We modeled global time trends in median CD4 cell counts at combination antiretroviral therapy initiation in human immunodeficiency virus–infected adults. These counts have increased in all country income groups since 2002 but generally remained below 350/μL in 2015.
Keywords: antiretroviral therapy, CD4 cell count, WHO guidelines
Abstract
Background
Early initiation of combination antiretroviral therapy (cART), at higher CD4 cell counts, prevents disease progression and reduces sexual transmission of human immunodeficiency virus (HIV). We describe the temporal trends in CD4 cell counts at the start of cART in adults from low-income, lower-middle-income, upper-middle-income, and high-income countries (LICs, LMICs, UMICs, and HICs, respectively).
Methods
We included HIV-infected individuals aged ≥16 years who started cART between 2002 and 2015 in a clinic participating in the International epidemiology Databases to Evaluate AIDS (IeDEA) or the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE). Missing CD4 cell counts at the start of cART were estimated through multiple imputation. Weighted mixed-effect models were used to smooth trends in median CD4 cell counts.
Results
A total of 951855 adults from 16 LICs, 11 LMICs, 9 UMICs, and 19 HICs were included. Overall, the modeled median CD4 cell count at the start of cART increased from 2002 to 2015, from 78/µL (95% confidence interval, 58–104/µL) to 287/µL (250–328/µL) in LICs, from 99/µL (71–140/µL) to 234/µL (192–285/µL) in LMICs, from 71/µL (49–104/µL) to 311/µL (255–379/µL) in UMICs, and from 161/µL (143–181/µL) to 327/µL (286–372/µL) in HICs. In LICs, LMICs, and UMICs, the increase was more pronounced in women; in HICs, the opposite was observed.
Conclusions
Median CD4 cell counts at the start of cART increased in all income groups, but generally remained below 350/μL in 2015. Substantial additional efforts and resources are required to achieve earlier diagnosis, linkage to care, and initiation of cART.
Modeling by the Joint United Nations Programme on HIV/AIDS (UNAIDS) indicates that there is a window of opportunity to end the human immunodeficiency virus (HIV)/AIDS epidemic by reaching the “90-90-90” targets, meaning that 90% of HIV infections are diagnosed, 90% of persons known to be HIV infected are receiving combination antiretroviral therapy (cART), and 90% of individuals receiving cART are virologically suppressed [1, 2]. In response, the World Health Organization (WHO) in its consolidated 2016 guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommended “lifelong cART to all children, adolescents and adults, including all pregnant and breastfeeding women living with HIV, regardless of CD4 cell count” [3].
Many individuals who live with HIV continue to enter care late. A previous analysis of cART programs and HIV cohort studies from low-income countries (LICs), lower-middle-income countries (LMICs), upper-middle-income countries (UMICs), and high-income countries (HICs) showed that median CD4 cell counts at the start of cART increased from 2000 to 2009 but remained below 200/µL in LICs and middle-income countries (MICs) and below 300/µL in HICs [4]. Similarly, a study published in Morbidity and Mortality Weekly Report [5] found that the percentage of patients starting cART with a CD4 cell count below 200/µL had decreased in 10 LICs and MICs but continued to be substantial in recent years, for example, 37% in Mozambique in 2014, or 34% in Haiti in 2015 [5]. A meta-analysis of African studies showed that the mean estimated CD4 cell count in 2012 was 309/µL at presentation to care and 140/µL at cART initiation [6]. Similarly, a meta-regression analysis of studies in developed countries showed only a small increase in the CD4 cell count at presentation from 1992 to 2011 [7].
For the present study, the International epidemiology Databases to Evaluate AIDS (IeDEA), a large collaboration of cART treatment programs and HIV cohort studies in the Americas, sub-Saharan Africa, and Asia-Pacific joined forces with the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) to examine global trends in CD4 cell counts at cART initiation.
METHODS
Data Sources
IeDEA is a consortium structured through regional centers to pool clinical and epidemiological data on persons living with HIV and receiving cART. COHERE is a collaboration of European HIV cohorts. Regional cohorts of IeDEA and COHERE have been described in detail elsewhere [8–12]. Institutional review boards approved the pooling of data and their use in collaborative analyses.
Inclusion Criteria and Definitions
We included all individuals aged ≥16 years if they had a recorded cART starting date and sex, were treatment naive, and started therapy between 2002 and 2015. We excluded countries that contributed <100 patients with CD4 cell counts at therapy start and individual patients who started therapy in a year and country for which <10 CD4 cell counts were reported. cART was defined as ≥3 antiretroviral drugs, from 2 drug classes. The CD4 cell count at the start of cART was the count nearest to the date of starting cART, within a window of 3 months before and 1 week after initiation of therapy. CD4 cell counts >5000/μL (>3 times above the upper reference range [13]) were considered invalid. Countries were grouped according to the World Bank classification of gross national income per capita in 2015 [14], as LICs (≤$1025), LMICs ($1026–$4035), UMICs ($4036–$12475), and HICs (≥$12476). Severe immunodeficiency was defined as a CD4 cell count <200/μL [15]. Regions were defined according to IeDEA and COHERE conventions [8–11, 16].
Multiple Imputation of Missing CD4 Cell Counts
We imputed square roots of CD4 cell counts using predictive mean matching, adjusting for country and year of cART start and stratifying by sex, country income group, and region. We generated 50 imputed data sets and combined these using Rubin’s rule [17].
Weighted Analysis of Temporal Trends
We aggregated data by calendar year (3–14 years, depending on the country), country (55 countries), and sex (2 groups), and we calculated the median CD4 cell count at the start of cART for each of the resulting data cells. We assigned a weight to each data cell that consisted of 2 components, which were multiplied. The first component corresponded to the number of observations, divided by the average number of observations in data cells of the same country income group (and was thus normalized by country income group), and captured the precision of the aggregated values in each data cell. The second component corresponded to the ratio of the number of patients who were newly enrolled in that cohort and year to the number of patients starting cART in that country and year, as estimated by UNAIDS [18] and was also normalized by country income group.
We used weighted additive mixed models to analyze temporal trends in median CD4 cell counts at the start of cART. The covariates sex and country income group, as well as their interaction, were included as fixed effects, country as a random intercept, and yearly trends smoothed by sex and country income group. Similarly, we analyzed the median CD4 cell count according to region instead of country income group. For this analysis, weights were normalized by region. We also modeled the proportion of patients starting cART with severe immunodeficiency (CD4 cell count <200/µL), using generalized additive mixed models, and we fitted this model to other CD4 cell count thresholds (<50/µL, <100/µL, <350/µL, and <500/µL). We used the data set that included imputed CD4 cell counts. In sensitivity analyses, we fitted models to the data set consisting of complete cases only. We also fitted models including only cohorts that contributed data each year from 2005 to 2014.
We present CD4 cell counts as observed or modeled median CD4 cell counts with interquartile ranges (IQRs) or 95% confidence intervals (CIs). All analyses were done using R software, version 3.2.3 (R Core Team). The appendix gives further technical details (see Supplementary Digital Content).
