Summary
Existing tuberculosis diagnostics fail to distinguish active tuberculosis from latent or cured disease. Indoleamine 2, 3-dioxygenase, an interferon γ–inducible enzyme, is a blood-based tuberculosis biomarker that performed with high accuracy in patients with human immunodeficiency virus infection.
Keywords: Tuberculosis, HIV, indoleamine 2, 3-dioxygenase, IDO, diagnostic
Abstract
Background
There is no biomarker for diagnosing active tuberculosis in patients with human immunodeficiency virus (HIV) infection. Indoleamine 2, 3-dioxygenase (IDO) is an immunoregulatory enzyme that breaks down tryptophan (Trp) to metabolites known as kynurenines (Kyns). We investigated whether IDO activity, as measured by the ratio of Kyn to Trp, could be used to diagnose or predict active tuberculosis disease in HIV-infected adults.
Methods
Kyn and Trp concentrations were measured using ultraperformance liquid chromatography mass spectrometry in plasma samples from 32 HIV-infected patients in whom active tuberculosis developed and who were followed up prospectively. We compared to 70 HIV-infected control subjects from the same cohort in whom tuberculosis did not develop, matched by age, sex, and CD4 cell count, and 37 unmatched HIV-infected patients with a diagnosis of pneumonia. Clinical parameters, including body mass index, CD4 cell count, HIV load, and C-reactive protein levels were analyzed.
Results
At the time of tuberculosis diagnosis, IDO activity was significantly higher in patients with tuberculosis than in controls (P < .001). Six months before tuberculosis diagnosis, IDO activity was significantly higher in all patients who later developed tuberculosis (P < .001) than controls. After 6 months of tuberculosis treatment, IDO activity in patients with tuberculosis declined to levels similar to those in controls. IDO activity was 4-fold higher in patients with tuberculosis than in those with pneumonia, and could be used to distinguish them. With a receiver operating characteristic curve, IDO activity had a sensitivity of 97%, a specificity of 99%, and positive and negative predictive values of 89% and 100% for detecting active tuberculosis disease.
Conclusion
Plasma IDO activity is suitable as a biomarker of active tuberculosis in HIV-positive patients.
Tuberculosis in patients with human immunodeficiency virus (HIV) infection is a global health concern. Tuberculosis causes death in 1.3 million persons annually, many of whom are HIV infected [1]. Although recent diagnostic techniques using tuberculosis nucleic acid amplification and interferon γ release assays have improved tuberculosis diagnosis, the lack of a biomarker for diagnosing active tuberculosis disease hampers tuberculosis control. In HIV-infected individuals, these diagnostic challenges are compounded by paucibacilliary tuberculosis and high rates of disseminated tuberculosis [2]. Current tests to determine latent tuberculosis infection are insufficiently sensitive to identify those likely to progress to tuberculosis disease [3, 4]. A challenge is to identify sensitive and specific markers with potential for use in diagnosing tuberculosis disease and measuring responses to treatment.
Many candidate biomarkers for tuberculosis diagnosis and response to treatment have been proposed [5–7]. None have been validated for application in a tuberculosis-endemic area. A plausible candidate biomarker is activity of the enzyme indoleamine 2, 3-dioxygenase (IDO). IDO is an interferon γ–inducible cytosolic enzyme that catalyses degradation of tryptophan (Trp) to kynurenines (Kyns) [8]. By reducing local Trp and producing immunomodulatory metabolites, IDO suppresses T-cell proliferation and function, resulting in immune suppression and tolerance [9]. IDO regulates physiological functions, such as pregnancy [10], and modulates pathogenesis of diverse pathological conditions, including cancer [11] and infectious diseases [12, 13].
In HIV, elevated IDO activity has been associated with neurocognitive disorders and rapid progression to AIDS [14]. IDO activity has been reported to be up-regulated in HIV-negative adults with tuberculosis disease [15, 16]. In animal models, IDO expression and activity was high in granulomatous lesions in mice with tuberculosis [17]. Almeida et al [18] reported up-regulated IDO messenger RNA in sputum samples from patients with tuberculosis, with values that declined >500-fold after tuberculosis treatment. Suzuki et al [15, 16] reported serum IDO activity significantly higher in patients with tuberculosis than in control subjects and IDO activity correlated with time to death. These data imply that elevated IDO activity may play a role in the pathogenesis of active tuberculosis disease. In HIV-infected individuals, the diagnostic significance of IDO activity is uncertain. Using ultraperformance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS), we determined the ratio of Kyn to Trp to investigate the diagnostic accuracy of IDO activity as a biomarker for active tuberculosis disease in HIV-infected patients.
