Monthly vitamin D3 50000 IU increased spine bone mineral density in youth with HIV treated with tenofovir disoproxil fumarate as part of combination antiretroviral therapy.
Keywords: tenofovir disoproxil fumarate, vitamin D supplementation, bone mineral density, parathyroid hormone, HIV infection
Abstract
Background
Tenofovir disoproxil fumarate (TDF) decreases bone mineral density (BMD). We hypothesized that vitamin D3 (VITD3) would increase BMD in youth receiving TDF.
Methods
This was a randomized, double-blind, placebo-controlled trial of directly observed VITD3 vs placebo every 4 weeks for 48 weeks in youth aged 16–24 years with HIV, RNA load <200 copies/mL, taking TDF-containing combination antiretroviral therapy (TDF-cART) for ≥180 days. Participants (N = 214) received a daily multivitamin containing VITD3 400 IU and calcium 162 mg, plus monthly randomized VITD3 50000 IU (n = 109) or placebo (n = 105). Outcome was change from baseline to week 48 in lumbar spine BMD (LSBMD). Data presented are median (Q1, Q3).
Results
Participants were aged 22.0 (21.0, 23.0) years, 84% were male, and 74% were black/African American. At baseline, 62% had 25-hydroxy vitamin D (25-OHD) <20 ng/mL. Multivitamin adherence was 49% (29%, 69%), and VITD3/placebo adherence 100% (100%, 100%). Vitamin D intake was 2020 (1914, 2168) and 284 (179, 394) IU/day, and serum 25-OHD concentration was 36.9 (30.5, 42.4) and 20.6 (14.4, 25.8) ng/mL at 48 weeks in VITD3 and placebo groups, respectively (P < .001). From baseline to week 48, LSBMD increased by 1.15% (–0.75% to 2.74%) in the VITD3 group (n = 99; P < .001) and 0.09% (–1.49% to 2.61%) in the placebo group (n = 89; P = .25), without between-group difference (P = .12). VITD3 group changes occurred with baseline 25-OHD <20 ng/mL (1.17% [–.82% to 2.90%]; P = .004) and ≥20 ng/mL (0.93% [–.26% to 2.15%]; P = .033).
Conclusions
For youth taking TDF-cART, LSBMD increased through 48 weeks with VITD3 plus multivitamin, but not with placebo plus multivitamin, independent of baseline vitamin D status.
Clinical Trials Registration
Tenofovir disoproxil fumarate (TDF) is widely used in human immunodeficiency virus (HIV) treatment as part of combination antiretroviral therapy (cART) and in HIV preexposure prophylaxis combined with emtricitabine (FTC) [1]. While initiation of any cART for HIV treatment decreases dual-energy X-ray absorptiometry (DXA)–measured bone mineral density (BMD) [2], TDF use results in greater BMD decline than comparator nucleoside reverse transcriptase inhibitors, including stavudine [3, 4], zidovudine [4], abacavir [5–7], and tenofovir alafenamide (TAF) [8, 9]. During chronic cART treatment, switching from TDF to a regimen without TDF improves BMD [10, 11], another demonstration of TDF’s effect on bone.
Although the mechanisms of TDF-related bone toxicity are unknown, TDF increases parathyroid hormone (PTH) [12–15], decreases fibroblast growth factor 23 (FGF23) [15], and increases serum bone turnover markers (eg, C-terminal telopeptides [CTX], bone alkaline phosphatase [BAP], and osteocalcin [OC]) [5], changes also observed in persons with vitamin D (25-hydroxy vitamin D [25-OHD]) deficiency [16].
Independent of TDF use or HIV infection, in persons with 25-OHD deficiency, vitamin D supplementation improves endocrine and bone turnover changes, and increases BMD [17]. Vitamin D supplementation also improves the endocrine and BMD changes associated with TDF use. At initiation of TDF-containing cART in adults with HIV, randomization to daily high-dose vitamin D3 (4000 IU) plus twice-daily calcium lessened BMD decline, independent of baseline 25-OHD status [18]. During chronic treatment with TDF-containing cART, vitamin D3 supplementation (50000 IU) every 4 weeks decreased PTH over 12 weeks of treatment in youth with HIV [19]. In calcium- and/or 25-OHD-deficient adults with HIV infection on TDF-containing cART, vitamin D and/or calcium supplementation improved both PTH and BMD at 48 weeks [20].
We hypothesized that an “adolescent-friendly” monthly dose of vitamin D3 (50000 IU [19]) would increase BMD and decrease PTH more effectively than a daily multivitamin containing vitamin D3 (400 IU) and calcium in youth with HIV being chronically treated with TDF-containing cART, independent of baseline vitamin D status.
METHODS
Overview
Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) study 109 was a 48-week randomized, double-blind, placebo-controlled, multicenter trial performed between October 2012 and May 2016 at 13 ATN and 6 International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) sites in the United States and Puerto Rico. Study version 1.0, designed for 96 weeks, was modified to version 2.0 in August 2014, with 48 weeks of follow-up, due to ATN financial constraints. The study was approved by each participating center’s institutional review board, and appropriate written informed consent/assent was obtained prior to enrollment.
Participants
We enrolled persons ages 16 through 24 years with behaviorally acquired HIV-1 infection, treated with TDF-containing cART for ≥180 days, with viral load <200 copies/mL ≤90 days before and at the time of screening. Subjects were excluded for body weight >159 kg (DXA requirement), hepatitis B or C infection, clinical renal diseases, sarcoidosis, arteriosclerosis, osteoporosis and/or other bone diseases, parathyroid disorders, current or recent pregnancy or lactation, pregnancy planned during the study, or gastrointestinal disease potentially interfering with study agent absorption; use of medicines affecting BMD, interfering with vitamin D absorption or TDF excretion, or causing nephrotoxicity; or estimated glomerular filtration rate (eGFR) <70 mL/minute/1.73 m2, or serum calcium above the upper limits of laboratory norms. Subjects taking multivitamins, calcium, or vitamin D could enroll if supplements were discontinued at screening and thereafter (Figure 1). Because pretreatment vitamin D concentration was not an eligibility criterion, there was no exclusion based on pretreatment vitamin D status [21].
