant attention recently as a potential adjunctive agent for TB, as it has been shown to enhance autophagy, an immune process crucial for the control of TB [13–15]. Patients with DM are thus a key group in which to assess the potential of metformin as an adjunctive therapy for TB.
Accordingly, we performed a retrospective cohort study to assess the effect of DM and metformin use on all-cause mortality during TB treatment, and 2- and 6-month sputum TB culture conversion rates, adjusting for common confounders often accompanying DM.
METHODS
Study Design and Population
This was a retrospective cohort study. Patients aged ≥13 years with culture-confirmed, drug-susceptible pulmonary TB undergoing treatment at the National Taiwan University Hospital (NTUH) in Taipei, Taiwan, between 2000 and 2013 were selected for inclusion. There were no exclusion criteria. NTUH is one of 19 teaching hospitals serving the 9.2 million people in northern Taiwan. More than 90% of patients with TB were treated in the ambulatory setting. Patients were treated according to the American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America clinical practice guidelines [16]. Repeat TB sputum cultures were not routinely obtained until 2006. All patient data were de-identified. The study was approved by the institutional review boards of the National Taiwan University Hospital and Johns Hopkins University.
Clinical Variables
The first exposure was DM, defined by any one of the following criteria prior to TB treatment initiation: International Classification of Diseases (ICD) coding for DM; prescription of oral hypoglycemic agents and/or insulin; fasting blood glucose (FBG) >7.0 mmol/L; non-FBG >11.1 mmol/L; or hemoglobin A1c (HbA1c) >6.5%. The second exposure was metformin use among patients with DM during TB treatment. Metformin use was obtained from data on prescriptions filled during TB treatment, and was categorized in 2 ways. In the first, if a person was on metformin within 30 days of starting TB treatment, they were classified as using metformin throughout TB treatment, similar to an intention-to-treat analysis. In the second classification, only those patients using metformin during ≥80% of TB treatment were classified as metformin users. Patients with chronic kidney disease (CKD) stage 3b or higher were excluded from the metformin analysis, as this is a relative contraindication to metformin use and could have a confounding effect [17].
Data on the following known TB-associated risk factors and potential confounders were collected: age, sex (males as reference group), body mass index (BMI), CKD (ICD-9 coding or glomerular filtration rate <60 mL/minute/1.73 m2 for >3 months), history of or active malignancy, hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, autoimmune disease, history of tobacco use, initial sputum acid-fast smear status, cavitary lesions on chest radiograph, and TB treatment adherence. Poor TB treatment adherence was defined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation [16] or completing >80% of TB treatment up to the time of death during TB treatment. Data was also collected on FBG and HbA1c. Human immunodeficiency virus (HIV) serostatus and hyperglycemia data were only available for a subset of the total 2416 patients in the cohort; therefore, these parameters were not included in the analysis.
The primary clinical outcome was all-cause mortality during TB treatment. Secondary microbiological outcomes included 2- and 6-month sputum culture status.
Statistical Analysis
Data were analyzed using Stata version 13.0 software (StataCorp, College Station, Texas). The distributions of characteristics between patients with and without DM were compared using t test or χ2 test. Univariate logistic regression with mortality and 2-month culture status as the outcome was used to obtain crude odds ratios for DM, age, elderly (aged ≥65 years), sex, BMI, CKD, cancer, HBV, HCV, autoimmune disease, cavitary disease, and poor treatment adherence. Multivariate logistic regression was performed with mortality and 2-month culture status as the outcome variables predicted by DM status using all significant covariates (P < .05) in the crude logistic regression model for either outcome variable. The effect of metformin on time to mortality during TB treatment was analyzed using survival analysis and Cox regression modeling using the above criteria.
RESULTS
Demographics and Comorbidities of Active Tuberculosis Cases Stratified by Diabetes Mellitus Status
There were a total of 2416 cases of culture-confirmed pulmonary TB diagnosed between 2000 and 2013 in the cohort. Baseline demographic data and clinical characteristics are shown in Table 1. The median age was 66.0 years (interquartile range [IQR], 47.6–77.2 years) and 68.3% were male. Of the 2416 patients, 699 (28.9%) had DM. Patients with DM were older and more were male relative to patients without DM. Among the 987 subjects for whom BMI data were available, patients with DM had a higher mean BMI, a lower proportion was underweight, and a higher proportion was overweight. Among comorbidities assessed, patients with DM were more likely to have CKD, cancer, HCV, and a history of tobacco use. There was no significant difference between the 2 groups in the proportion of patients with cavitary disease, positive sputum acid-fast smear at the start of TB treatment, or TB treatment adherence. Only 35% of the patients were tested for HIV, of whom 5.9% were positive.