METHODS
Subjects
The Lung Cohort study enrolled HIV-infected adults from 2008 to 2012 in Soweto, Johannesburg. A total of 754 adults were followed up every 6 months for up to 4 years to detect interval events. Antiretroviral therapy (ART) was initiated according to routine care, with a threshold CD4 cell count of 200/µL as the ART initiation criterion. Blood, urine, and sputum samples were collected at each visit. Patients were screened for tuberculosis symptoms and investigated if symptomatic. Chest radiographs were obtained when indicated. During follow-up, active tuberculosis disease developed in 34 patients. These subjects had no history or clinical signs compatible with active tuberculosis at the time of enrollment. Active tuberculosis disease was bacteriologically confirmed or diagnosed based on clinical signs and symptoms, including protracted cough, fever, weight loss, and suggestive radiological findings (clinical tuberculosis). Bacterial pneumonia was diagnosed according to routine clinical care, including respiratory rate, fever, productive sputa, signs of consolidation at clinical examination or chest radiography, and/or microbiological culture results. Tuberculosis treatment was conducted at nurse-run community clinics and not at the study site.
After study completion, we selected all subjects with incident tuberculosis with available samples. One patient was excluded owing to diagnosis of nontuberculous mycobacterial disease, and 1 had no plasma sample available. We evaluated 32 patients with tuberculosis at enrollment, 6 months before tuberculosis diagnosis, at tuberculosis diagnosis, and at all available time points after tuberculosis diagnosis. Control subjects who did not have tuberculosis diagnosed during follow-up were matched at a 2:1 ratio from the same cohort (n = 70). Controls were matched based on CD4 stratum, sex, and the visit at which tuberculosis was diagnosed. We also selected 37 subjects from the same cohort who had pneumonia diagnosed. One patient had a diagnosis of pneumonia 6 months before the tuberculosis diagnosis and was therefore included in both pneumonia and tuberculosis groups at different times. Controls and patients with pneumonia were all HIV-infected adults.
Measurement of Plasma Kynurenine and Tryptophan
L-Trp, L-Kyn, deuterated Trp-d5, and Kyn-d4 were used as reference compounds and internal standards respectively (Sigma-Aldrich). Kyn and Trp were measured with UPLC-MS/MS (Acquity UPLC and Xevo TQ-S Tandem mass spectrometer, Waters, USA) using a modification of a method described elsewhere [19]. Briefly, ethylenediaminetetraacetic acid–anticoagulated plasma samples stored at −70oC were thawed at room temperature, spiked with internal standards (Trp-d5 and Kyn-d4), deproteinized with absolute methanol, vortexed for 10 minutes, and centrifuged at 3156 g for 10 minutes. The supernatant was then analyzed on the UPLC-MS/MS system. Analytes were separated using an isocratic elution of injected samples within 2 minutes. Kyn and Trp were detected in multiple reaction mode using electrospray ionization mass spectrometry in positive mode. The IDO concentration was calculated as the ratio of measured Kyn concentration to measured Trp concentration. C-reactive protein (CRP), CD4 cell count, and HIV viral load tests were performed by the National Health Laboratory Service.
Statistical Analysis
Normally distributed data are shown as means with standard deviations. Nonnormally distributed data are expressed as median values with interquartile range (IQR). Student t tests were used to compare 2 parametric groups, Mann-Whitney tests for nonparametric unpaired groups, Wilcoxon tests for paired groups, and Kruskal-Wallis tests with Dunn posttests for multiple nonparametric groups. All comparisons were 2 sided. Categorical data were analyzed using Fisher exact tests. IDO activity fold change was plotted to assess within-person variation in subjects with ≥5 available time points. Fold change was the ratio of IDO activity level at a particular time point to the level at baseline. The baseline was the first visit for controls and 12 months before tuberculosis diagnosis for patients with tuberculosis.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated and a receiver operating characteristic curve plotted to evaluate the most suitable cutoff, giving the highest area under the curve (AUC). For correlations, Spearman correlation coefficient was used. Differences were considered significant at P < .05. Analyses used GraphPad Prism 6.01 software (GraphPad Software).
Study Approval
The Lung Cohort Study was approved by the Johns Hopkins Medicine Institutional Review Board and the University of the Witwatersrand Human Research Ethics Committee; the nested study described herein was approved by the latter. Participants signed informed consent.
RESULTS
Demographics and Clinical Parameters
Demographic and clinical characteristics of 102 patients with tuberculosis and controls are summarized in Table 1. Of the 32 tuberculosis cases, 18 were laboratory confirmed and 14 were clinical tuberculosis. One patient had extrapulmonary tuberculosis (cutaneous tuberculosis without systemic involvement), and 2 had multidrug-resistant tuberculosis. Body mass index (BMI), CD4 cell count, CRP, and viral load differed significantly at the time of tuberculosis diagnosis, compared with controls at the corresponding visit 3 (Table 2). Lower BMI and higher viral load were apparent in patients with tuberculosis, even at baseline.
Table 1.