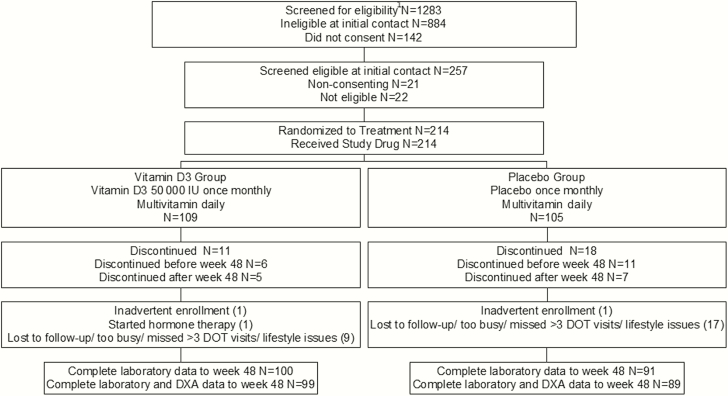
Figure 1.
Participant flow diagram. 1See Methods for eligibility criteria. Abbreviations: DOT, directly observed therapy; DXA, dual-energy X-ray absorptiometry.
Treatments
Participants were randomized to receive vitamin D3, 50000 IU (Tishcon, Westbury, New York) or a matching placebo gelatin capsule [22]. Randomization to vitamin D3 or placebo was stratified by sex at birth, age (<20 vs ≥20 years), and race (black/African American vs other), in a 1:1 ratio.
Capsules, manufactured yearly, had content confirmed by independent analysis before and at the end of use for each batch. Treatment assignment was blinded to all study participants and personnel except the site pharmacist. Directly observed oral treatment was administered at baseline and every 4 weeks while on study.
All participants were also supplied with a multivitamin and mineral supplement (Nature’s Bounty Multi-Day Vitamins plus Minerals, Nature’s Bounty, Bohemia, New York) and instructed to take 1 tablet daily. Among other constituents, this contained vitamin D3 400 IU and calcium 162 mg (as dicalcium phosphate and calcium carbonate). This vitamin D dose was chosen to assure that total vitamin D intake (dietary plus supplement) in the group randomized to placebo would approximate the recommended daily intake of 600 IU [21]. Thus, the vitamin D3 group received a daily multivitamin plus randomized directly observed vitamin D3 50000 IU every 4 weeks; the placebo group received a daily multivitamin plus randomized placebo every 4 weeks.
Percentage adherence to multivitamin use was measured by pill count. Adherence to randomized treatment was identified by missed visits for directly observed therapy. Vitamin D/calcium dose received was calculated as prescribed dose multiplied by adherence [17]. Total vitamin D/calcium doses received were the sum of dietary, multivitamin, and randomized treatment amounts.
Study Visits
After screening, study visits occurred at baseline and weeks 12, 24, and 48. Treatment/safety visits occurred every 4 weeks. Spine, hip, and whole body DXA scans were performed at baseline and weeks 24 and 48 and analyzed centrally. With few exceptions, participants were scanned on the same instrument throughout the study. Six sites had GE/Lunar (Madison, Wisconsin) scanners, and 13 sites had Hologic devices (Waltham, Massachusetts). Machine-generated z scores were used. A standard phantom was scanned on each DXA instrument.
Urine and blood samples were collected at baseline and weeks 12, 24, and 48 after a minimum 8-hour fast. Serum samples were used for measurement of PTH, FGF23, vitamin D binding protein (VDBP), 25-OHD, 1,25-dihydroxy vitamin D (1,25-OH(2)D), CTX, BAP, OC, calcium, creatinine, phosphate, and albumin. Spot urine samples for creatinine (UCr), calcium, phosphate, glucose, protein, and retinol-binding protein (URBP) were collected upon arrival. A second urine sample for β-2 microglobulin was collected 1 hour later after participants drank 8–12 ounces of water. All samples were processed at the study sites, frozen, and sent for storage and batch analysis at the US Department of Agriculture, Agricultural Research Service, Western Human Nutrition Research Center, Davis, California (see Supplementary Materials).
Dietary or supplemental intake of calcium, vitamin D, and total energy were assessed at baseline and week 48 using the Block Dietary Systems Food Frequency Questionnaire [23] (Nutritionquest, Berkeley, California). Information on exercise frequency, tobacco and alcohol use, and attempts to lose weight was collected by questionnaire at baseline and week 48.
Statistical Analysis
The primary outcome measure was the percentage change from baseline to week 48 in lumbar spine (L1–L4) BMD (LSBMD), with evaluable participants defined as those with BMD data available at both baseline and at week 48. The study was designed to enroll 214 participants, to have 100 evaluable participants in each randomized treatment group, giving a power of 90% to detect a between-group difference of increase in LSBMD ≥1.8% from baseline to week 48 [24]. Data are presented as median (Q1, Q3) unless otherwise specified. Analyses compared differences between participants in randomized groups, using an intent-to-treat analysis cohort for baseline data, and the evaluable cohort for the data on change from baseline to week 48. We report all available biochemical data for participants who had baseline and week 48 data. The Wilcoxon signed-rank test was used to test for significance of differences from baseline to a specified study week within each group. Between-group differences were assessed by the Wilcoxon rank-sum test. Multivariable analyses were used to assess the effects of covariates on the relationship of randomized group or 25-OHD to change in LSBMD.
RESULTS
Participants and Baseline Data
Altogether, 214 participants were randomized and completed a baseline visit, 191 completed week 48 with complete laboratory data, and 188 completed week 48 with complete laboratory and DXA data (Figure 1). There were 2 inadvertent enrollments. One hundred two participants enrolled in protocol version 1.0 and 112 in protocol version 2.0.