Table 1.
Baseline Characteristics of Patients With Culture-Confirmed Pulmonary Tuberculosis Treated at National Taiwan University Hospital, 2000–2013, Stratified by Diabetes Mellitus Status (N = 2416)
| Characteristic | DM | Without DM | Total | P Value |
|---|---|---|---|---|
| (n = 699) | (n = 1717) | (N = 2416) | ||
| Demographics | ||||
| Age, y | <.001 | |||
| Median (IQR) | 71.6 (58.7–79.3) | 62.1 (41.3–76.4) | 66.0 (47.6–77.2) | |
| Mean (SD) | 68.6 (14.6) | 58.2 (21.1) | 61.2 (20.0) | |
| Elderly (>65 y), % | 64.4 | 46.1 | 51.4 | <.001 |
| Male sex, % | 76.4 | 64.9 | 68.3 | <.001 |
| BMI, kg/m2 (n = 495; 1254) | <.001 | |||
| Mean (SD) | 22.0 (3.8) | 20.8 (3.3) | 21.2 (3.5) | |
| Underweight (BMI <18.5), % (n = 444; 1309) | 17.6 | 24.7 | 22.7 | .001 |
| Overweight (BMI >25), % (n = 444; 1309) | 18.6 | 10.1 | 12.5 | <.001 |
| Comorbidities, % | ||||
| Chronic kidney disease | 24.2 | 9.0 | 13.4 | <.001 |
| Cancer | 20.3 | 13.2 | 15.3 | <.001 |
| Hepatitis B virus | 6.9 | 6.1 | 6.3 | .470 |
| Hepatitis C virus | 4.3 | 2.3 | 2.9 | .007 |
| Autoimmune | 2.4 | 3.9 | 3.5 | .072 |
| Alcoholism | 2.9 | 3.2 | 3.1 | .660 |
| History of tobacco use | 37.9 | 29.4 | 31.9 | <.001 |
| Cavitary disease, % | 16.2 | 13.7 | 14.4 | .116 |
| Initial sputum acid-fast smear-positive, % | 40.2 | 42.4 | 41.8 | .314 |
| TB treatment adherencea, % | 75.8 | 76.5 | 76.3 | .711 |
Abbreviations: BMI, body mass index; DM, type 2 diabetes mellitus; IQR, interquartile range; SD, standard deviation; TB, tuberculosis.
aDefined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation or completing >80% of TB treatment up to the time of death during TB treatment.
Effect of Diabetes Mellitus and Comorbidities on Mortality During Tuberculosis Treatment
During TB treatment, a higher proportion of patients with DM than those without DM experienced the primary clinical outcome, death (29.0% vs 13.7%; P < .01). Crude and adjusted odds ratios for the logistic regression model with mortality during TB treatment as the outcome are shown in Table 2. In unadjusted logistic regression, patients with DM had 2.57 times higher odds of death during TB treatment than patients without DM (95% confidence interval [CI], 2.07–3.18; P < .001). After adjusting for age, sex, CKD, cancer, HCV, history of tobacco use, cavitary disease, and poor TB treatment adherence, patients with DM had 1.91 times higher odds of mortality during TB treatment than those without DM (95% CI, 1.51–2.40; P < .01).
Table 2.
Crude and Adjusted Odds Ratios, Based on a Logistic Regression Model, of Mortality During Tuberculosis Treatment (N = 2416)
| Characteristic | Crude OR | (95% CI) | P Value | Adjusted ORa | (95% CI) | P Value |
|---|---|---|---|---|---|---|
| DM | 2.57 | (2.07–3.18) | <.001 | 1.91 | (1.51–2.40) | <.001 |
| Age | 1.04 | (1.04–1.05) | <.001 | 1.04 | (1.03–1.05) | <.001 |
| Male sex | 1.92 | (1.50–2.46) | <.001 | 1.57 | (1.18–2.10) | .002 |
| CKD | 2.40 | (1.85–3.13) | <.001 | 1.42 | (1.07–1.90) | .017 |
| Cancer | 3.63 | (2.85–4.63) | <.001 | 3.14 | (2.42–4.08) | <.001 |
| HCV | 1.62 | (.94–2.81) | .083 | 1.13 | (.62–2.05) | .686 |
| History of tobacco use | 1.24 | (1.00–1.54) | .053 | 0.87 | (.68–1.12) | .288 |
| Cavitary disease | 1.23 | (.93–1.64) | .143 | 1.59 | (1.16–2.18) | .004 |
| TB treatment adherenceb | 2.10 | (1.68–2.62) | <.001 | 2.18 | (1.70–2.78) | <.001 |
Abbreviations: CKD, chronic kidney disease; CI, confidence interval; DM, type 2 diabetes mellitus; HCV, hepatitis C virus infection; OR, odds ratio; TB, tuberculosis.