RESULTS
Descriptive Analyses
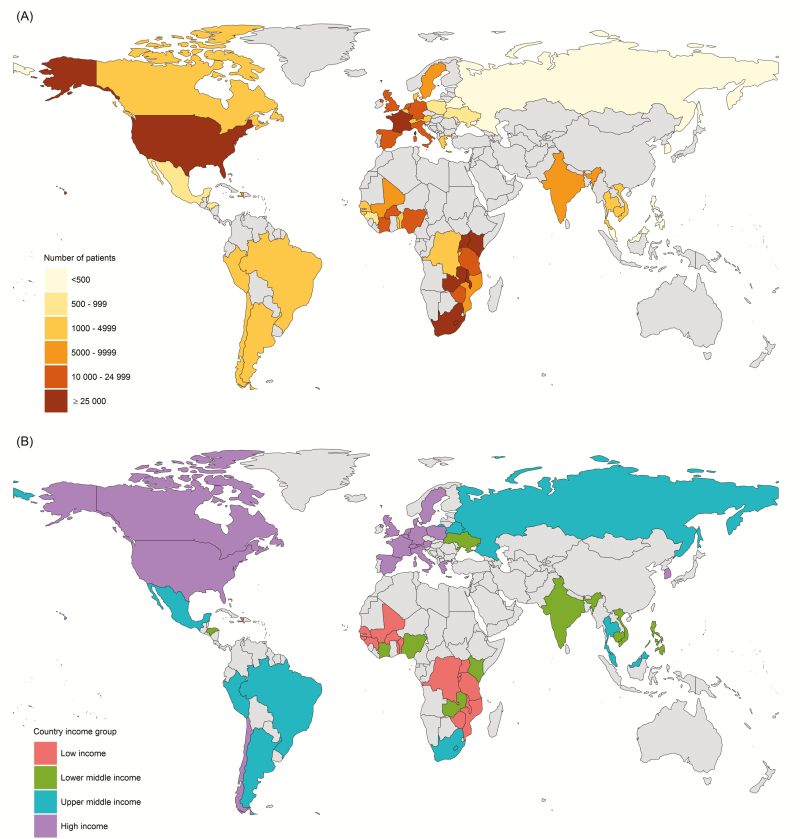
We received data from 1472098 patients. We excluded a total of 520243 patients and 22 countries who did not meet the inclusion criteria. Supplementary Figure S1 (see Supplementary Digital Content) shows the inclusion of patients. A total of 951 855 individuals from 55 countries (16 LICs, 11 LMICs, 9 UMICs, and 19 HICs) were included (Table 1 and Figure 1). Five countries contributed 160–499 persons; 6 countries, 500–999; 20 countries, 1000–4999; 5 countries, 5000–9999; 12 countries, 10000–24999; and 7 countries, ≥25000. The number of individuals included in each country ranged from 160 (Malaysia) to 350595 (Zambia).
Table 1.
Characteristics of Persons Living With Human Immunodeficiency Virus Starting Combination Antiretroviral Therapy by World Bank Income Group (2015), Country, and Sex
| Country by Income Status | Patients, No. | Age, Median, y | Calendar Year of cART Initiation, Median | Range of Data, Calendar Years | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||
| Low income | |||||||
| Benin | 2542 | 1559 | 33 | 40 | 2009 | 2008 | 2002–2014 |
| Burkina Faso | 7832 | 3312 | 35 | 41 | 2008 | 2008 | 2002–2014 |
| Burundi | 3123 | 1711 | 35 | 43 | 2012 | 2012 | 2009–2015 |
| Democratic Republic of the Congo | 1425 | 303 | 33 | 41 | 2011 | 2011 | 2005–2014 |
| Guinea | 640 | 323 | 32 | 41 | 2012 | 2012 | 2008–2014 |
| Guinea-Bissau | 1941 | 1002 | 35 | 40 | 2010 | 2010 | 2007–2014 |
| Haiti | 3422 | 2287 | 34 | 40 | 2013 | 2013 | 2003–2015 |
| Malawi | 29 965 | 20 219 | 31 | 37 | 2011 | 2011 | 2007–2015 |
| Mali | 3222 | 1800 | 33 | 41 | 2009 | 2009 | 2002–2014 |
| Mozambique | 6314 | 2925 | 29 | 36 | 2013 | 2013 | 2006–2015 |
| Rwanda | 7730 | 4443 | 32 | 38 | 2008 | 2008 | 2004–2015 |
| Senegal | 603 | 407 | 37 | 43 | 2009 | 2009 | 2002–2014 |
| United Republic of Tanzania | 8798 | 3999 | 36 | 41 | 2009 | 2009 | 2005–2014 |
| Togo | 2649 | 1351 | 33 | 40 | 2009 | 2009 | 2005–2009 |
| Uganda | 27 644 | 15 841 | 32 | 37 | 2009 | 2009 | 2003–2014 |
| Zimbabwe | 15 652 | 7195 | 36 | 40 | 2012 | 2012 | 2004–2015 |
| Overall (IQR)a | 123 502 | 68 677 | 33 (28–40) | 38 (32–45) | 2011 (2008–2013) | 2010 (2008–2012) | 2002–2015 |
| Lower middle income | |||||||
| Cambodia | 1136 | 1003 | 33 | 36 | 2009 | 2009 | 2005–2014 |
| Cote d’Ivoire | 14 819 | 7725 | 35 | 42 | 2008 | 2008 | 2002–2014 |
| Honduras | 436 | 562 | 33 | 38 | 2006 | 2007 | 2002–2015 |
| India | 2802 | 6100 | 33 | 36 | 2009 | 2008 | 2002–2014 |
| Kenya | 72 329 | 33 311 | 33 | 39 | 2010 | 2010 | 2003–2014 |
| Lesotho | 6870 | 3638 | 35 | 40 | 2011 | 2011 | 2005–2015 |
| Nigeria | 14 729 | 7587 | 32 | 39 | 2008 | 2008 | 2005–2014 |
| Philippines | 16 | 191 | 36 | 30 | 2010 | 2009 | 2008–2010 |
| Ukraine | 570 | 264 | 29 | 34 | 2008 | 2009 | 2004–2014 |
| Vietnam | 554 | 918 | 30 | 34 | 2012 | 2012 | 2004–2014 |
| Zambia | 217 525 | 133 070 | 33 | 37 | 2011 | 2011 | 2003–2015 |
| Overall (IQR)a | 331 786 | 194 369 | 33 (28–40) | 38 (32–44) | 2010 (2008–2013) | 2010 (2007–2013) | 2002–2015 |
| Upper middle income | |||||||
| Argentina | 888 | 2161 | 35 | 37 | 2008 | 2009 | 2002–2015 |
| Belarus | 235 | 258 | 32 | 34 | 2009 | 2008 | 2006–2013 |
| Brazil | 774 | 1941 | 38 | 35 | 2010 | 2010 | 2002–2015 |
| Malaysia | 31 | 129 | 37 | 37 | 2008 | 2009 | 2004–2010 |
| Mexico | 104 | 858 | 35 | 33 | 2009 | 2009 | 2002–2015 |
| Peru | 872 | 2328 | 34 | 33 | 2010 | 2011 | 2004–2015 |
| Russian Federation | 159 | 159 | 29 | 32 | 2008 | 2008 | 2003–2012 |
| South Africa | 45 359 | 24 240 | 33 | 38 | 2010 | 2010 | 2003–2015 |
| Thailand | 451 | 586 | 36 | 37 | 2008 | 2008 | 2003–2010 |
| Overall (IQR)a | 48 873 | 32 660 | 33 (28–40) | 37 (32–44) | 2010 (2007–2012) | 2010 (2007–2012) | 2002–2015 |
| High income | |||||||
| Austria | 627 | 1774 | 33 | 38 | 2008 | 2010 | 2002–2014 |
| Belgium | 1205 | 1303 | 31 | 39 | 2007 | 2010 | 2002–2014 |
| Canada | 295 | 818 | 36 | 39 | 2008 | 2009 | 2003–2013 |
| Chile | 160 | 1415 | 37 | 35 | 2006 | 2009 | 2002–2014 |
| Denmark | 609 | 1313 | 35 | 42 | 2007 | 2008 | 2002–2013 |
| France | 9036 | 18 094 | 35 | 40 | 2006 | 2007 | 2002–2014 |