At baseline, VITD3 and placebo groups were balanced by age, sex at birth, race, and most HIV-related and lifestyle variables (Table 1). The placebo group had a lower BMI. While prestudy multivitamin use was lower in the placebo group, supplements were used infrequently in either group. Sixty-two percent of participants had serum 25-OHD concentrations in the inadequate or deficient range, using Institute of Medicine (IOM) criteria (25-OHD <20 ng/mL [21]). There were no differences in baseline measurements between evaluable and nonevaluable participants (data not shown), and no treatment-related reasons for discontinuation (Figure 1).
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Overall | Randomized Group | P Value | |
|---|---|---|---|---|
| Vitamin D3 | Placebo | |||
| No.a | 212 | 108 | 104 | |
| Enrolled in protocol version 1 | 101 | 51 | 50 | |
| Enrolled in protocol version 2 | 111 | 57 | 54 | |
| Age, yb | .58 | |||
| Median | 22.0 | 22.0 | 22.0 | |
| Q1, Q3 | 21.0, 23.0 | 20.5, 24.0 | 21.0, 23.0 | |
| Sex at birth, No. (%) | .71 | |||
| Male | 179 (84) | 90 (83) | 89 (86) | |
| Female | 33 (16) | 18 (17) | 15 (14) | |
| Sexual maturity rating (Tanner stage)c, No. (%) | 1.00 | |||
| 2 | 1 (<1) | 1 (1) | 0 (0) | |
| 3 | 2 (1) | 1 (1) | 1 (1) | |
| 4 | 5 (2) | 3 (3) | 2 (2) | |
| 5 | 204 (96) | 103 (95) | 101 (97) | |
| Race, No. (%) | .64 | |||
| Black/African American | 157 (74) | 78 (72) | 79 (76) | |
| Other race | 55 (26) | 30 (28) | 25 (24) | |
| Hispanic ethnicity, No. (%) | 45 (21) | 21 (19) | 24 (23) | .51 |
| BMI, kg/m2 | .024 | |||
| Median | 23.6 | 24.5 | 22.7 | |
| Q1, Q3 | 21.2, 28.8 | 22.1, 28.7 | 20.9, 28.9 | |
| BMI, No. (%) | .26 | |||
| Underweight (<18.5 kg/m2) | 8 (4) | 3 (3) | 5 (5) | |
| Normal (18.5–24.9 kg/m2) | 116 (55) | 54 (50) | 62 (60) | |
| Overweight (25–29.9 kg/m2) | 43 (20) | 27 (25) | 16 (15) | |
| Obese (≥30 kg/m2) | 45 (21) | 24 (22) | 21 (20) | |
| Lifestyle, No. (%) | ||||
| Exercise once weekly or more | 136 (64) | 70 (65) | 66 (63) | .89 |
| Trying to lose weight | 73 (34) | 38 (35) | 35 (34) | .89 |
| Smoke cigarettes | 52 (25) | 26 (24) | 26 (25) | 1.00 |
| Drink alcohol | 77 (38) | 48 (45) | 29 (29) | .021 |
| Uses multivitamin or mineral supplement, No. (%) | 53 (25) | 34 (32) | 19 (18) | .028 |
| Daily energy intake, kcal/kg/day | .79 | |||
| Median | 35.4 | 36.4 | 35.0 | |
| Q1, Q3 | 23.3, 54.8 | 22.9, 53.3 | 23.3, 58.7 | |
| Calcium intake, mg/day | ||||
| Dietary | .65 | |||
| Median | 1014 | 1024 | 988 | |
| Q1, Q3 | 573, 1545 | 609, 1560 | 534, 1494 | |
| Supplementald | .15 | |||
| Median | 200 | 200 | 123 | |
| Q1, Q3 | 45, 210 | 100, 257 | 40, 200 | |
| Total | .47 | |||
| Median | 1023 | 1045 | 988 | |
| Q1, Q3 | 581, 1545 | 636, 1560 | 534, 1494 | |
| Inadequate total daily calcium intake for age, No. (%)e | 104 (49) | 51 (47) | 53 (51) | .68 |
| Vitamin D intake, IU/day | ||||
| Dietary | .20 | |||
| Median | 138 | 150 | 116 | |
| Q1, Q3 | 77, 239 | 75, 278 | 78, 216 | |
| Supplementalf | .82 | |||
| Median | 600 | 600 | 600 | |
| Q1, Q3 | 400, 1000 | 400, 1000 | 400, 1500 | |
| Total vitamin D intake, IU/day | .051 | |||
| Median | 147 | 174 | 119 | |
| Q1, Q3 | 82, 320 | 82, 377 | 82, 245 | |