Demographic Characteristics of Patients With Tuberculosis and Controls
| Demographic Data | Patients, No. (%)a | P Valueb | ||
|---|---|---|---|---|
| Tuberculosis (n = 32) | Controls (n = 70) | Pneumonia (n = 37) | ||
| Female sex | 22 (69) | 52 (74) | 32 (86) | .19 |
| Age, mean (SD), y | 38 (8) | 37 (6) | 38 (8) | .48 |
| Mode of tuberculosis diagnosis | ||||
| Microscopy or culture | 18 (56) | … | … | … |
| Clinical symptoms and chest radiography only | 14 (44) | … | … | … |
| Tuberculosis site or type | ||||
| Pulmonary | 31 (97) | … | … | … |
| Extrapulmonary | 1 (3) | … | … | … |
| Multidrug resistant | 2 (6) | … | … | … |
Abbreviation: SD, standard deviation.
aData represent No. (%) of patients except where otherwise specified (for age)
b P values were determined with χ2 test for sex and with 1-way analysis of variance for age.
Table 2.
Comparison of BMI, CD4 Cell Count, CRP Level, and HIV Viral Load in Patients and Controls
| Variable | Baseline, Median (IQR)a | P Valueb | Time of Tuberculosis Diagnosis, Median (IQR)a | P Valueb | ||
|---|---|---|---|---|---|---|
| Patients With Tuberculosis | Controls (Visit 1) | Patients With Tuberculosis | Controls (Visit 3) | |||
| BMI, kg/m2 | 23 (20–27) | 26 (23–30) | .03c | 22 (16–27) | 25 (16–31) | .03c |
| CD4 cell count, cells/mL | 329 (240–403) | 372 (273–511) | .057 | 249 (211–401) | 417 (300–593) | .01c |
| CRP, mg/L | 6 (1–15) | 3 (1–11) | .33 | 9 (2–14) | 5 (1–10) | .03c |
| HIV viral load, copies/mL | 21 289 (49–41 870) | 1581 (49–8338) | .01c | 5368 (101–49 022) | 525 (49–6813) | .01c |
| Patients receiving ART, No. (%) | 8/32 (25) | 21/70 (30) | .64d | 16/32 (50) | 28/70 (40) | .39d |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CRP, C-reactive protein; HIV, human immunodeficiency virus; IQR, interquartile range.
aData represent median value (IQR) except where otherwise specified.
b P values were determined using Mann-Whitney tests, except where otherwise noted.
cSignificant at P < .05.
d P values for proportion receiving ART were determined using Fisher exact tests.
Plasma Indoleamine 2, 3-Dioxygenase Activity in Human Immunodeficiency Virus-Infected Patients With Tuberculosis and Controls
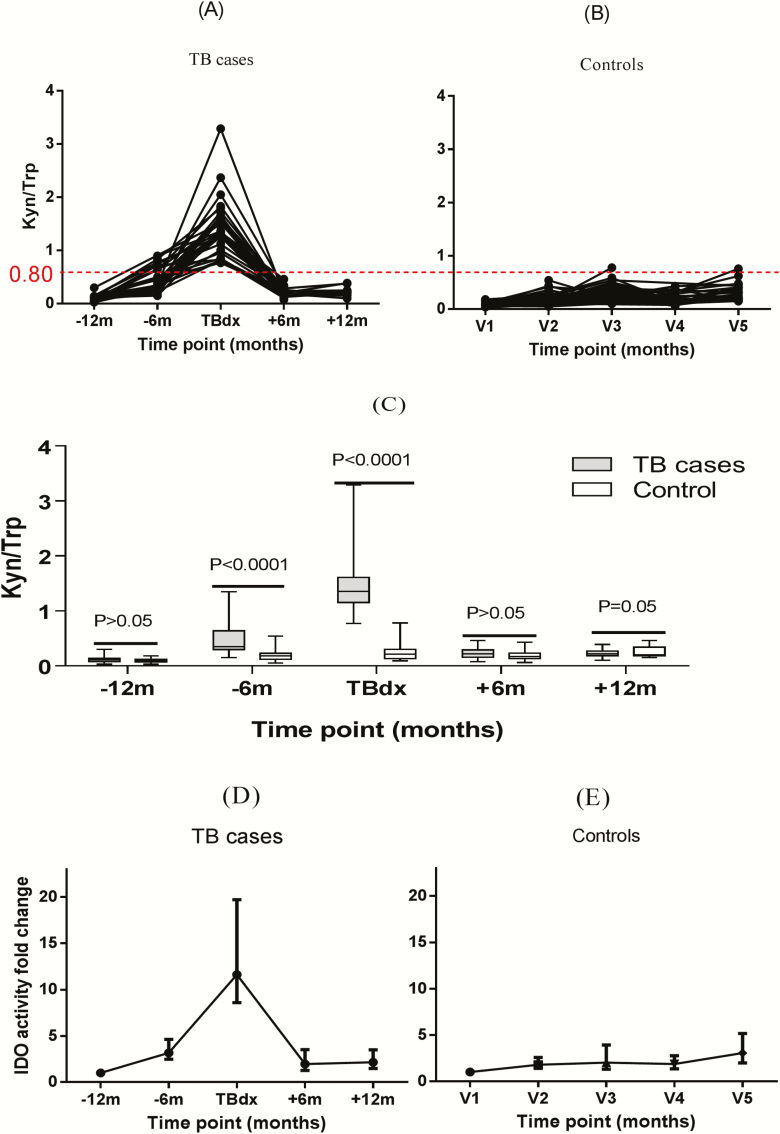
We evaluated plasma IDO activity from samples taken from 12 months before 12 months after tuberculosis diagnosis. Figure 1A and 1B show that for controls, the median IDO activity (Kyn/Trp ratio) over 5 time points (2½ years) was 0.16 (IQR, 0.11–0.24). IDO activity was significantly higher in patients with tuberculosis at the time of tuberculosis diagnosis (median, 1.35; IQR, 1.15–1.60) than in controls at the corresponding visit, visit 3 (0.21; 0.14–0.30; P < .001). Figure 1C shows that even 6 months before tuberculosis diagnosis, IDO activity was significantly elevated in those who later progressed to tuberculosis (median, 0.35; IQR, 0.29–0.60), compared with controls at the corresponding visit, visit 2 (0.17; 0.12–0.23; P < .001). IDO activity declined in all patients with tuberculosis after tuberculosis treatment to levels comparable to those in controls at visit 4 (median, 0.20 [IQR, 0.16–0.28] vs 0.17 [0.13–0.23]; P > .05). Results were due to a combination of increased Kyn and decreased Trp levels and were less marked with use of either analyte alone (data not shown).