| Germany | 2709 | 10 686 | 34 | 40 | 2008 | 2009 | 2002–2015 |
| Greece | 555 | 2997 | 36 | 36 | 2008 | 2010 | 2002–2014 |
| Hong Kong | 133 | 574 | 36 | 41 | 2009 | 2010 | 2003–2013 |
| Italy | 3631 | 10 627 | 37 | 40 | 2009 | 2009 | 2002–2015 |
| Republic of Korea | 18 | 364 | 41 | 37 | 2011 | 2010 | 2002–2015 |
| Netherlands | 2806 | 11 078 | 33 | 41 | 2008 | 2009 | 2002–2015 |
| Poland | 142 | 427 | 31 | 33 | 2007 | 2008 | 2002–2013 |
| Singapore | 117 | 1643 | 41 | 42 | 2010 | 2010 | 2006–2014 |
| Spain | 2218 | 9006 | 36 | 37 | 2008 | 2009 | 2002–2014 |
| Sweden | 2138 | 3095 | 33 | 41 | 2009 | 2009 | 2002–2015 |
| Switzerland | 882 | 2881 | 36 | 40 | 2008 | 2009 | 2002–2014 |
| United Kingdom | 5823 | 13 976 | 35 | 38 | 2007 | 2008 | 2002–2013 |
| United States | 4187 | 22 626 | 42 | 44 | 2008 | 2008 | 2003–2014 |
| Overall (IQR)a | 37 291 | 114 697 | 35 (29–43) | 40 (33–48) | 2007 (2005–2010) | 2008 (2006–2011) | 2002–2015 |
Abbreviations: IQR, interquartile range.
aIQRs provided for median values.
Figure 1.
Map of countries contributing patients to the collaborative analysis by number of patients (A) and country income group (B).
The percentage of women was 57% overall and ranged from 5% in South Korea to 82% in the Democratic Republic of the Congo. In LICs, LMICs, and UMICs, the median (IQR) age of individuals starting cART was 35 (29–42) years; in HICs, it was 39 (32–47) years. The median year of cART initiation ranged from 2007 in France and Honduras to 2013 in Haiti and Mozambique. The median CD4 cell count at cART initiation ranged from 106/µL in Senegal, Thailand, and Vietnam to 275/µL in Belgium, and it was 182/μL overall; it was 179/μL (IQR, 85–288/μL) in LICs, 172/μL (85–279/μL) in LMICs, 141/μL (60–227/μL) in UMICs, and 251/μL (128–370/μL) in HICs. The proportion of patients starting cART with severe immunodeficiency (CD4 cell count <200/µL) was 55%, ranging from 31% in Switzerland to 77% in Senegal; this proportion was 56% in LICs, 58% in LMICs, 68% in UMICs, and 38% in HICs. Tables 1 and 2 and Supplementary Table S3 show detailed results by country and sex.
Table 2.
Median CD4 Cell Count and Proportion of Persons Living With Human Immunodeficiency Virus Starting Combination Antiretroviral Therapy With Severe Immunodeficiency in 2002–2015 by World Bank Income Group (2015), Country, and Patient Sex
| Country by Income Status | Proportion of Patients Missing CD4 cell Count Measurements, % | CD4 Cell Count at cART Initiation, Median, Cells/µL | Proportion Starting cART With CD4 Cell Count <200/µL, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete Case Analysis | Imputed Data | Complete Case Analysis | Imputed Data | |||||||
| Female Patients | Male Patients | Female Patients | Male Patients | Female Patients | Male Patients | Female Patients | Male Patients | Female Patients | Male Patients | |
| Low income | ||||||||||
| Benin | 35 | 35 | 153 | 97 | 155 | 100 | 63 | 77 | 62 | 76 |
| Burkina Faso | 37 | 37 | 212 | 159 | 211 | 163 | 47 | 58 | 47 | 57 |
| Burundi | 64 | 63 | 252 | 233 | 263 | 242 | 34 | 41 | 33 | 40 |
| Democratic Republic of the Congo | 16 | 20 | 237 | 198 | 241 | 200 | 41 | 51 | 41 | 50 |
| Guinea | 38 | 42 | 196 | 167 | 195 | 167 | 51 | 58 | 51 | 59 |
| Guinea-Bissau | 21 | 18 | 164 | 153 | 162 | 153 | 60 | 64 | 61 | 64 |
| Haiti | 31 | 31 | 267 | 213 | 269 | 211 | 35 | 48 | 35 | 48 |
| Malawi | 71 | 64 | 187 | 154 | 196 | 157 | 54 | 63 | 51 | 62 |
| Mali | 23 | 22 | 165 | 119 | 165 | 119 | 56 | 70 | 57 | 70 |
| Mozambique | 38 | 23 | 270 | 214 | 275 | 214 | 35 | 47 | 34 | 47 |
| Rwanda | 21 | 22 | 246 | 198 | 246 | 198 | 39 | 50 | 39 | 51 |
| Senegal | 44 | 42 | 109 | 101 | 112 | 106 | 75 | 80 | 74 | 79 |
| United Republic of Tanzania | 34 | 33 | 126 | 113 | 130 | 115 | 72 | 75 | 71 | 74 |
| Togo | 95 | 94 | 154 | 150 | 144 | 156 | 71 | 72 | 69 | 63 |
| Uganda | 37 | 34 | 176 | 146 | 172 | 138 | 57 | 65 | 58 | 67 |
| Zimbabwe | 31 | 29 | 197 | 149 | 208 | 154 | 51 | 65 | 48 | 63 |
| Overall (IQR)a | 44 | 43 | 192 (97–303) | 156 (68–258) | 193 (98–304) | 156 (68–255) | 52 | 62 | 52 | 62 |
| Lower middle income | ||||||||||