| Daily total vitamin D intake, No. (%) | .013 | |||
| <300 IU | 157 (74) | 72 (67) | 85 (82) | |
| 300–600 IU | 34 (16) | 25 (23) | 9 (9) | |
| >600 IU | 21 (10) | 11 (10) | 10 (10) | |
| Season enrolled, No. (%) | .91 | |||
| Winter | 83 (39) | 44 (41) | 39 (38) | |
| Spring | 85 (40) | 43 (40) | 42 (40) | |
| Summer | 33 (16) | 15 (14) | 18 (17) | |
| Fall | 11 (5) | 6 (6) | 5 (5) | |
| Geographic latitude (degrees north), No. (%) | .81 | |||
| ≤35 | 73 (34) | 36 (33) | 37 (36) | |
| >35 to 40 | 72 (34) | 39 (36) | 33 (32) | |
| >40 | 67 (32) | 33 (31) | 34 (33) | |
| Serum vitamin D concentration, ng/mL | .41 | |||
| Median | 16.4 | 15.7 | 16.8 | |
| Q1, Q3 | 11.4, 23.9 | 12.1, 24.9 | 10.2, 22.7 | |
| Serum vitamin D concentration category, No. (%)e | .57 | |||
| Deficient (25-OHD <12 ng/mL) | 57 (27) | 27 (25) | 30 (29) | |
| Inadequate (25-OHD 12 to <20 ng/mL) | 75 (35) | 37 (34) | 38 (37) | |
| Adequate (25-OHD ≥20 to 50 ng/mL) | 78 (37) | 42 (39) | 36 (35) | |
| Excess (25-OHD >50 ng/mL) | 2 (1) | 2 (2) | 0 (0) | |
| Time since HIV diagnosis, y | .61 | |||
| Median | 2.0 | 2.0 | 2.0 | |
| Q1, Q3 | 1.0, 3.0 | 1.0. 3.0 | 1.0, 3.0 | |
| CD4 cell count, cells/μL | .32 | |||
| Median | 651 | 636 | 663 | |
| Q1, Q3 | 491, 832 | 473, 826 | 507, 844 | |
| CD4 cells (cells/μL), No. (%) | ||||
| 0 to <200 | 1 (<1) | 0 (0) | 1 (1) | .56 |
| 200 to ≤350 | 19 (9) | 12 (11) | 7 (7) | |
| 351 to ≤500 | 35 (17) | 18 (17) | 17 (16) | |
| >500 | 157 (74) | 78 (72) | 79 (76) | |
| Duration of prior TDF treatment, mo | .37 | |||
| Median | 19.0 | 20.5 | 17.5 | |
| Q1, Q3 | 11.0, 33.0 | 11.0, 33.5 | 12.0, 31.5 | |
| Antiretrovirals, No. (%) | .87 | |||
| Protease inhibitor | 66 (31) | 33 (31) | 33 (32) | |
| NNRTI | 87 (41) | 47 (44) | 40 (38) | |
| PI + NNRTI | 22 (10) | 11 (10) | 11 (11) | |
| INSTI | 37 (17) | 17 (16) | 20 (19) | |
| Efavirenz in ART, No. (%)g | 78 (37) | 44 (41) | 34 (33) | .26 |
| Ritonavir in ART, No. (%)g | 88 (42) | 44 (41) | 44 (42) | .89 |
Abbreviations: 25-OHD, 25-hydroxy vitamin D; ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor, NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
aA total of 214 participants were enrolled. Two inadvertent enrollees are excluded from this table.
bData are shown as median (Q1, Q3) unless otherwise specified. Q1 and Q3 represent the first and third quartiles of the distribution, respectively.
cSexual maturity rating (Tanner stage) was determined by physical examination or self-assessment.
dThe median supplemental calcium intake was calculated only for those who used supplements (n = 23): 17 vitamin D3; 6 placebo.
eInstitute of Medicine criteria [21].
fThe median supplemental vitamin D intake was calculated only for those who used supplements (n = 24): 16 vitamin D3; 8 placebo.
gEver used efavirenz or ritonavir.
Calcium and Vitamin D Intake on Study
Over 48 weeks, median (Q1, Q3) daily multivitamin adherence by pill count was 49% (27%, 69%), and directly observed randomized treatment adherence was 100% (100%, 100%), with no difference between VITD3 and placebo groups (Supplementary Table 1). At week 48, the total vitamin D intake received was 2020 (1914, 2168) and 284 (179, 394) IU/day in the VITD3 and placebo groups, respectively (P < .001).