Figure 1.
A, B, Plasma indoleamine 2, 3-dioxygenase (IDO) activity in patients with human immunodeficiency virus (HIV) infection in whom active tuberculosis disease developed (A) and in HIV-infected controls (B). IDO activity showed elevation from 6 months before tuberculosis diagnosis (−6 mo). At the time of tuberculosis diagnosis (TBdx), all patients with tuberculosis had IDO activity value >0.80 (red dotted line), compared with 1.5% of controls. Kyn, kynurenine; Trp, tryptophan; V1 (etc), visit 1 (etc). C, Plasma IDO activity as a measure of Kyn/Trp ratio in HIV-infected patients with tuberculosis compared with HIV-infected controls. IDO activity was significantly elevated both at tuberculosis diagnosis and 6 months earlier. D, E, Plasma IDO activity plotted as fold change in patients with tuberculosis (D) and controls (E), showing median and interquartile ranges at each time point (lower and upper portions of boxes represent 25th and 75th percentiles, respectively; horizontal lines within box plots, medians). P values were determined using Kruskal-Wallis tests with Dunn posttests. For patients with tuberculosis, there were 25, 26, 32, 27, and 21 patients, respectively, at the 5 time points shown; for controls, 55, 62, 70, 59, and 33 patients, respectively.
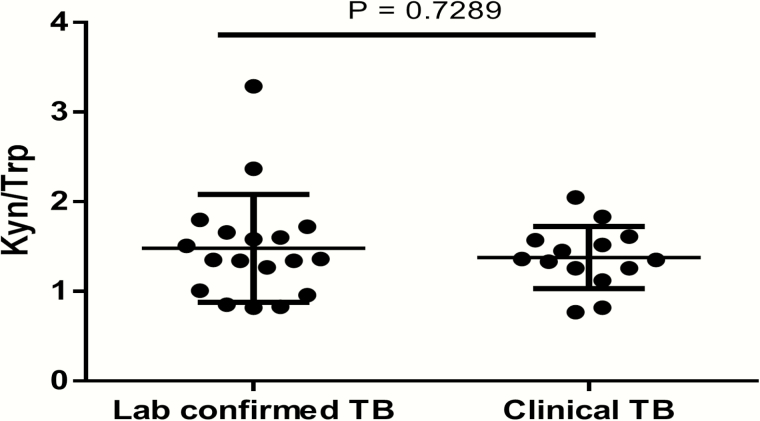
At the time of tuberculosis diagnosis, patients with tuberculosis had a median 12-fold [IQR, 9–20-fold] increase in IDO activity from baseline, whereas controls had median 2-fold [IQR, 1–4-fold] change over the entire study period (Figure 1D and 1E). Six months before tuberculosis diagnosis, patients who later progressed to active tuberculosis had a median 4-fold (IQR, 2.5–4.6-fold) increase in IDO activity compared with their baseline value, indicating that IDO activity increased ≥6 months before tuberculosis diagnosis. There was no difference in median IDO activity between patients with laboratory-confirmed tuberculosis and those with clinical tuberculosis (1.35 [IQR, 0.99–1.67] vs 1.34 [1.22–1.58]; P = .73; Figure 2).
Figure 2.
Plasma indoleamine 2, 3-dioxygenase (IDO) activity in patients with laboratory-confirmed (n = 18) or clinical (n = 14) tuberculosis. There was no difference in IDO activity dependent on mode of tuberculosis diagnosis. P values were determined using Mann-Whitney tests. Medians and interquartile ranges are shown. Kyn, kynurenine; TB, tuberculosis; Trp, tryptophan.