| Cambodia | 6 | 6 | 178 | 115 | 179 | 115 | 57 | 68 | 56 | 68 |
| Cote d’Ivoire | 36 | 40 | 176 | 144 | 172 | 142 | 57 | 64 | 58 | 65 |
| Honduras | 22 | 19 | 120 | 110 | 123 | 110 | 77 | 74 | 77 | 74 |
| India | 11 | 9 | 166 | 122 | 167 | 122 | 64 | 74 | 63 | 74 |
| Kenya | 36 | 32 | 177 | 120 | 182 | 124 | 57 | 73 | 55 | 71 |
| Lesotho | 20 | 19 | 226 | 169 | 234 | 173 | 44 | 57 | 42 | 56 |
| Nigeria | 27 | 26 | 203 | 152 | 205 | 154 | 49 | 62 | 49 | 61 |
| Philippines | 12 | 3 | 204 | 192 | 209 | 192 | 50 | 52 | 48 | 52 |
| Ukraine | 34 | 58 | 246 | 200 | 240 | 199 | 34 | 49 | 36 | 51 |
| Vietnam | 13 | 9 | 170 | 70 | 168 | 71 | 59 | 77 | 60 | 78 |
| Zambia | 35 | 31 | 188 | 158 | 195 | 161 | 54 | 62 | 51 | 61 |
| Overall (IQR)a | 34 | 30 | 186 (97–297) | 149 (70–248) | 191 (100–307) | 152 (71–255) | 54 | 64 | 52 | 63 |
| Upper middle income | ||||||||||
| Argentina | 35 | 33 | 209 | 196 | 208 | 193 | 48 | 51 | 48 | 51 |
| Belarus | 28 | 26 | 196 | 171 | 196 | 185 | 52 | 57 | 53 | 55 |
| Brazil | 17 | 16 | 239 | 227 | 236 | 226 | 41 | 45 | 42 | 45 |
| Malaysia | 13 | 19 | 175 | 151 | 175 | 151 | 59 | 64 | 58 | 64 |
| Mexico | 22 | 12 | 131 | 160 | 132 | 160 | 67 | 59 | 66 | 59 |
| Peru | 24 | 18 | 145 | 113 | 151 | 113 | 60 | 68 | 60 | 68 |
| Russian Federation | 50 | 43 | 209 | 196 | 211 | 196 | 44 | 52 | 43 | 51 |
| South Africa | 28 | 27 | 149 | 114 | 154 | 116 | 67 | 76 | 65 | 75 |
| Thailand | 10 | 11 | 123 | 97 | 125 | 99 | 75 | 75 | 73 | 75 |
| Overall (IQR)a | 28 | 26 | 151 (70–232) | 123 (48–218) | 156 (72–242) | 125 (49–220) | 66 | 71 | 64 | 70 |
| High income | ||||||||||
| Austria | 15 | 17 | 237 | 266 | 236 | 264 | 40 | 35 | 40 | 35 |
| Belgium | 36 | 32 | 266 | 280 | 265 | 280 | 34 | 30 | 35 | 29 |
| Canada | 18 | 17 | 224 | 238 | 227 | 243 | 43 | 38 | 42 | 37 |
| Chile | 29 | 29 | 201 | 191 | 191 | 181 | 48 | 52 | 53 | 54 |
| Denmark | 29 | 30 | 231 | 234 | 232 | 238 | 39 | 40 | 38 | 39 |
| France | 18 | 17 | 249 | 266 | 250 | 266 | 36 | 35 | 36 | 35 |
| Germany | 29 | 26 | 223 | 237 | 220 | 235 | 43 | 41 | 44 | 42 |
| Greece | 20 | 22 | 192 | 249 | 192 | 249 | 52 | 38 | 51 | 38 |
| Hong Kong | 1 | 2 | 112 | 111 | 109 | 111 | 70 | 66 | 70 | 66 |
| Italy | 27 | 27 | 252 | 258 | 254 | 257 | 39 | 39 | 38 | 39 |
| Republic of Korea | 6 | 4 | 207 | 221 | 207 | 221 | 47 | 44 | 47 | 44 |
| Netherlands | 28 | 25 | 230 | 260 | 230 | 260 | 42 | 35 | 42 | 35 |
| Poland | 55 | 51 | 203 | 238 | 217 | 228 | 50 | 39 | 48 | 41 |
| Singapore | 9 | 9 | 138 | 128 | 134 | 133 | 62 | 62 | 62 | 61 |
| Spain | 19 | 18 | 229 | 260 | 229 | 260 | 43 | 36 | 43 | 36 |
| Sweden | 29 | 27 | 230 | 240 | 225 | 240 | 43 | 38 | 43 | 39 |
| Switzerland | 16 | 13 | 259 | 270 | 259 | 270 | 34 | 30 | 34 | 30 |
| United Kingdom | 34 | 34 | 220 | 245 | 220 | 244 | 44 | 37 | 44 | 37 |
| United States | 14 | 14 | 274 | 272 | 276 | 273 | 36 | 37 | 36 | 36 |
| Overall (IQR)a | 24 | 22 | 241 (128–360) | 254 (128–372) | 240 (128–360) | 253 (130–370) | 40 | 37 | 40 | 37 |
Abbreviations: IQR, interquartile range.
aIQRs provided for median values.
Multiple Imputation of Missing CD4 Cell Counts
The CD4 cell count measurement at the start of cART was missing in 311647 patients, in 44% of individuals in LICs, 33% in LMICs, 27% in UMICs, and 22% in HICs (Table 2). Compared with them, the 640208 individuals who had a CD4 cell count reported at the start of cART were more likely to be female and less likely to be from a LIC (Supplementary Table S1). Five countries from Southern Africa provided information about the WHO stage of patients at cART initiation. The WHO stage distributions were similar overall in patients with and those without reported CD4 cell counts (Supplementary Table S2).
Medians of imputed CD4 cell counts from the main analysis and the complete cases (sensitivity analysis) were similar (Table 2 and Supplementary Table S3). Differences in CD4 cell counts ranged from −10/μL in Ukraine to +10.5/μL in Burundi. Similarly, the proportion of patients starting cART with counts <200/μL were similar for imputed and complete data. The differences ranged from −4.7% in Togo to +3.4% in Ukraine.