Serum Vitamin D Concentrations
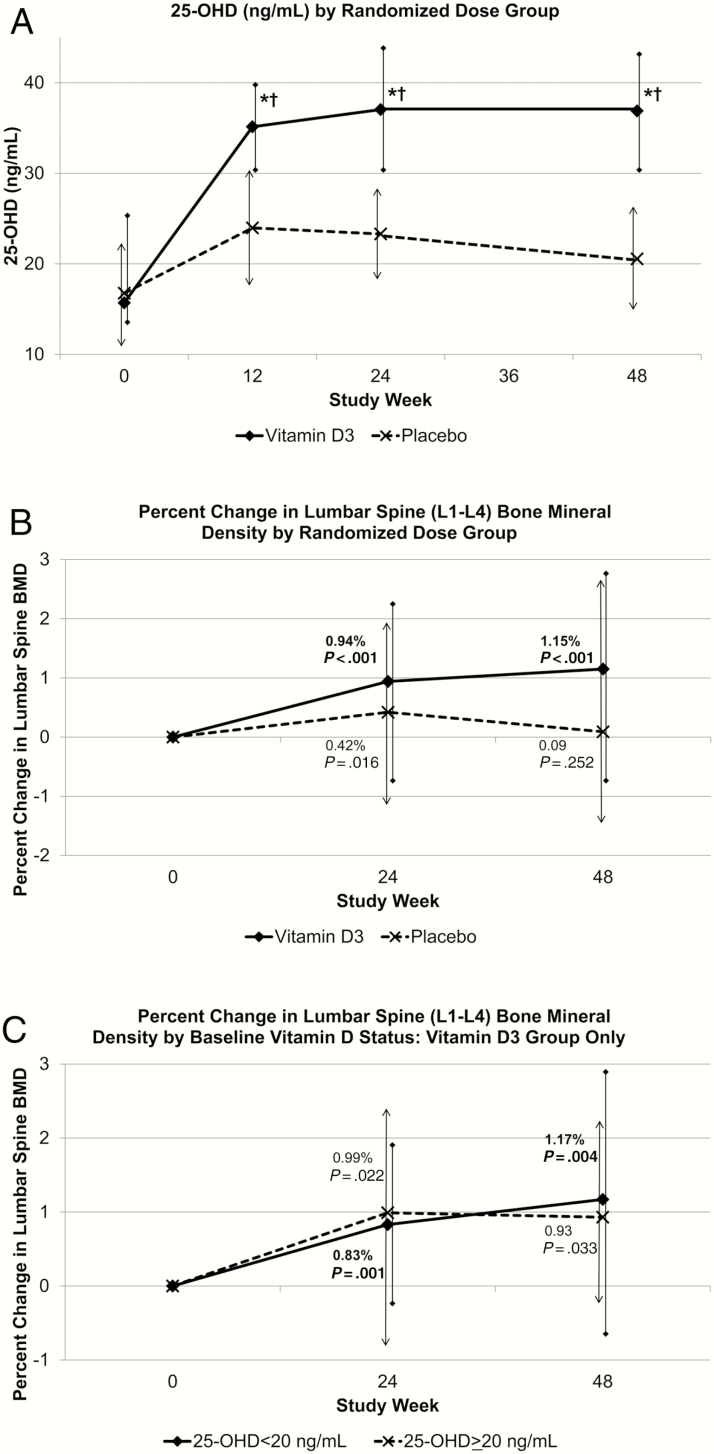
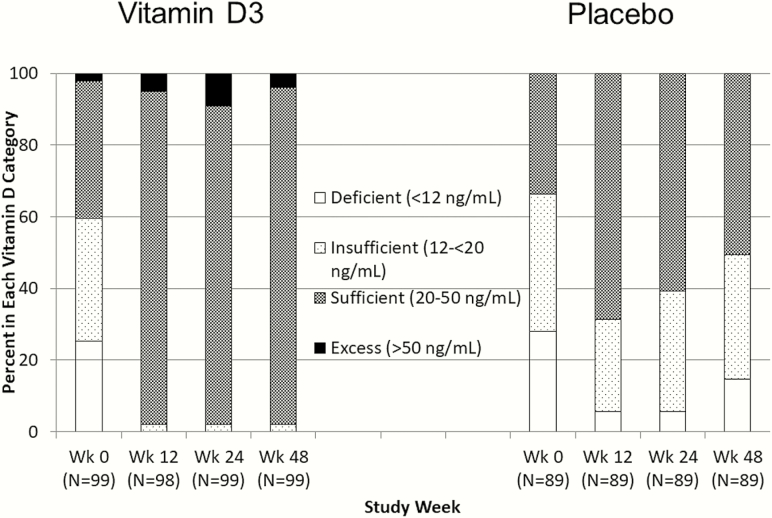
Serum 25-OHD increased by week 12 in both groups, with a greater increase in the VITD3 than the placebo group at week 48 (Figure 2A; P < .001). At week 48, median 25-OHD concentrations were 36.9 (30.5, 42.4) ng/mL in the VITD3 and 20.6 (14.4, 25.8) ng/mL in the placebo group (Figure 2A and Supplementary Table 2; change from baseline P < .001 and P = .001, respectively). By week 48, 25-OHD was >20 ng/mL in 98% and 51% of VITD3 and placebo groups, respectively (Figure 3). Changes from baseline to week 48 in 1,25-OH(2)D and free 1,25-OD(2)D were greater in the VITD3 compared to placebo group (Supplementary Table 2) (between-group P = .014 and P = .021, respectively). Serum VDBP decreased 4% over 48 weeks in the VITD3 group (Supplementary Table 2).
Figure 2.
Serum vitamin D (25-OHD) concentration and change in lumbar spine (L1–L4) bone mineral density (BMD) by study week and vitamin D randomized dose group. Data are median and bars are Q1, Q3. P values shown in the figures are for the change from baseline to each study week. A, For both the vitamin D3 and placebo group, 25-OHD values at weeks 12, 24, and 48 were significantly higher than baseline. *25-OH-D change from baseline differed between dose groups (P < .001). †25-OHD at weeks 12, 24, and 48 differed between dose groups (P < .001). Vitamin D3, n = 100; placebo, n = 91. B, At week 48, the change from baseline was significant in the vitamin D3 group but not in the placebo group. There was no significant difference in the change from baseline to week 48 between the vitamin D3 and placebo groups (P = .117). C, Vitamin D3, n = 99; placebo, n = 89; 25-OHD <20 ng/mL, n = 59; 25-OHD >20 ng/mL, n = 40.
Figure 3.
Vitamin D status (serum 25-OHD category) by study week and vitamin D randomized dose group. Classification of vitamin D status based on Institute of Medicine categories [21]. At baseline, both vitamin D3 and placebo groups had about 60% of participants with vitamin D deficiency/insufficiency (25-OHD <20 ng/mL).
Bone Mineral Density and Anthropometric Variables
Baseline median z scores in the group as a whole were ≤ –0.5 for spine, hip, and whole-body BMD (data not shown). From baseline to week 48, LSBMD increased by 1.15% (–0.75%, 2.74%) in the VITD3 group (P < .001) and 0.09% (–1.49%, 2.61%) in the placebo group (P = .25), without a significant difference between the 2 groups (P = .12) (Figure 2B). This increase occurred in VITD3 participants with baseline 25-OHD <20 ng/mL (1.17% [–0.82%, 2.90%]; P = .004) and in those with baseline 25-OHD ≥20 ng/mL (0.93% [–0.26%, 2.15%]; P = .033) (Figure 2C). LSBMD z score declined in the placebo group (0.10 [–0.30, 0.20]; P < .05). There were no other significant changes in DXA-measured variables in either group, including hip and whole-body BMD (Supplementary Table 2). Body weight increased in the placebo group.
In multivariable models, change in LSBMD from baseline to week 48 did not differ between males and females, but was most strongly associated with baseline 25-OHD (P < .05; Table 2).
Table 2.