aAdjusted for all variables included in the table.
bDefined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation or completing >80% of TB treatment up to the time of death during TB treatment.
BMI data were only available for 1749 of the 2416 patients. In an unadjusted logistic regression model with BMI as a continuous variable, increasing BMI was significantly associated with decreased odds of death during TB treatment (odds ratio [OR], 0.92 [95% CI, .89–.96]; P < .01). When BMI was included in the multivariate model above, DM remained significantly associated with death during TB treatment (OR, 2.02 [95% CI, 1.49–2.73]; P < .01).
Effect of Diabetes Mellitus and Comorbidities on 2-Month and 6-Month Sputum-Culture Conversion
Sputum culture data were only available for 1323 of the 2416 patients, as they were not routinely collected at NTUH until 2006. The 1323 patients for whom follow-up TB sputum culture data were available did not significantly differ from the patients for whom follow-up culture data were not available in any of the clinical variables above except cancer (16.7% vs 13.5%; P = .03) and autoimmune disease (4.3% vs 2.5%; P = .01). During TB treatment, a higher proportion of patients with DM than patients without DM had a 2-month sputum culture positive for Mycobacterium tuberculosis (23.9% vs 14.2%; P < .01). There was no difference in 6-month TB sputum culture positivity between DM and non-DM patients (0.3% vs 0.8%; P = .39). Crude and adjusted ORs for the logistic regression model with positive 2-month sputum culture as the outcome are shown in Table 3. In unadjusted logistic regression, patients with DM had 1.89 times higher odds of having positive sputum cultures after 2 months of TB treatment than patients without DM (95% CI, 1.40–2.55; P < .001). When adjusting for age, sex, CKD, cancer, HCV, history of tobacco use, cavitary disease, and poor TB treatment adherence, patients with DM still had significantly increased odds of remaining sputum culture positive at 2 months (OR, 1.72 [95% CI, 1.25–2.38]; P = .001). BMI was not associated with sputum culture positivity after 2 months of TB treatment (OR, 1.02 [95% CI, .98–1.07]; P = .34).
Table 3.
Crude and Adjusted Odds Ratios, Based on a Logistic Regression Model, of 2-Month Sputum Culture Positivity for Mycobacterium tuberculosis (n = 1323)
| Characteristic | Crude OR | (95% CI) | P Value | Adjusted ORa | (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Type 2 diabetes mellitus | 1.89 | (1.40–2.55) | <.001 | 1.72 | (1.25–2.38) | .001 |
| Age | 1.00 | (1.00–1.01) | .510 | 1.00 | (.99–1.01) | .693 |
| Male | 1.52 | (1.10–2.10) | .012 | 1.43 | (.98–2.08) | .062 |
| Chronic kidney disease | 1.14 | (.77–1.69) | .510 | 1.07 | (.70–1.65) | .751 |
| Cancer | 0.90 | (.61–1.34) | .612 | 0.78 | (.51–1.18) | .242 |
| Hepatitis C virus | 1.55 | (.72–3.33) | .258 | 1.40 | (.63–3.13) | .410 |
| History of tobacco use | 1.42 | (1.06–1.91) | .020 | 1.05 | (.75–1.48) | .762 |
| Cavitary disease | 4.04 | (2.90–5.65) | <.001 | 4.03 | (2.84–5.71) | <.001 |
| Poor TB treatment adherence | 1.06 | (.72–1.57) | .764 | 1.16 | (.77–1.75) | .490 |
Abbreviations: CI, confidence interval; OR, odds ratio; TB, tuberculosis.
aAdjusted for all variables included in the table.
bDefined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation or completing >80% of TB treatment up to the time of death during TB treatment.