Temporal Trends in CD4 Cell Counts
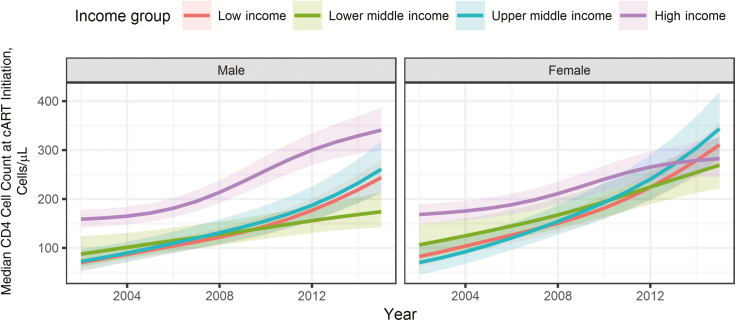
The estimated median CD4 cell count at the start of cART from 2002 to 2015 varied across income groups (Figure 2). The modeled median CD4 cell count at cART initiation increased in LICs by 268%, from 78/µL (95% CI, 58–104/µL) to 287/µL (250–328/µL); in LMICs by 136%, from 99/µL (71–140/µL) to 234/µL (192–285/µL); in UMICs by 338%, from 71/µL (49–104/µL) to 311/µL (255–379/µL); and in HICs by 103%, from 161/µL (143–181/µL) to 327/µL (286–372/µL). In LICs, LMICs, and UMICs the increase was more pronounced in women (+277% in LICs, +153% in LMICs, and +391% in UMICs) than in men (+248% in LICs, +99% in LMICs, and +261% in UMICs); in HICs the opposite was the case (+68% in women and +115% in men). Results of the complete case analysis and analysis restricted to cohorts contributing data from 2005–2014 were similar (Supplementary Figure S2A and S2B, Supplementary Digital Content).
Figure 2.
Median CD4 cell count in adults at the start of combination antiretroviral therapy (cART) by sex and country income group. Results from additive mixed-effects model based on 951855 adults after imputation of missing data. 95% confidence intervals are shown as shaded areas.
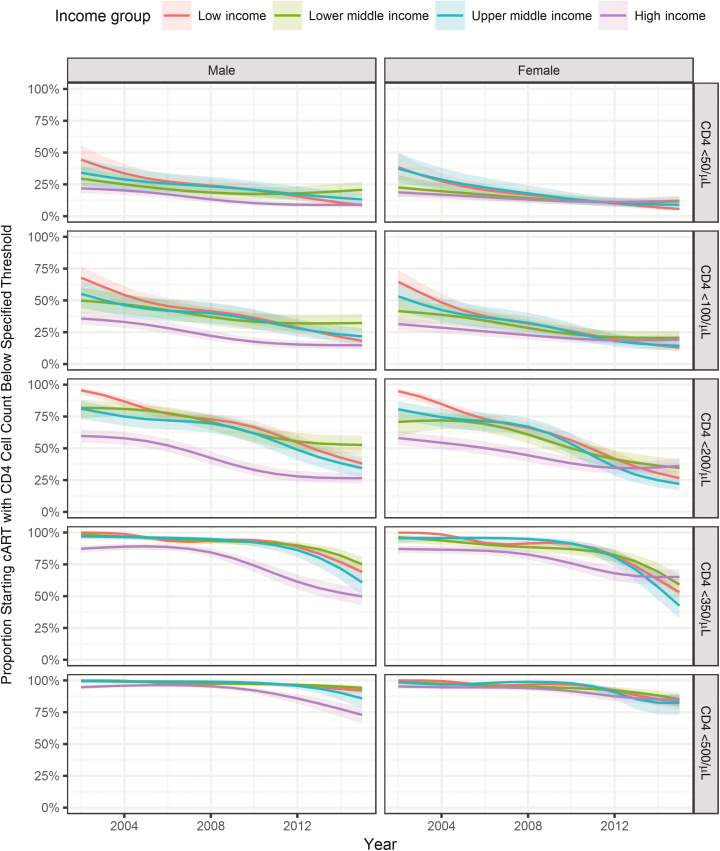
Figure 3 shows modeled temporal trends in the proportion of patients starting cART with severe immunodeficiency (CD4 cell count <200/µL) and below other thresholds. In LICs, the estimated proportion of adults starting with severe immunodeficiency declined from 95% (95% CI, 90%–97%) in 2002 to 31% (26%–36%) in 2015. Corresponding declines were from 75% (95% CI, 65%–83%) to 40% (33%–47%) in LMICs, from 79% (71%–86%) to 26% (20%–33%) in UMICs, and from 59% (54%–64%) to 29% (24%–34%) in HICs. For the lowest 3 CD4 thresholds (<50/µL, <100/µL, and <200/µL) the proportions of patients starting cART below the threshold declined over the study-period. However, trends plateaued toward the end of the study period, for example, for individuals from HICs or LMICs who started therapy with CD4 cell counts below 100/µL or 200/µL. The proportions for the 2 highest CD4 thresholds (<350/µL and <500/µL) were constant over the first few years and then started to decrease. Results of the complete case analysis and analysis restricted to cohorts contributing data from 2005–2014 were similar (see Figure S3A and S3B, Supplementary Digital Content).
Figure 3.
Proportion of patients starting combination antiretroviral therapy (cART) with CD4 cell counts below 50/µL, 100/µL, 200/µL, 350/µL, and 500/µL (rows) by sex (columns) and country income group (colors). Results from generalized additive mixed effects models based on 951855 adults after imputation of missing data. 95% confidence intervals are shown as shaded areas.
Supplementary Figure S4 shows the modeled temporal trends in median CD4 cell count at the start of cART by sex and region. Regions showed different trends, with the largest increases in median CD4 cell count at the start of cART from 2003 to 2014 seen in Southern Africa (from 93/µL [95% CI, 60–146/µL] to 259/µL [224–300/µL]) and North America (from 172/µL [131–227/µL] to 435/µL [317–597/µL]) and the smallest increases seen in West Africa (from 118/µL [88–158/µL] to 186/µL [160–217/µL]) and East Europe (from 160/µL [101–254/µL] to 261/µL [199–342/µL]). Results from complete case analysis and analysis restricted to cohorts contributing data from 2005–2014 were similar (see Supplementary Figure S4A and S4B, Supplementary Digital Content).
DISCUSSION
This global analysis of the CD4 cell count at cART initiation included almost 1 million individuals living with HIV in North America, Latin America and the Caribbean, Asia-Pacific, sub-Saharan Africa, and Europe. The median CD4 cell count substantially increased in all 4 groups of countries defined by per capita income, with steeper increases in LICs and UMICs than in LMICs or HICs. In 2015, these counts were highest in HICs, followed by UMICs, LICs, and LMICs. There were also important differences between regions. For example, the estimated median CD4 cell count in individuals starting cART in North America rose to 435/µL in 2014; at the other end of the spectrum, it was 186/µL in individuals starting cART in West Africa in the same year. Median CD4 cell counts were higher and increases steeper in women than in men, except in HICs, where in recent years women started cART with lower counts than men. The proportion starting therapy with severe immunodeficiency decreased substantially, but trends seemed to have plateaued in recent years, especially in HICs.