Multivariable Models of Change in Lumbar Spine (L1–L4) Bone Mineral Density From Baseline to Week 48, by Randomized Treatment Group
| Model | Variables in Model | Estimated Effect Coefficient (Standard Deviation) | P Valuea |
|---|---|---|---|
| 1 | Randomized treatment group (vitamin D3 vs placebo) | 0.491 (0.423) | .246 |
| 2 | Randomized treatment group (vitamin D3 vs placebo) | 0.575 (0.414) | .166 |
| Baseline 25-OHD (ng/mL) | –0.048 (0.021) | .026 | |
| Baseline lumbar spine BMD (g/cm2) | –2.541 (1.321) | .056 | |
| 3 | Randomized treatment group (vitamin D3 vs placebo) | 0.494 (0.418) | .238 |
| Baseline 25-OHD (ng/mL) | –0.044 (0.022) | .049 | |
| Baseline lumbar spine BMD (g/cm2) | –2.406 (1.471) | .103 | |
| Covariatesb |
Abbreviations: 25-OHD, 25-hydroxy vitamin D; BMD, bone mineral density.
a P value from likelihood ratio χ2 test.
bCovariates in the model (continuous variables unless otherwise specified) were as follows: age at enrollment (years); race (Black/African American vs other race); sex at birth (male or female); body mass index (kg/m2); lean body mass (g); efavirenz use ever (yes/no); duration of tenofovir disporoxil fumarate use (months); CD4 at entry (cells/μL); viral load (detectable vs below limit of quantitation); weight-bearing exercise (yes or no by questionnaire at baseline). In the full model, none of these covariates was statistically significantly associated with change in lumbar spine BMD from baseline to week 48 (P > .05).
Endocrine, Bone Turnover, and Renal Variables
At baseline, no endocrine, renal, or bone turnover markers differed significantly by treatment group (Supplementary Table 2). An effect of vitamin D3 on PTH was seen in the VITD3, but not the placebo group, with decline in PTH at 12 weeks sustained at week 48 in the VITD3 group (P = .016). Vitamin D3 effect on FGF23 was seen at 12 weeks, with sustained increases in both groups to week 48. Serum bone turnover markers declined in both groups. There were no significant changes in urine glucose, URBP/UCr ratio, or urine B2MG, markers of integrity of renal tubular function. Tubular reabsorption of phosphate declined only in the VITD3 group, reaching statistical significance at week 48.
Safety
There was no clinically meaningful change in markers of renal glomerular function (Supplementary Table 2). Serum creatinine increased in both groups, while eGFR decreased slightly in the VITD3 group. There was no identified clinical vitamin D toxicity in either group, and no difference in any clinical toxicity between the 2 treatment groups over the entire study duration. In the VITD3 group, 2 participants had 25-OHD >50 ng/mL at baseline, and 4 had 25-OHD concentrations above that value at week 48. One participant in the placebo group, whose cART regimen included atazanavir, developed nephrolithiasis. Study treatments were withheld until symptom resolution, then restarted and well tolerated for the remainder of the trial. There was 1 traumatic fracture in each group during the study.
DISCUSSION
For adolescents and young adults with HIV being chronically treated with TDF-containing cART, vitamin D3 administration improved LSBMD within 24 weeks of initiation. This LSBMD increase continued to 48 weeks in the VITD3 group, with a median serum 25-OHD concentration of 36.9 (30.5, 42.4) ng/mL at that time. The placebo group, receiving a multivitamin plus vitamin D3 placebo, showed a decline in LSBMD z score and no significant increase in LSBMD by 48 weeks, and had a lower week 48 serum 25-OHD concentration (median, 20.6 [14.4, 25.8] ng/mL). In the VITD3 group, the increase in LSBMD occurred in participants with and without baseline vitamin D deficiency/insufficiency (baseline 25-OHD <20 or ≥20 ng/mL) [21].
In the VITD3 group, LSBMD increased by 1.15% over 48 weeks, an effect size approaching that of other interventions for mitigating TDF-associated bone toxicity in HIV-infected adults. For example, LSBMD increased by 1.6% when switching from TDF-containing cART to a regimen containing TAF [10], and by 2.6% when switching from TDF-FTC-efavirenz to ritonavir-boosted darunavir alone [11]. In an open-label study, after 48 weeks of vitamin D and/or calcium treatment, LSBMD increased by 2.4% in calcium and/or vitamin D-deficient persons on TDF-containing cART [20]. The effect size in our study may be smaller because we did not preselect for vitamin D or calcium deficiency at study entry.
While not directly comparable [2], further perspective on the magnitude of LSBMD change comes from randomized studies of cART initiation, which show differences in 48-week LSBMD decline associated with TDF-containing vs comparator regimens that range from 0.8% (–2.4% [TDF] vs –1.6% [abacavir]) [7] to about 2.4% (–3.4% [TDF] vs –1.0% [TAF]) [9]. At initiation of TDF-containing cART in adults with HIV, daily vitamin D3 4000 IU plus twice-daily calcium supplementation resulted in LSBMD decline at 48 weeks of –1.4% compared to –2.9% in a placebo group, about 1.5% difference [18].
We saw a vitamin D3 treatment effect only on LSBMD and not hip or total body BMD. TDF is known to decrease both hip and lumbar spine BMD [3, 6–11, 18]. In a study in which vitamin D3 50000 IU was administered monthly in adults with HIV treated with cART not containing TDF, LSBMD increased by 2.6% at 2 years while total hip BMD decreased by 0.8% and total body BMD decreased by 0.5% [25], suggesting that the greater effect of vitamin D3 on spine compared to hip BMD is not TDF-related. This differential effect of vitamin D3 on spine (trabecular bone) and hip (cortical bone) may be related to study duration, with a visible effect in the more metabolically active trabecular bone in the spine in studies of relatively short duration.