Effect of Metformin on Mortality Among Patients With Diabetes Mellitus Undergoing Tuberculosis Treatment
Among the 699 patients with DM in the cohort, 65 were excluded because of CKD stage 3b or higher, a contraindication to metformin use, to eliminate potential confounding. Of the remaining 634 patients with DM in the cohort, 216 received metformin and 418 received other antidiabetic agents at TB treatment initiation, which was defined as days 0–30 of TB treatment. Patients receiving metformin had statistically significant lower median age and proportion of cancer, and statistically significant higher BMI, proportion of cavitary disease, and proportion of positive initial sputum acid-fast stain (Table 4). Metformin users also had a statistically higher mean HbA1c measured within 3 months of initiating TB treatment.
Table 4.
Baseline Characteristics of 634 Diabetes Patients With Culture-Confirmed Tuberculosis, Stratified by Metformin Use
| Characteristic | Metformin (n = 216) | Non-Metformin (n = 418) | Total (n = 634) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | .016 | |||
| Median (IQR) | 69.0 (54.5–77.0) | 71.6 (60.1–80.3) | 71.0 (57.5–79.2) | |
| Mean (SD) | 66.1 (14.5) | 69.1 (15.0) | 68.1 (14.9) | |
| Elderly (>65 y), % | 56.9 | 65.6 | 62.6 | .034 |
| Male sex, % | 77.8 | 77.0 | 77.3 | .882 |
| BMI, kg/m2 (n = 166; 283) | .008 | |||
| Mean (SD) | 22.6 (3.9) | 21.6 (3.6) | 21.9 (3.8) | |
| Comorbiditya, % | ||||
| Chronic kidney disease | 18.4 | 12.5 | 13.4 | .056 |
| Cancer | 24.2 | 14.8 | 21.0 | .006 |
| Hepatitis B virus | 8.3 | 6.2 | 6.3 | .321 |
| Hepatitis C virus | 4.2 | 3.3 | 3.6 | .602 |
| Autoimmune | 2.8 | 1.9 | 2.2 | .483 |
| Alcoholism | 2.3 | 2.9 | 2.7 | .681 |
| History of tobacco use | 41.7 | 38.3 | 39.4 | .409 |
| Cavitary disease, % | 24.1 | 13.4 | 18.8 | .001 |
| Initial sputum acid-fast smear-positive, % | 51.9 | 34.2 | 40.2 | <.001 |
| Poor TB treatment adherenceb, % | 78.2 | 75.4 | 76.3 | .418 |
| Blood glucose controlc | ||||
| FBG (n = 128; 250) | ||||
| Mean (SD), mmol/L | 9.5 (4.5) | 8.6 (4.5) | 8.9 (4.5) | .064 |
| HbA1c (n = 141; 154) | ||||
| Mean % (SD) | 8.9 (2.5) | 8.2 (2.4) | 8.5 (2.5) | .020 |
Abbreviations: BMI, body mass index; FBG, 2-hour fasting blood glucose; HbA1c, hemoglobin A1c; IQR, interquartile range; SD, standard deviation; TB, tuberculosis.
aForty-six patients with CKD stage 3b or higher were excluded from the study due to concerns for confounding.
bDefined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation or completing >80% of TB treatment up to the time of death during TB treatment.
cMeasured within 3 months of TB treatment start. Patients with FGB >7 mmol/L or HbA1c >6.5% are considered to have diabetes.