Plasma Indoleamine 2, 3-Dioxygenase Activity in Human Immunodeficiency Virus-Infected Patients With Pneumonia
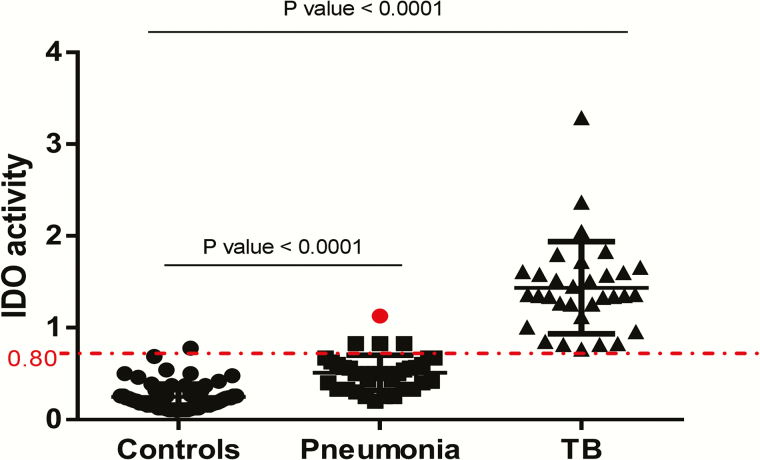
To determine whether plasma IDO activity could discriminate between active tuberculosis and other causes of lung disease, we evaluated 37 patients who presented with pneumonia (Figure 3). The median plasma IDO activity in patients with pneumonia was 0.50 (IQR, 0.35–0.60), intermediate between that for patients with tuberculosis (median, 1.35; IQR, 1.15–1.60) and controls (0.16; 0.14–0.30).
Figure 3.
Plasma indoleamine 2, 3-dioxygenase (IDO) activity human immunodeficiency virus (HIV)–infected controls (n = 70), HIV-infected patients with pneumonia (n = 37), and HIV-infected patients with active tuberculosis (n = 32). Patients with active tuberculosis had significantly higher IDO values than patients with pneumonia. At a cutoff of 0.80, IDO activity classified all but 4 pneumonia cases as “not tuberculosis.” The red dot indicates a patient in whom pneumonia was first diagnosed and who later progressed to active tuberculosis disease within 6 months. Medians and interquartile ranges are shown. P values were determined using Kruskal-Wallis tests with Dunn posttests.
Diagnostic Significance of Plasma Indoleamine 2, 3-Dioxygenase Activity
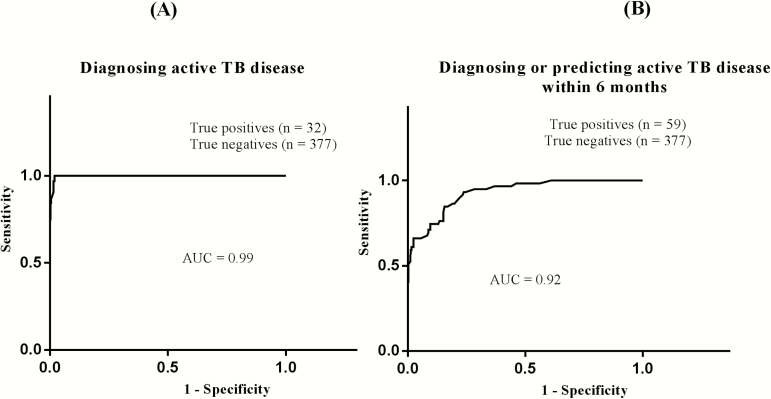
We evaluated different cutoffs to determine sensitivity, specificity, PPV, and NPV, and we performed receiver operating characteristic analysis. We used both laboratory-confirmed and clinical tuberculosis at the time of tuberculosis diagnosis as true-positives (n = 32). Controls at all time points, patients with pneumonia, and patients with tuberculosis (at time points other than tuberculosis diagnosis or 6 months earlier) were used as true-negatives (n = 377). At a cutoff of 0.80, IDO activity had a sensitivity of 97% (confidence interval, 83%–99%), a specificity of 99% (96%–99%), a PPV of 89%, and an NPV of 100% for detecting active tuberculosis (AUC, 0.99; P = .001; Table 3, Figure 4, and Supplementary Table S1). Comparing patients with tuberculosis alone as true-positives (n = 32) with patients with pneumonia alone as true-negatives (n = 37), the sensitivity, specificity, PPV, and NPV were 97%, 89%, 100%, and 97%, respectively, at the same cutoff (AUC, 0.98; Supplementary Table S2)
Figure 4.
Receiver operating characteristic curves for the use of indoleamine 2, 3-dioxygenase (IDO) activity in diagnosing (A) and predicting (B) active tuberculosis disease in patients with human immunodeficiency virus (HIV) infection. A, Using a cutoff of 0.80, plasma IDO activity had a diagnostic sensitivity of 97.0% (confidence interval, 83%–99%) and a specificity of 98.9% (96%–99%; Supplementary Table S1). B, For predicting active tuberculosis disease within 6 months, using the same cutoff, IDO activity had a sensitivity of 61% (confidence interval, 42%–68%) and a specificity of 99% (97%–99%; Supplementary Table S3). Patients with active tuberculosis were combined with those who went on to develop tuberculosis because pragmatically the whole group could potentially benefit from antituberculosis therapy. (For similar analysis excluding time of tuberculosis diagnosis, see Supplementary Table S4.) AUC, area under the curve; TB, tuberculosis.