The decreases in the proportion of patients starting therapy below the different CD4 thresholds mirror the WHO guidelines to some extent. For example, the proportion starting with a CD4 cell count below 350/µL was close to 100% in LICs, LMICs, and UMICs until about 2010, and started declining after that point, possibly owing to the implementation of the 2009 guideline [19]. In HICs the decline had already started before the guideline expansion, in 2008. This reflects the fact that national guidelines in resource-limited settings generally echoed WHO guidelines [20], whereas HICs have more rapidly increased the CD4 cell count threshold for initiation of cART. For example, in 2012 North American guidelines converged in their recommendation that cART should be offered to all HIV-infected individuals, irrespective of CD4 cell count [21, 22]. The WHO followed suit in 2016, recommending “lifelong cART for all children, adolescents and adults, including all pregnant and breastfeeding women living with HIV, regardless of CD4 cell count” [3]. The impact of these recommendations will be the subject of future collaborative analyses.
It is likely that the substantial rise in HIV testing in many countries, supported by governments, the US President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund, and other donors contributed to increasing CD4 cell counts at the start of cART [23], but this may not have been the case in all settings [24, 25]. The steeper increase in CD4 cell count among women compared with men in LICs and MICs may be explained by increased testing coverage after scale-up of programs to prevent mother-to-child transmission. UNAIDS estimates that 90% of pregnant women living with HIV in Eastern and Southern Africa, 48% in Central and West Africa and 41% in Asia and the Pacific received antiretroviral drugs [26], up from <5% in 2002 [27]. However, among the 22 UNAIDS priority countries [28], several still had coverage rates below 50% in 2015 for programs to prevent mother-to-child transmission, including India, Chad and Nigeria [26].
Analyses were based on raw data from many HIV-infected individuals starting cART, which is an important strength of this study. Such individual patient data meta-analyses have been described as the “yardsticks” against which the quality of other reviews should be judged [29]. Our results are consistent with an earlier analysis of IeDEA and European data, based on individual patient data from 379865 patients in 23 countries, which showed that CD4 cell counts in LICs and MICs increased from about 90/µL in 2002 to about 150/µL in 2009 [4]. Our results are also in line with analyses of individual patient data from a smaller number of countries [5, 30, 31].
The weighting of estimates was another strength, with more weight given to the more precise estimates of median CD4 cell count, and by the number of patients starting cART in a given country and year [18], so that countries with many patients starting cART were adequately represented in our analysis. Our study also had several limitations. We included data up to 2015, but not all countries contributed data spanning the entire period from 2002 to 2015. It is reassuring that results were very similar when we restricted analyses to the cohorts that contributed data for each year from 2005 to 2014.
Another limitation was that many individuals had missing CD4 cell counts at cART initiation, which we addressed by multiple imputation. Results including the imputed values were very similar to those of complete case analyses. If some of the CD4 cell counts were missing owing to poorer health, this would violate the assumption of values missing at random and lead to overestimation of the median count. For example, some patients with missing CD4 cell counts may have started therapy immediately because of an opportunistic infection and might thus be more likely to have a lower count, especially in LICs. Data on opportunistic infections and clinical stage was incomplete, and we could not use this information in our imputation models. However, for the 5 Southern African countries, which provided information on clinical stage, the WHO stage distribution overall was similar in patients with reported and those with missing CD4 cell counts. These data indicate that, at least in Southern Africa, only a small portion of missing counts are due to poorer health.
Data from some countries were limited to a small number of patients from a single clinic. We excluded these data sets because the data were probably unrepresentative of all patients receiving cART in those countries. Some data included in modeling of time trends may also not be representative of all patients receiving cART in the country. In particular, the clinics from LICs and MICs participating in IeDEA are mainly urban and capture data in electronic databases, indicating a higher level of resources. They may more closely reflect best practice in urban settings than in the country as a whole [8]. Nevertheless, our collaborative study is a unique source of information on trends and determinants of the CD4 cell count in adult patients starting cART across the globe.
In conclusion, median CD4 cell counts at the start of cART have increased in all country income groups over the last few years, and the proportion of individuals starting cART with severe immunodeficiency has decreased. However, the median CD4 cell count at cART start generally remained below 350/μL in 2015 and the decline in severe immunodeficiency appears to have plateaued in some countries. Clearly, substantial additional efforts and resources will be needed to achieve early diagnosis, rapid linkage to care, and prompt initiation of cART globally.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Writing committee. The writing committee included the following: Nanina Anderegg (Institute of Social and Preventive Medicine, University of Bern, Switzerland), Klea Panayidou (Institute of Social and Preventive Medicine, University of Bern, Switzerland), Yao Abo (Programme PAC-CI, Centre Hospitalier Universitaire de Treichville, Abidjan, Côte d’Ivoire), Belen Alejos (National Center of Epidemiology, Instituto de Salud Carlos III, Madrid, Spain), Keri N. Althoff (Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland), Kathryn Anastos (Departments of Medicine and Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx NY), Andrea Antinori (HIV/AIDS Department, National Institute for Infectious Diseases L. Spallanzani, IRCCS, Rome, Italy), Eric Balestre (Centre INSERM U1219, Bordeaux Population Health, Université de Bordeaux, France), Renaud Becquet (INSERM, Centre de Recherche INSERM U1219 and Institut de Santé Publique Epidémiologie Développement, Université Bordeaux, France), Antonella Castagna (Department of Infectious Diseases, San Raffaele Scientific Institute, University Vita-Salute San Raffaele, Milan, Italy), Barbara Castelnuovo (Infectious Diseases Institute, Makerere University, Mulago Hospital, Kampala, Uganda), Geneviève Chêne (INSERM, ISPED, Centre INSERM U1219-Bordeaux Population Health, Bordeaux, France), Lara Coelho (Instituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil), Intira Jeannie Collins (Medical Research Council [MRC] Clinical Trials Unit, Institute of Clinical Trials & Methodology, University College London, United Kingdom), Dominique Costagliola (Sorbonne Universites, UPMC Université