Adherence with the daily multivitamin was poor in both groups. The VITD3 group received a total daily dose of vitamin D3 equal to about 2000 IU/day and achieved serum 25-OHD concentrations of 37 ng/mL by week 48, similar to the 25-OHD concentration attained using monthly vitamin D3 50000 IU without a daily multivitamin [22]. The monthly regimen in the VITD3 group supplies a dose of vitamin D3 considered adequate for fracture prevention [17], and meets the Endocrine Society recommendation for vitamin D dose and serum 25-OHD concentration (>30 ng/mL) for persons with HIV infection [16], while the placebo group achieved the IOM target (25-OHD concentration 20 ng/mL) [21]. Our data suggest that the low dose of vitamin D3 in standard multivitamin preparations is insufficient to affect bone turnover, BMD, and other variables that reflect skeletal health in an adolescent population with HIV. The safety of the higher dose, and the effect observed over a broad range of baseline 25-OHD concentrations, suggests that such dosing could be used in practice without measuring serum 25-OHD concentrations, as long as other vitamin D supplements were discontinued at the time of regimen initiation.
A similar effect on LSBMD might be seen in the absence of calcium supplementation. Calcium intake from the multivitamin was <10% of the total daily calcium intake. As high calcium intake is less important in persons with high serum 25-OHD [17, 26], the LSBMD change would likely be similar even if the multivitamin were omitted from the regimen.
The decrease in PTH and serum bone turnover markers, seen especially in the VITD3 group, suggest a physiologic state similar to vitamin D deficiency that is corrected with vitamin D administration [27]. While some studies have suggested this “functional 25-OHD deficiency” may be mediated through a TDF effect on decreasing free 1,25-OH(2)D [28] by increasing VDBP [29, 30], TDF was not associated with changes in VDBP in other studies [15, 31], and current study results do not support a central role for VDBP.
Low FGF23 has been associated with TDF [15, 30, 32]. The increase in FGF23 in both groups is larger than was seen in TDF-treated vitamin D or calcium-deficient adults with HIV [20]. In severe vitamin D deficiency, FGF23 may initially fall in response to vitamin D treatment when there is associated hypophosphatemia [33], but with less severe deficiency, FGF23 concentrations rise in response to vitamin D repletion [34]. The initial FGF23 decline in severe vitamin D deficiency and later rise in response to high concentrations of 1,25-OH(2)D was previously found in TDF-treated youth [35]. In the current study, the decline in TRP in the absence of broader evidence of renal tubular dysfunction suggests phosphate loss may be an FGF23-mediated effect, not primarily a renal tubular effect.
A strength of this study was enrollment of youth who had viral load <200 copies/mL for ≥180 days on unchanged cART, an index of good adherence to cART. A “youth-friendly” dosing scheme with directly observed monthly vitamin D3/placebo administration was another key component of our study design. The challenges of adherence with the daily multivitamin in our adolescent/young adult cohort suggest that the total vitamin D3 received dose would have been lower with daily dosing, even if a higher daily dose were used. We note that LSBMD was rising through 48 weeks. Longer follow-up perhaps could have shown a greater difference in BMD between the 2 treatment groups.
In summary, monthly high-dose vitamin D3 supplementation significantly increased LSBMD over 48 weeks in HIV-infected youth on stable TDF-containing cART regimens, regardless of baseline vitamin D status. This safe regimen merits consideration for managing TDF-associated LSBMD loss.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the ATN from the National Institutes of Health (grant numbers U01 HD 040533 and U01 HD 040474) through the NICHD (B. K.), with supplemental funding from the National Institute on Drug Abuse (S. Kahana) and National Institute of Mental Health (P. Brouwers, S. Allison). The study was scientifically reviewed by the ATN’s Therapeutic Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at the University of Alabama at Birmingham. Network operations and analytic support was provided by the ATN Data and Operations Center at Westat (D. R. Harris, B. Driver).
Acknowledgments. We acknowledge the contributions of the Adolescent Trials Network for HIV/AIDS Interventions (ATN) study coordinators who participated in this study, and Kavya Vellala, a Westat Protocol Specialist, for her activities in protocol development and implementation. We are also grateful to Andrea Miller, Justin Wheeler, and Roger Fielding at the Tufts Body Composition Analysis Center for analysis of DXA scans. We thank the following staff at the USDA, Agricultural Research Service, Western Human Nutrition Research Center: Tammy Freytag, Joseph Domek, and Erik Gertz. We thank George Siberry of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) for his careful input in study design and performance. We acknowledge the contribution of the investigators and staff at the following sites that participated in this study: University of South Florida, Tampa (Patricia Emmanuel, Diane Straub, Elizabeth Enriquez-Bruce), Children’s Hospital of Los Angeles (Marvin Belzer, Diane Tucker), Children’s National Medical Center (Larry D’Angelo, Connie Trexler), Children’s Hospital of Philadelphia (Steve Douglas, Mary Tanney), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Miguel Martinez, Lisa Henry-Reid, Kelly Bojan), Montefiore Medical Center (Donna Futterman, Maria Campos), Tulane University Health Sciences Center (Sue Ellen Abdalian, Leslie Kozina), University of Miami School of Medicine (Larry Friedman, Donna Maturo), St. Jude’s Children’s Research Hospital (Pat Flynn, Aditya Guar, Mary Dillard), Baylor College of Medicine, Texas Children’s Hospital (Mary Paul, Jane Head); Wayne State University (Liz Secord, Angulique Outlaw, Charnell Cromer); Johns Hopkins University School of Medicine (Allison Agwu, Renata Sanders, Thuy Anderson); The Fenway Institute (Ken Mayer, Julian Dormitzer); and University of Colorado (Dan Reirden, Carrie Chambers); University of Southern California (Andrea Kovacs, Eva Operskalski, James Homans, Allison Bearden, Susie Sanchez); Children’s Diagnostic & Treatment Center, Inc (Ana Puga, Zulma Eysallenne); San Juan City Hospital PR (Midnela Acevedo, Nicolas Rosario, Lourdes Angeli Nieves); Jacobi Medical Center Bronx (Andrew Wiznia, Jacobo Abadi, Michael Rosenberg, Joanna Dobroszycki, Marlene Burey). The investigators are grateful to the members of the local youth Community Advisory Boards for their insight and counsel and are indebted to the youth who participated in this study.