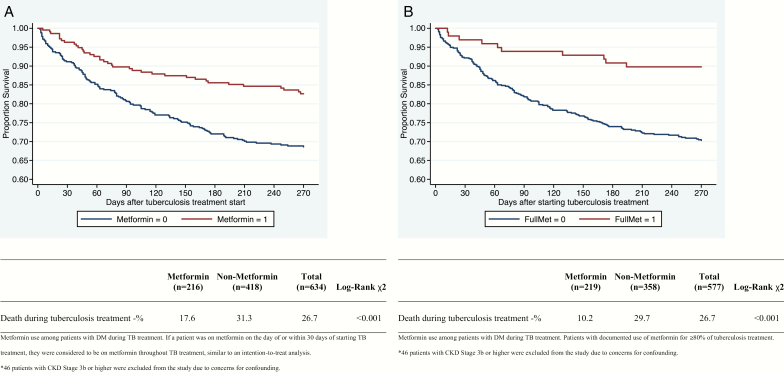
Overall mortality was 17.6% among metformin users and 31.3% among non-metformin users, and survival was significantly higher in the metformin group in a log-rank test of Kaplan-Meier survival distributions (P = .01; Figure 1). In a Cox proportional hazards model adjusting for age, sex, CKD, cancer, cavitary disease, and poor TB treatment adherence, metformin users had 0.56 hazard of death compared with non-metformin users (95% CI, .39–.82; P = .002; Table 5). BMI data were only available for 449 of the patients. In the unadjusted Cox regression model with BMI as a continuous variable, increasing BMI was significantly associated with decreased hazard of death during TB treatment (hazard ratio [HR], 0.88 [95% CI, .84–.93]; P < .01). When BMI was included in the multivariate model above, metformin use remained significantly associated with decreased death during TB treatment (HR, 0.53 [95% CI, .35–.81]; P < .01).
Figure 1.
Kaplan-Meier estimates of time to mortality among diabetic patients during tuberculosis treatment according to metformin use (n = 634). Patients with chronic kidney disease stage 3b or higher were excluded due to concerns for confounding. A, Intention-to-treat analysis. If a person was on metformin within 30 days of starting tuberculosis treatment, they were classified as using metformin throughout tuberculosis treatment. B, Documented metformin use for 80% of tuberculosis treatment. Only those patients with prescription data showing metformin use during equal to or more than 80% of tuberculosis treatment were classified as metformin users.
Table 5.
Crude and Adjusted Hazard Ratios, Based on Cox Proportional Hazards Model, of Survival Among Diabetic Patients During Tuberculosis Treatment (n = 634)
| Characteristic | Intention-to-Treat Analysis | Documented Metformin Use for 80% of Tuberculosis Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude HR | (95% CI) | P Value | Adjusted HRa | (95% CI) | P Value | Crude HR | (95% CI) | P Value | Adjusted HRa | (95% CI) | P Value | |
| Metformin use | 0.50 | (.35–.72) | <.001 | 0.56 | (.39–.82) | .002 | 0.31 | (.16–.58) | <.001 | 0.41 | (.21–.78) | .007 |
| Age | 1.03 | (1.02–1.04) | <.001 | 1.03 | (1.02–1.05) | <.001 | 1.03 | (1.02–1.04) | <.001 | 1.03 | (1.02–1.04) | <.001 |
| Male sex | 1.18 | (.81–1.72) | .390 | 1.27 | (.87–1.85) | .223 | 1.18 | (.81–1.72) | .390 | 1.24 | (.85–1.81) | .263 |
| CKDb | 1.32 | (.90–1.93) | .158 | 1.11 | (.75–1.63) | .611 | 1.32 | (.90–1.93) | .158 | 1.14 | (.77–1.68) | .517 |
| Cancer | 1.92 | (1.39–2.65) | <.001 | 1.79 | (1.29–2.48) | <.001 | 1.92 | (1.39–2.65) | <.001 | 1.83 | (1.32–2.53) | <.001 |
| Cavitary disease | 1.12 | (.76–1.65) | .577 | 1.55 | (1.04–2.32) | .033 | 1.12 | (.76–1.65) | .577 | 1.49 | (1.00–2.23) | .050 |
| TB treatment adherencec | 1.36 | (.97–1.90) | .071 | 1.31 | (.93–1.83) | .119 | 1.36 | (.97–1.90) | .071 | 1.19 | (.85–1.68) | .303 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; TB, tuberculosis.
aAdjusted for all variables included in table.
bForty-six patients with CKD stage 3b or higher were excluded from the study due to concerns for confounding.
cDefined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation or completing >80% of TB treatment up to the time of death during TB treatment.
Next, patients were reclassified as having used metformin throughout TB treatment if metformin use was documented for ≥80% of TB treatment. Using this definition, among the 634 patients with DM in the cohort, 98 received metformin throughout TB treatment and 536 did not. Metformin use was significantly associated with lower median age and higher BMI, proportion of HBV, proportion of cavitary disease, and proportion adherent to TB treatment. Patients receiving metformin also had a statistically higher mean FBG or HbA1c measured within 3 months of initiating TB treatment (Supplementary Table 1).