Paris 06, INSERM, Institut Pierre Louis d’Epidemiologie et de Sante Publique, Paris, France), Brenda Crabtree-Ramírez (Department of Infectious Diseases, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico), Francois Dabis (INSERM, Centre de Recherche INSERM U1219 and Institut de Santé Publique Epidémiologie Développement, Université Bordeaux, France), Antonella d’Arminio Monforte (Clinic of Infectious and Tropical Diseases, Department of Health Sciences, ASST Santi Paolo e Carlo, University of Milan, Italy), Mary-Ann Davies (Centre for Infectious Disease Epidemiology and Research, School of Public Health and Family Medicine, University of Cape Town, South Africa), Stéphane De Wit (Department of Infectious Diseases, St Pierre University Hospital, Université Libre de Bruxelles, Brussels, Belgium), Valérie Delpech (Public Health England, London, United Kingdom), Nicole L. De La Mata (The Kirby Institute, UNSW Sydney, New South Wales, Australia), Stephany Duda (Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee), Aimee Freeman (Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland), Stephen J. Gange (Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland), Katharina Grabmeier-Pfistershammer (Division of Immunology, Allergy and Infectious Diseases, Department of Dermatology, Medical University of Vienna, Austria), Barbara Gunsenheimer-Bartmeyer (Robert Koch Institute, Berlin, Germany), Awachana Jiamsakul (The Kirby Institute, UNSW Sydney, New South Wales, Australia), Mari M. Kitahata (Center for AIDS Research, University of Washington, Seattle), Matthew Law (The Kirby Institute, UNSW Sydney, New South Wales, Australia), Christian Manzardo (Infectious Diseases Service, Hospital Clinic-IDIBAPS, University of Barcelona, Spain), Catherine McGowan (Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee), Laurence Meyer (Université Paris Sud, Le Kremlin-Bicêtre, France), Richard Moore (Department of Medicine, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland), Cristina Mussini (Clinic of Infectious Diseases, University of Modena and Reggio Emilia, Italy), Gertrude Nakigoz (Rakai Health Sciences Program, Uganda), Denis Nash (Institute for Implementation Science in Population Health, City University of New York and Graduate School of Public Health and Health Policy, City University of New York), Oon Tek Ng (Tan Tock Seng Hospital, Singapore), Niels Obel (Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Denmark), Nikos Pantazis (Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Greece), Armel Poda (Institut Supérieur des Sciences de la Santé, Université Polytechnique de Bobo-Dioulasso, Bobo-Dioulasso, Burkina Faso), Dorthe Raben (Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Denmark), Peter Reiss (Stichting HIV Monitoring and Department of Global Health and Division of Infectious Diseases, Academic Medical Center, University of Amsterdam, the Netherlands), Larry Riggen (Department of Biostatistics, Indiana University Fairbanks School of Public Health, Indianapolis), Caroline Sabin (Research Department of Infection and Population Health, University College London, United Kingdom), Jean d’Amour Sinayobye (Division of Research and Clinical Education, The Rwanda Military Hospital, Kanombe, Kigali), Anders Sönnerborg (Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden), Marcel Stoeckle (Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel and University Basel, Switzerland), Claire Thorne (Great Ormond Street Institute of Child Health, University College London, United Kingdom), Carlo Torti (Infectious and Tropical Diseases Unit, Department of Medical and Surgical Sciences, University “Magna Graecia” of Catanzaro, Italy), Christella Twizere (Centre Hospitalo-Universitaire de Kamenge, Bujumbura, Burundi), Jan-Christian Wasmuth (Department of Internal Medicine I, University of Bonn, Germany), Linda Wittkop (INSERM, Centre de Recherche INSERM U1219 and Institut de Santé Publique Epidémiologie Développement, Université Bordeaux, France), Kara Wools-Kaloustian (Division of Infectious Diseases, Indiana University School of Medicine, Indianapolis), Marcel Yotebieng (Division of Epidemiology, College of Public Health, Ohio State University, Columbus), Ole Kirk (CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Denmark), and Matthias Egger (Institute of Social and Preventive Medicine, University of Bern, Switzerland, and (Centre for Infectious Disease Epidemiology and Research, School of Public Health and Family Medicine, University of Cape Town, South Africa)
Acknowledgments. The IeDEA and COHERE collaborations are grateful to all patients, caregivers, and data managers involved in the participating cohorts and treatment programs.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. The African regions for IeDEA are supported by the National Cancer Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Allergy and Infectious Diseases (NIAID) as part of the IeDEA (grants U01AI069919, U01AI069924, U01AI096299, and U01AI069911). The Caribbean, Central, and South America Network for HIV Epidemiology (CCASAnet), a member cohort of IeDEA (grant U01AI069923), is funded by the following institutes: NICHD, Office of the Director, NIH, NIAID, the National Cancer Institute, and the National Institute of Mental Health. The North American AIDS Cohort Collaboration on Research and Design of IeDEA is supported by the NIH (grants U01AI069918, F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01AG053100, R01CA165937, R01DA011602, R01DA012568, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794, U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214, and Z01CP010176); the US Centers for Disease Control and Prevention (CDC; contracts CDC-200-2006-18797 and CDC-200-2015-63931); from the US Agency for Healthcare Research and Quality (contract 90047713); from the US Health Resources and Services Administration (contract 90051652); the Canadian Institutes of Health Research (grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118); Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Cancer Institute, National Institute for Mental Health, and National Institute on Drug Abuse. The TREAT Asia HIV Observational Database and the Australian HIV Observational Database are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the NIAID, the NICHD, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse, as part of IeDEA (grant U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing and affiliated with the Faculty of Medicine, UNSW Sydney.
The COHERE study gr oup has received unrestricted funding from Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), France; the HIV Monitoring Foundation, the Netherlands; and the Augustinus Foundation, Denmark. The research leading to these results received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under EuroCoord grant agreement 260694. Icona Foundation is sponsored by unrestricted grants from Gilead, Bristol-Myers Squibb (BMS), ViiV, and MSD Italy. ANRS HIV cohorts are funded by ANRS. The Collaborative HIV Paediatric Study is funded by NHS England and has received additional support from the PENTA Foundation and the Medical Research Council programme (MC_UU_12023/26), as well as Abbott, Boehringer Ingelheim, BMS, Gilead Sciences, GlaxoSmithKline, Janssen, and Roche. A list of other funders of the participating cohorts can be found at www.cohere.org.