Prognostic Significance of Plasma Indoleamine 2, 3-Dioxygenase Activity in Human Immunodeficiency Virus-Infected Patients
We determined the prognostic significance of IDO activity to diagnose or predict active tuberculosis 6 months before tuberculosis diagnosis in HIV-infected patients. We used patients with laboratory-confirmed or clinical tuberculosis at 6 months before and at the time of tuberculosis diagnosis as true-positives (n = 59). As true-negatives, we used controls at all time points, patients with pneumonia, and patients with tuberculosis at time points other than diagnosis or 6 months earlier (n = 377). At cutoff of 0.80, IDO activity had a sensitivity, specificity, PPV, and NPV of 61%, 99%, 90%, and 94%, respectively, in predicting progress to active tuberculosis within 6 months (AUC, 0.92; Supplementary Table S3 and Figure 4). Using the same cutoff but excluding patients at the time point of active tuberculosis, this analysis gives a sensitivity, specificity, PPV, and NPV of 21%, 100%, 100%, and 87%, respectively (AUC, 0.93; Supplementary Table S4).
No Correlation Between Plasma Indoleamine 2, 3-Dioxygenase Activity and Other Clinical Parameters
IDO activity showed no significant correlation with CD4 cell count, viral load, or CRP level in patients with tuberculosis (at baseline or at diagnosis) or controls (at baseline or visit 3) (data not shown). We evaluated the diagnostic significance of CRP for diagnosing active tuberculosis disease. Again we merged bacteriologically confirmed and clinical tuberculosis at the time of tuberculosis diagnosis as true-positives (n = 32). We used all controls at all time points, patients with pneumonia, and patients with tuberculosis (at time points other than tuberculosis diagnosis or 6 months earlier) as true-negatives (n = 368). At a cutoff of 8.0, CRP values had a sensitivity, specificity, PPV, and NPV of 60%, 56%, 27%, and 93%, respectively, for indicating active tuberculosis in HIV infection (AUC, 0.56; Supplementary Table S4 and Supplementary Figure S1).
DISCUSSION
We report a striking association between increased IDO activity and active tuberculosis disease among persons with HIV. Studies have proposed elevated IDO activity as prognostic in tuberculosis disease; however, no reports have discussed its diagnostic significance in HIV-infected patients. We assessed the diagnostic potential of elevated IDO activity in a longitudinal HIV-infected cohort followed up from 12 months before to 12 months after tuberculosis diagnosis.
In controls (all HIV infected), followed up for >2 years, the median IDO activity was 0.16, ranging 2-fold from baseline. At tuberculosis diagnosis, the median IDO activity was 1.35, which was 12-fold different from baseline. IDO activity was mildly elevated with pneumonia but of lower magnitude than in tuberculosis disease. Our findings are consistent with the available literature [15, 16, 18] and may be linked to the immunosuppressive roles of IDO [13]. IDO activity changes were due to increased product (Kyn) and decreased substrate (Trp) concentrations, but neither Kyn nor Trp levels alone had high diagnostic potential. Enzyme activity may not be detected with molecular, proteomic, or genomic analysis, which may explain why certain prior studies have not identified IDO as a candidate biomarker [5]. Weiner et al [17], however, also detected elevated Kyn and decreased Trp levels using metabolic profiling of >400 small molecules [17].
Compared with patients with pneumonia, those with tuberculosis had about a 4-fold increase in plasma IDO activity, consistent with findings in HIV-negative individuals [15, 16]. IDO activity, using a cutoff of 0.80, correctly classified all but 4 of 37 pneumonia cases as “not tuberculosis”; 1 of the 4 cases progressed to active tuberculosis within 6 months.
We determined diagnostic sensitivity, specificity, PPV and NPV. We merged patients with laboratory-confirmed or clinical tuberculosis as true-positives and used patients with pneumonia, controls at all time points, and patients with tuberculosis at time points other than tuberculosis diagnosis (excluding 6 months before tuberculosis diagnosis) as true-negatives. IDO activity had a sensitivity, specificity, PPV, and NPV of 97%, 99%, 89%, and 100%. It performed excellently for both ruling in and ruling out active tuberculosis. Sputum microscopy and culture have relatively low sensitivity and specificity in HIV-infected individuals [20].
IDO activity showed no difference between bacteriologically proven and clinical tuberculosis, nor between pulmonary and extrapulmonary tuberculosis, although our sample size was small. Our results suggest that IDO activity may be useful for indicating the presence or absence of active tuberculosis disease in either site [21]. Unlike interferon γ release assays, however, IDO activity seems to discriminate between active tuberculosis disease and latent or cured infection. Our data demonstrating IDO activity elevation in certain patients even 6 months before diagnosis suggest that, as a screening tool, IDO activity, with a cutoff of >0.8, could identify 1 in 5 patients (21%) who would progress to active disease within 6 months, decreasing morbid effects and transmission risk.