Overall mortality was 10.2% among patients who received metformin and 29.7% among patients who did not, and survival was significantly higher in the metformin group in a log-rank test of Kaplan-Meier survival distributions (P < .01; Figure 2). In a Cox proportional hazards model adjusting for age, sex, CKD, cancer, cavitary disease, and poor TB treatment adherence, metformin use was associated with 0.41 hazard of death (95% CI, .21–.78; P = .007; Table 5).
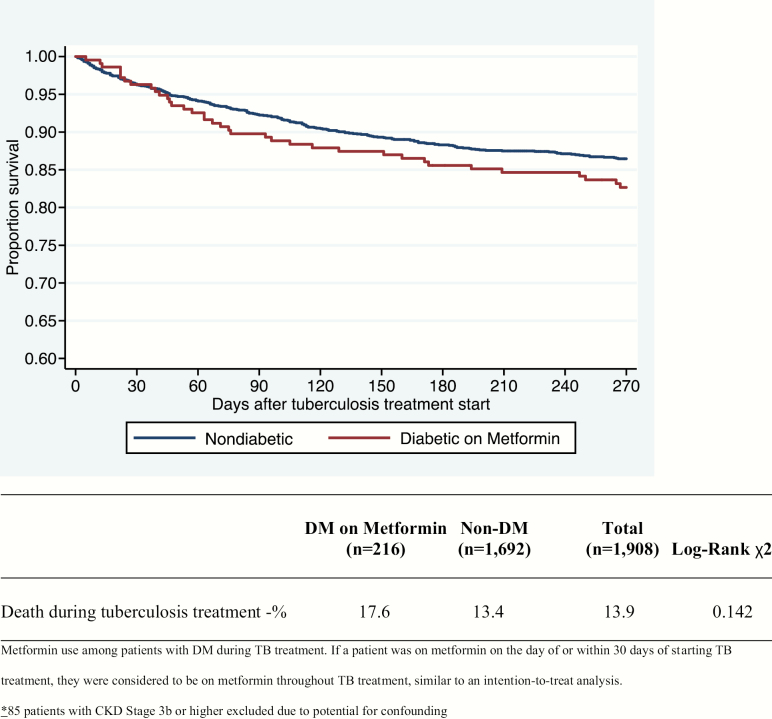
Figure 2.
Kaplan-Meier estimates of time to mortality during tuberculosis treatment, comparing diabetic patients on metformin to nondiabetic patients (n = 1894). Patients with chronic kidney disease stage 3b or higher were excluded due to concerns for confounding. Abbreviation: DM, type 2 diabetes mellitus.
Finally, using the intention-to-treat classification of metformin use, and once again excluding all patients with CKD stage 3b or higher, the remaining 216 DM patients receiving metformin during TB treatment were compared to the remaining 1678 non-DM patients. Overall mortality was 17.6% among diabetic patients receiving metformin and 13.4% among nondiabetic patients, and survival was not significantly different between the 2 groups in a log-rank test of Kaplan-Meier survival distributions (P = .14; Figure 2). In a Cox proportional hazards model adjusting for age, sex, CKD, cancer, cavitary disease, and TB treatment completion, DM patients receiving metformin had no difference in hazard of death compared to non-DM during TB treatment (HR, 1.02 [95% CI, .72–1.45]; P = .91; Table 6).
Table 6.
Crude and Adjusted Hazard Ratios, Based on Cox Proportional Hazards Model, of Mortality During Tuberculosis Treatment, Comparing Diabetic Patients on Metformin to Nondiabetic Patients (n = 1894)
| Characteristic | Crude HR | (95% CI) | P Value | Adjusted HRa | (95% CI) | P Value |
|---|---|---|---|---|---|---|
| DM on metformin during TB treatment | 1.30 | (.92–1.84) | .143 | 1.03 | (.73–1.46) | .875 |
| Age | 1.04 | (1.03–1.05) | <.001 | 1.04 | (1.03–1.05) | <.001 |
| Male sex | 1.99 | (1.48–2.68) | <.001 | 1.54 | (1.11–2.12) | .009 |
| CKDb | 1.52 | (1.03–2.25) | .037 | 0.96 | (.64–1.44) | .852 |
| Cancer | 3.81 | (2.95–4.92) | <.001 | 3.08 | (2.38–4.00) | <.001 |
| HCV | 1.82 | (1.00–3.32) | .053 | 1.12 | (.61–2.06) | .723 |
| History of tobacco use | 1.28 | (1.00–1.65) | .054 | 0.90 | (.69–1.18) | .456 |
| Cavitary disease | 1.28 | (.93–1.76) | .125 | 1.64 | (1.18–2.27) | .003 |
| Poor TB treatment adherencec | 2.35 | (1.83–3.01) | <.001 | 2.11 | (1.64–2.72) | <.001 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; DM, type 2 diabetes mellitus; HCV, hepatitis C virus; HR, hazard ratio; TB, tuberculosis.