Potential conflicts of interest. K. N. A. is a board member of TrioHealth and has received grants and other financial support from the NIH and Gilead Sciences. K. A. has received grants and other financial support from NIAID, Brown University, and the NIH. A. A. has received consultancy fees, grants or travel expenses from Gilead Sciences, BMS, ViiV Healthcare, Merck, Janssen Cilag, and Abbvie. B. C. has received grants and financial support from Infectious Disease Institute and the NIH. G. C. has received grants and other support from ANRS and the European Commission (FP7/2007–2013). I. J. C. has received grants from NHS England. D. C. was a member of the HIV board of Gilead France until December 2015 and has received consultancy fees, grants, and other financial support from Innavirvax, Janssen-Cilag, Merck Sharp & Dohme–Chibret, ViiV, and Gilead. A. d. M. was a board member of Gilead, Jansen, Merck Sharp & Dohme (MSD), and ViiV. S. D. W. has received consultancy fees, grants, or other financial support from ViiV, MSD, Gilead, Janssen, and BMS. K. G.-P. is a board member of Gilead Sciences and has received financial support from BMS, Gilead Sciences, and GSK-ViiV. O. K. is a board member for Gilead and ViiV and has received financial support from Gilead, BMS, and ViiV. L. M. has received grants and other financial support from ANRS, Framework Program 7 through Medical Research Council. C. M. is a board member for MSD, Gilead, BMS, and ViiV and has received grants and other financial support from Gilead, ViiV, Janssen, and MSD. P. R. is a board member for Gilead Sciences and Janssen Pharmaceutica and has received grants and other financial support from Gilead, ViiV, Janssen, and Merck & Co. C. S. is a board member for ViiV, Gilead, and Janssen-Cilag and has received grants or financial support from MRC, Gilead, ViiV, and Janssen-Cilag. A. S. has received consultancy fees, grants and other financial support from Immune System Regulation AB, Octapharma, Gilead, Jansen-Cilag, BMS, and GlaxoSmithKline/ViiV. M. S. is a board member for Abbvie, Janssen Cilag, MSD, Gilead, and ViiV and has received consultancy fees or grants from Roche, Gilead, Janssen Cilag, and MSD. C. T. has received grants from the European Commission, Abbvie, Public Health England, and the Medical Research Council. C. T. has received reimbursement of expenses for participation to international conferences from Gilead. J.-C. W. has received financial support from Gilead, Abbvie, and MSD. L. W. was a board member of BMS until 2015 and has received grants from ANRS and payments for lectures from Gilead and Janssen. B. C.-R. has received financial support from Janssen, MSD, Abbvie, and Gilead. M.-A. D. has received grants from the NIH, International AIDS Society, and the CDC. N. L. D. L. M. has received financial support from the NIH, the University of Sydney, and the NSW Ministry of Health. M. L. has received grants and other financial support from Gilead Sciences, Boehringer Ingelheim, MSD, BMS, Janssen-Cilag, ViiV HealthCare, and Sirtex. R. M. has received payments from Medscape. O. T. N. has received grants from Singapore Medical Research Council. L. R. and K. W.-K. have received grants from the CDC and the NIH. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
The IeDEA and COHERE Cohort Collaborations:
Nanina Anderegg, Klea Panayidou, Yao Abo, Belen Alejos, Keri N Althoff, Kathryn Anastos, Andrea Antinori, Eric Balestre, Renaud Becquet, Antonella Castagna, Barbara Castelnuovo, Geneviève Chêne, Lara Coelho, Intira Jeannie Collins, Dominique Costagliola, Brenda Crabtree-Ramírez, Francois Dabis, Antonella d’Arminio Monforte, Mary-Ann Davies, Stéphane De Wit, Valérie Delpech, Nicole L De La Mata, Stephany Duda, Aimee Freeman, Stephen J Gange, Katharina Grabmeier-Pfistershammer, Barbara Gunsenheimer-Bartmeyer, Awachana Jiamsakul, Mari M Kitahata, Matthew Law, Christian Manzardo, Catherine McGowan, Laurence Meyer, Richard Moore, Cristina Mussini, Gertrude Nakigoz, Denis Nash, Oon Tek Ng, Niels Obel, Nikos Pantazis, Armel Poda, Dorthe Raben, Peter Reiss, Larry Riggen, Caroline Sabin, Jean d’Amour Sinayobye, Anders Sönnerborg, Marcel Stoeckle, Claire Thorne, Carlo Torti, Christella Twizere, Jan-Christian Wasmuth, Linda Wittkop, Kara Wools-Kaloustian, Marcel Yotebieng, Ole Kirk, and Matthias Egger
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS). “15 by 15”—A global target achieved 2015. Available at: http://www.unaids.org/en/resources/documents/2015/15_by_15_a_global_target_achieved. Accessed 1 May 2016. [PubMed]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: An ambitious treatment target to help end the AIDS epidemic 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed 5 April 2015. [PubMed]
- 3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed 2016. Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 4. Avila D, Althoff KN, Mugglin C et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2014; 65:e8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auld AF, Shiraishi RW, Oboho I et al. Trends in prevalence of advanced HIV disease at antiretroviral therapy enrollment: 10 countries, 2004–2015. Morb Mortal Wkly Rep 2017; 66:558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in Sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2014; 60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4+ cell count at presentation to HIV care, 1992–2011. Clin Infect Dis 2013; 57:1027–37. [DOI] [PubMed] [Google Scholar]
- 8. Egger M, Ekouevi DK, Williams C et al. Cohort profile: the International Epidemiological Databases to Evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGowan CC, Cahn P, Gotuzzo E et al. Cohort profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol 2007; 36:969–76. [DOI] [PubMed] [Google Scholar]
- 10. Gange SJ, Kitahata MM, Saag MS et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou J, Kumarasamy N, Ditangco R et al. The TREAT Asia HIV observational database. J Acquir Immune Defic Syndr 2005; 38:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chêne G, Phillips A, Costagliola D et al. Cohort profile: Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. Int J Epidemiol 2017; 46:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bofill M, Janossy G, Lee CA et al. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol 1992; 88:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The World Bank. How we classify countries. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed November 2017. [Google Scholar]
- 15. World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. 2003 Revision. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
- 16. Mocroft A, Lundgren JD, Sabin ML et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med 2013; 10:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley, 1987. [Google Scholar]
- 18.AIDSinfo online database. Available at: www.aidsinfo.unaids.org. Accessed November 2017.
- 19. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 revision. Geneva, Switzerland: World Health Organization, 2010:1–359. [PubMed] [Google Scholar]
- 20. Beck EJ, Vitoria M, Mandalia S, Crowley S, Gilks CF, Souteyrand Y. National adult antiretroviral therapy guidelines in resource-limited countries: concordance with 2003 WHO guidelines?AIDS 2006; 20:1497–502. [DOI] [PubMed] [Google Scholar]
- 21. Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 29 January 2008. Washington, DC: Department of Health and Human Services, 2008. [Google Scholar]
- 22. Thompson MA, Aberg JA, Hoy JF et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 23. Marum E, Taegtmeyer M, Parekh B et al. “What took you so long?” the impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. J Acquir Immune Defic Syndr 2012; 60(suppl 3):S63–9. [DOI] [PubMed] [Google Scholar]
- 24. Liggett A, Medina N, Samayoa B et al. Is expanded HIV testing associated with earlier HIV diagnosis? results from an HIV clinic in Guatemala City. J Int Assoc Provid AIDS Care 2016; 15:201–4. [DOI] [PubMed] [Google Scholar]
- 25. Wanyenze RK, Kamya MR, Fatch R et al. Missed opportunities for HIV testing and late-stage diagnosis among HIV-infected patients in Uganda. PLoS One 2011; 6:e21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS data. Geneva, Switzerland: UNAIDS, 2016. [Google Scholar]
- 27. Prendergast AJ, Essajee S, Penazzato M. HIV and the millennium development goals. Arch Dis Child 2015; 100(suppl 1):S48–52. [DOI] [PubMed] [Google Scholar]
- 28. Joint United Nations Programme on HIV/AIDS (UNAIDS). Global plan towards the elimination of new HIV infections, 2011–2015. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 29. Chalmers I. The Cochrane collaboration: preparing, maintaining, and disseminating systematic reviews of the effects of health care. Ann N Y Acad Sci 1993; 703:156–63; discussion 163–5. [DOI] [PubMed] [Google Scholar]
- 30. Mutimura E, Addison D, Anastos K et al. Trends in and correlates of CD4+ cell count at antiretroviral therapy initiation after changes in national ART guidelines in Rwanda. AIDS 2015; 29:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lahuerta M, Wu Y, Hoffman S et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four Sub-Saharan African Countries. Clin Infect Dis 2014; 58:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.