IDO activity declined in all patients with tuberculosis within 6 months to levels comparable to those in controls, consistent with the literature [18]. Administration of ART may also decrease IDO [22] but would probably not explain the difference between our 2 groups, because both were receiving ART. Plasma IDO activity may perhaps be used to monitor tuberculosis treatment. Our patients had no relapses with which to assess successful versus unsuccessful treatment. Currently, tuberculosis treatment response is monitored by sputum or culture conversion at 2 months. Smear microscopy is insensitive, is nonspecific for M. tuberculosis, and does not identify viable bacilli [23]. M. tuberculosis culture is time-consuming and reliant on sputum quality and quantity. IDO activity offers potential to monitor treatment response because it is determined with a blood-based rather than sputum-based test and declines in response to treatment. This aspect requires follow-up studies of larger cohorts, including patients with treatment failure.
We found that patients who progressed to active tuberculosis disease had metabolic changes even at baseline. Patients with tuberculosis had significantly lower BMI and higher viral loads than controls at baseline, despite no significant difference in CD4 cell counts or CRP levels. At diagnosis, they had lower BMI and CD4 cell counts with higher viral loads and CRP levels than controls, consistent with other reports [24–26]. Metabolic changes in HIV infection are predictors of early wasting and progression to AIDS [27].
Consistent with several studies [28, 29], patients with pneumonia or active tuberculosis had elevated CRP levels. CRP levels were not significantly higher in patients with tuberculosis than in those with pneumonia. CRP did not perform as well as IDO activity as a marker of active tuberculosis disease. Many potential tuberculosis biomarkers are indicators of infection and immune activation rather than specific for tuberculosis. Further research is needed to evaluate IDO activity in conditions clinically resembling tuberculosis, such as sarcoidosis [30].
The current study has particular strengths. Our samples were from a prospective, longitudinal cohort that evaluated patients from 12 months before to 12 months after tuberculosis diagnosis. Both study subjects and controls were HIV infected in a tuberculosis-endemic area. Study limitations include small sample size and lack of molecular confirmation of tuberculosis cases. UPLC-MS/MS requires specialized equipment and skills. Enzymatic activity, however, should be detectable with lower-cost, higher-throughput methods [31]. It is not clear whether our results apply to HIV-uninfected or pediatric populations. Utility for diagnosis of disseminated tuberculosis requires evaluation, particularly in patients with AIDS who have CD4 cell counts <200/µL. Conditions such as pregnancy and cancer may influence IDO activity, and its interpretation in such patients requires further study.
A validated tuberculosis biomarker would be of great impact in the fight against tuberculosis. Such a marker could be used to predict progress to active tuberculosis disease, to indicate active tuberculosis, and potentially to monitor anti-tuberculosis therapy. Early identification of active tuberculosis disease in HIV-infected patients could curtail transmission of tuberculosis. Plasma IDO activity shows strong potential advantage over other proposed biomarkers.
In conclusion, we found that plasma IDO activity, as measured by Kyn/Trp ratio using UPLC-MS/MS, had excellent validity in identifying active tuberculosis disease in HIV-infected adults. Furthermore, IDO activity seems useful for detecting tuberculosis disease before onset of symptoms.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. C. G. A. G. performed the literature search, conducted experiments, analyzed data, produced the figures, wrote and revised the manuscript. T. S. supervised laboratory work, assisted with data interpretation, and edited the manuscript. C. J. H. performed data mining, assisted with data interpretation, and edited the manuscript. N. A. M. assisted with trial design and patient recruitment and edited the manuscript. R. E. C. assisted with trial design and patient recruitment and edited the manuscript. J. A.G. supervised laboratory work, assisted with data interpretation, and edited the manuscript. M. S. S., senior author, designed the hypothesis, supervised data analysis and interpretation, and edited the manuscript.
Acknowledgments. We are grateful to the participants in the Soweto Lung Cohort.
Financial support. This work was supported by the Fogarty International Centre (South African TB AIDS Training grant U2RTW007373), the National Health Laboratory Services Research Trust, and a Discovery Foundation Academic Fellowship Award. Study enrollment and follow-up was funded by National Institutes of Health (grants R01HL090312 and P30AI094189).