aAdjusted for all variables included in table.
bEighty-five patients with CKD stage 3b or higher excluded due to potential for confounding.
cDefined as completing >80% of a 6-month TB treatment regimen within 9 months of TB treatment initiation or completing >80% of TB treatment up to the time of death during TB treatment.
DISCUSSION
The most striking finding of our study is that Taiwanese patients with DM and TB had nearly twice the odds of death during TB treatment compared to patients without DM. Our data indicate that despite the advanced age and multiple comorbidities of patients with DM, DM itself poses an increased risk of death during TB treatment. Furthermore, metformin use along with standard TB treatment appears to reverse the increased mortality associated with DM during TB treatment, suggesting a potential role as adjunctive TB treatment.
An estimated 52.1% and 66.7% of patients with DM in Asia and Africa, respectively, are unaware of their diagnosis [4]. This, together with the link between DM and TB susceptibility and poor TB treatment outcomes, has prompted the Bali Declaration, which calls for bidirectional screening for DM and TB [18]. As Asia will be the area of the world most affected by the DM epidemic [19], Taiwan, a country with a moderate incidence of TB (68 cases per 100000) [20], is an ideal and relevant clinical setting to evaluate the effect of DM on TB treatment outcomes. Indeed, 28.9% of our cohort had DM at the time of TB treatment, underscoring the importance of screening.
The link between DM and poor TB treatment outcomes is grounded in a review of the literature by Baker et al [6], which reveals that only 2 US-based studies have controlled for potential confounding variables when evaluating TB treatment outcomes among patients with DM [21, 22]. These findings raise the question of whether the increased DM-associated deaths could simply be a result of advanced age and/or immunocompromise from other comorbidities. The results of this Taiwan-based study, in which these confounders are controlled for in the analyses, therefore, are important in strengthening the evidence between DM and poor TB treatment outcomes. This finding persisted even after adjustment for BMI, which is important, as underweight status is associated with increased mortality and overweight status is associated with decreased mortality during TB treatment [23, 24].
DM was associated with increased sputum culture positivity after 2 months, but not after 6 months, of TB treatment. These data suggest that DM may reduce the efficacy of the initial phase of TB treatment, leaving patients with DM potentially infectious for a longer period of time. Although 2-month sputum-culture conversion is often used as a microbiological endpoint for assessing the efficacy of novel TB treatment regimens [25], it may in fact be a poor surrogate for relapse-free cure [26].
Finally, the antidiabetic agent metformin has received attention as a potential adjunctive, host-directed therapy for TB [12–15]. Metformin activates adenosine monophosphate–activated protein kinase, a regulator of cellular autophagy, which is crucial for effective control of intracellular pathogens such as Mycobacterium tuberculosis [27, 28]. Metformin was found to reduce M. tuberculosis growth and improve lung pathology in mice, and its use was associated with decreased mortality and number of lung cavities in humans in a retrospective cohort study [13]. Similarly, in our study, among patients with DM, concurrent metformin use was associated with significantly decreased mortality during TB treatment, even after adjusting for potential confounders, including CKD, a potential contraindication to metformin and a risk factor for poor TB treatment outcomes [9, 13]. Additionally, this effect was not due to improved blood glucose control, as DM patients on metformin in our cohort had equivalent or poorer blood glucose control compared to DM patients on other antidiabetic medications. Narrowing the classification of metformin users from those receiving metformin at TB treatment initiation to documented use of the agent throughout TB treatment (ie, increasing the dose response) resulted in an even further reduction in mortality, and, after adjustment for confounders, a lower hazard of death. Perhaps even more remarkable was that DM patients on metformin had the same hazard of death as non-DM patients, suggesting that metformin use in DM patients undergoing TB treatment potentially reverses the negative effect of DM on mortality during TB treatment.