Potential conflict of interest. N. A. M. reports receiving grants from Becton-Dickinson and grants from Roche and nonfinancial support from Abbott, outside the submitted work. M. S. S. reports receiving grants from South African TB AIDS Training, a Discovery Foundation Academic fellowship award, and grants from National Health Laboratory Services Research Trust; conference funding from Sanofi Pasteur, outside the submitted work; and funding from the World Health Organization, outside the submitted work. She has an expired provisional South African patent entitled “Method for diagnosing a disease”; the patent is in no way related to this work or to methods developed and used in the current study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization (WHO). Global tuberculosis report 2015. WHO/HTM/TB/2015.22. Geneva, Switzerland: WHO. 2015:192 Available at: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf [Google Scholar]
- 2. Padmapriyadarsini C, Narendran G, Swaminathan S. Diagnosis & treatment of tuberculosis in HIV co-infected patients. Indian J Med Res 2011; 134:850–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kussen GM, Dalla-Costa LM, Rossoni A, Raboni SM. Interferon-gamma release assay versus tuberculin skin test for latent tuberculosis infection among HIV patients in Brazil. Braz J Infect Dis 2016; 20:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wallis RS, Pai M, Menzies D et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 2010; 375:1920–37. [DOI] [PubMed] [Google Scholar]
- 5. Joosten SA, Fletcher HA, Ottenhoff TH. A helicopter perspective on TB biomarkers: pathway and process based analysis of gene expression data provides new insight into TB pathogenesis. PLoS One 2013; 8:e73230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallis RS, Kim P, Cole S et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis 2013; 13:362–72. [DOI] [PubMed] [Google Scholar]
- 7. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol 2011; 11:343–54. [DOI] [PubMed] [Google Scholar]
- 8. Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 2008; 222:206–21. [DOI] [PubMed] [Google Scholar]
- 9. Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007; 117:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munn DH, Zhou M, Attwood JT et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281:1191–3. [DOI] [PubMed] [Google Scholar]
- 11. Brandacher G, Perathoner A, Ladurner R et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 2006; 12:1144–51. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt SV, Schultze JL. New insights into IDO biology in bacterial and viral infections. Front Immunol 2014; 5:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond) 2015; 129:601–72. [DOI] [PubMed] [Google Scholar]
- 14. Kandanearatchi A, Brew BJ. The kynurenine pathway and quinolinic acid: pivotal roles in HIV associated neurocognitive disorders. FEBS J 2012; 279:1366–74. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki Y, Miwa S, Akamatsu T et al. Indoleamine 2,3-dioxygenase in the pathogenesis of tuberculous pleurisy. Int J Tuberc Lung Dis 2013; 17:1501–6. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki Y, Suda T, Asada K et al. Serum indoleamine 2, 3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012; 19:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiner J 3rd, Parida SK, Maertzdorf J et al. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS One 2012; 7:e40221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almeida AS, Lago PM, Boechat N et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol 2009; 183:718–31. [DOI] [PubMed] [Google Scholar]
- 19. Huang Y, Louie A, Yang Q et al. A simple LC-MS/MS method for determination of kynurenine and tryptophan concentrations in human plasma from HIV-infected patients. Bioanalysis 2013; 5:1397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desikan P. Sputum smear microscopy in tuberculosis: is it still relevant? Indian J Med Res 2013; 137:442–4. [PMC free article] [PubMed] [Google Scholar]
- 21. Herrera V, Perry S, Parsonnet J, Banaei N. Clinical application and limitations of interferon-γ release assays for the diagnosis of latent tuberculosis infection. Clin Infect Dis 2011; 52:1031–7. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Shao J, Cai R et al. Anti-retroviral therapy decreases but does not normalize indoleamine 2,3-dioxygenase activity in HIV-infected patients. PLoS One 2014; 9:e100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chegou NN, Hoek KG, Kriel M, Warren RM, Victor TC, Walzl G. Tuberculosis assays: past, present and future. Expert Rev Anti Infect Ther 2011; 9:457–69. [DOI] [PubMed] [Google Scholar]
- 24. Corbett EL, Charalambous S, Fielding K et al. Stable incidence rates of tuberculosis (TB) among human immunodeficiency virus (HIV)-negative South African gold miners during a decade of epidemic HIV-associated TB. J Infect Dis 2003; 188:1156–63. [DOI] [PubMed] [Google Scholar]
- 25. Hanrahan CF, Golub JE, Mohapi L et al. Body mass index and risk of tuberculosis and death. AIDS 2010; 24:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinson NA, Hoffmann CJ, Chaisson RE. Epidemiology of tuberculosis and HIV: recent advances in understanding and responses. Proc Am Thorac Soc 2011; 8:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smit E, Skolasky RL, Dobs AS et al. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987-1999. Am J Epidemiol 2002; 156:211–8. [DOI] [PubMed] [Google Scholar]
- 28. Breen RA, Leonard O, Perrin FM et al. How good are systemic symptoms and blood inflammatory markers at detecting individuals with tuberculosis? Int J Tuberc Lung Dis 2008; 12:44–9. [PubMed] [Google Scholar]
- 29. El-Shafey BI, Bahr HM, Ganna SA, Attia MS, Rakhawy M. The diagnostic value of serum levels of C-reactive protein and procalcitonin in differentiation between active pulmonary TB and CAP. Egypt J Bronchol 2015; 9:178. [Google Scholar]
- 30. Maertzdorf J, Weiner J, Mollenkopf HJ et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci USA 2012; 109:7853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Bakly WM, Hasanin AH. Hypericum perforatum decreased hippocampus TNF-α and corticosterone levels with no effect on kynurenine/tryptophan ratio in bilateral ovariectomized rats. Korean J Physiol Pharmacol 2014; 18:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.