This study has strengths and limitations. Its relatively large sample size allowed the effect of several different DM-associated comorbidities to be examined in a multivariate regression model. The single study site, NTUH, helped eliminate variance in clinical practice and environmental factors; however, the findings may not be readily generalizable to other populations. Other notable limitations include the retrospective study design, and that 2- and 6-month TB treatment sputum culture, BMI, HIV serostatus, FBG, and HbA1c data were not available for all patients in the study and therefore may represent a biased patient sample.
In summary, our study shows that following adjustment for age, sex, comorbidities, cavitary disease, and poor TB treatment adherence, DM is associated with increased mortality during TB treatment. Although DM is a risk factor for sputum culture–positive status after 2 months of TB treatment, it has no effect by 6 months, suggesting that DM delays, but does not prevent, sputum-culture conversion. Finally, our analysis shows that concurrent metformin use among patients with DM greatly decreased the odds of mortality during TB treatment, suggesting a potential role for this agent as an adjunctive, host-directed therapy for TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. N. R. D. and J.-Y. W. contributed equally to this work. J. E. G. and P. C. K. contributed equally to this work. N. R. D., J.-Y. W., J. E. G., and P. C. K. designed the study. N. R. D. and J.-Y. W. performed the analyses. J.-Y. W. collected the data. N. R. D. drafted the manuscript. All authors critically reviewed the manuscript.
Financial support. This work was supported by the National Institutes of Health/AIDS Clinical Trials Group Network (grant number UH2AI122309 and an administrative supplement for grant UM1AI068636) to P. C. K.; and the Centers for Disease Control, Taiwan (grant MOHW-105-CDC-C-114-000103 to J. Y. W.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. World Health Organization. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 3. World Health Organization. Global status report on noncommunicable diseases 2014. Geneva, Switzerland: WHO, 2015. [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation. IDF diabetes atlas. 7th ed Brussels: IDF, 2015. [Google Scholar]
- 5. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker MA, Harries AD, Jeon CY et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin PJ, Kent DM, Winn A, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–34. [PubMed] [Google Scholar]
- 8. García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013; 4:175–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baghaei P, Marjani M, Tabarsi P et al. Impact of chronic renal failure on anti-tuberculosis treatment outcomes. Int J Tuberc Lung Dis 2014; 18:352–6. [DOI] [PubMed] [Google Scholar]
- 10. Kobashi Y, Mouri K, Yagi S et al. Clinical features of immunocompromised and nonimmunocompromised patients with pulmonary tuberculosis. J Infect Chemother 2007; 13:405–10. [DOI] [PubMed] [Google Scholar]
- 11. Zumla A, Chakaya J, Centis R et al. Tuberculosis treatment and management—an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med 2015; 3:220–34. [DOI] [PubMed] [Google Scholar]
- 12. Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15:255–63. [DOI] [PubMed] [Google Scholar]
- 13. Singhal A, Jie L, Kumar P et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014; 6:263ra159. [DOI] [PubMed] [Google Scholar]
- 14. Marupuru S, Senapati P, Pathadka S, Miraj SS, Unnikrishnan MK, Manu MK. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz J Infect Dis 2017; 21:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vashisht R, Brahmachari SK. Metformin as a potential combination therapy with existing front-line antibiotics for tuberculosis. J Transl Med 2015; 13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nahid P, Dorman SE, Alipanah N et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312:2668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kapur A, Harries AD, Lönnroth K, Wilson P, Sulistyowati LS. Diabetes and tuberculosis co-epidemic: the Bali Declaration. Lancet Diabetes Endocrinol 2016; 4:8–10. [DOI] [PubMed] [Google Scholar]
- 19. Yoon KH, Lee JH, Kim JW et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368:1681–8. [DOI] [PubMed] [Google Scholar]
- 20. Liao CM, Hsieh NH, Huang TL et al. Assessing trends and predictors of tuberculosis in Taiwan. BMC Public Health 2012; 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis 2002; 34:752–9. [DOI] [PubMed] [Google Scholar]
- 22. Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009; 80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 23. Bhargava A, Chatterjee M, Jain Y et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One 2013; 8:e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanrahan CF, Golub JE, Mohapi L et al. Body mass index and risk of tuberculosis and death. AIDS 2010; 24:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurbatova EV, Cegielski JP, Lienhardt C et al. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir Med 2015; 3:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horne DJ, Royce SE, Gooze L et al. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010; 10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011; 13:1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.