Disclaimer. The comments and views of the authors do not necessarily represent the views of the NICHD.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 16 February 2017.
Contributor Information
Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 109 Study Team:
Kavya Vellala, Justin Wheeler, Roger Fielding, Tammy Freytag, Joseph Domek, Erik Gertz, Patricia Emmanuel, Diane Straub, Elizabeth Enriquez-Bruce, Marvin Belzer, Diane Tucker, Larry D’Angelo, Connie Trexler, Steve Douglas, Mary Tanney, John H Stroger, Jr., Miguel Martinez, Lisa Henry-Reid, Kelly Bojan, Donna Futterman, Maria Campos, Sue Ellen Abdalian, Leslie Kozina, Larry Friedman, Donna Maturo, Pat Flynn, Aditya Guar, Mary Dillard, Mary Paul, Jane Head, Liz Secord, Angulique Outlaw, Charnell Cromer, Allison Agwu, Renata Sanders, Thuy Anderson, Ken Mayer, Julian Dormitzer, Dan Reirden, Carrie Chambers, Andrea Kovacs, Eva Operskalski, James Homans, Allison Bearden, Susie Sanchez, Ana Puga, Zulma Eysallenne, Midnela Acevedo, Nicolas Rosario, Lourdes Angeli Nieves, Andrew Wiznia, Jacobo Abadi, Michael Rosenberg, Joanna Dobroszycki, and Marlene Burey
References
- 1. Grant RM, Anderson PL, McMahan V et al. ; iPrEx Study Team Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. [DOI] [PubMed] [Google Scholar]
- 3. Gallant JE, Staszewski S, Pozniak AL et al. ; 903 Study Group Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 4. Huang JS, Hughes MD, Riddler SA, Haubrich RH; AIDS Clinical Trials Group A5142 Study Team Bone mineral density effects of randomized regimen and nucleoside reverse transcriptase inhibitor selection from ACTG A5142. HIV Clin Trials 2013; 14:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moyle GJ, Stellbrink HJ, Compston J et al. ; ASSERT Team 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir Ther 2013; 18:905–13. [DOI] [PubMed] [Google Scholar]
- 6. McComsey GA, Kitch D, Daar ES et al. . Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stellbrink HJ, Orkin C, Arribas JR et al. ; ASSERT Study Group Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–72. [DOI] [PubMed] [Google Scholar]
- 8. Mills A, Crofoot G, McDonald C et al. . Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2015; 69:439–45. [DOI] [PubMed] [Google Scholar]
- 9. Sax PE, Zolopa A, Brar I et al. . Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014; 67:52–8. [DOI] [PubMed] [Google Scholar]
- 10. Mills A, Arribas JR, Andrade-Villanueva J et al. ; GS-US-292-0109 Team Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016; 16:43–52. [DOI] [PubMed] [Google Scholar]
- 11. Hamzah L, Tiraboschi JM, Iveson H et al. . Effects on vitamin D, bone and the kidney of switching from fixed-dose tenofovir disoproxil fumarate/emtricitabine/efavirenz to darunavir/ritonavir monotherapy: a randomized, controlled trial (MIDAS). Antivir Ther 2016; 21:287–96. [DOI] [PubMed] [Google Scholar]
- 12. Masiá M, Padilla S, Robledano C, López N, Ramos JM, Gutiérrez F. Early changes in parathyroid hormone concentrations in HIV-infected patients initiating antiretroviral therapy with tenofovir. AIDS Res Hum Retroviruses 2012; 28:242–6. [DOI] [PubMed] [Google Scholar]
- 13. Childs KE, Fishman SL, Constable C et al. . Short communication: Inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses 2010; 26:855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenvinge MM, Gedela K, Copas AJ et al. . Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr 2010; 54:496–9. [DOI] [PubMed] [Google Scholar]
- 15. Havens PL, Stephensen CB, Van Loan MD et al. . Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis 2016; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. ; Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–30. [DOI] [PubMed] [Google Scholar]
- 17. Bischoff-Ferrari HA. Vitamin D and fracture prevention. [Reprint of Endocrinol Metab Clin North Am 2010; 39(2):347–53, table of contents; PMID: 20511056]. Rheum Dis Clinics N Am 2012; 38:107–13. [DOI] [PubMed] [Google Scholar]
- 18. Overton ET, Chan ES, Brown TT et al. . Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med 2015; 162:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Havens PL, Stephensen CB, Hazra R et al. ; Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions 063 Study Team Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis 2012; 54:1013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bech A, Van Bentum P, Telting D, Gisolf J, Richter C, De Boer H. Treatment of calcium and vitamin D deficiency in HIV-positive men on tenofovir-containing antiretroviral therapy. HIV Clin Trials 2012; 13:350–6. [DOI] [PubMed] [Google Scholar]
- 21. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press, 2011. [PubMed] [Google Scholar]
- 22. Havens PL, Mulligan K, Hazra R et al. ; Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 063 Study Team Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50000 IU monthly in youth with HIV-1 infection. J Clin Endocrinol Metab 2012; 97:4004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cummings SR, Block G, McHenry K, Baron RB. Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol 1987; 126:796–802. [DOI] [PubMed] [Google Scholar]
- 24. Kalkwarf HJ, Gilsanz V, Lappe JM et al. . Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab 2010; 95:1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolland MJ, Grey AB, Horne AM et al. . Annual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: a randomized controlled trial. J Clin Endocrinol Metab 2007; 92:1283–8. [DOI] [PubMed] [Google Scholar]
- 26. Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003; 22:142–6. [DOI] [PubMed] [Google Scholar]
- 27. Holick MF. The D-batable parathyroid hormone plateau. Am J Med 2011; 124:1095–6. [DOI] [PubMed] [Google Scholar]
- 28. Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab 1985; 61:969–75. [DOI] [PubMed] [Google Scholar]
- 29. Hsieh E, Fraenkel L, Han Y et al. . Longitudinal increase in vitamin D binding protein levels after initiation of tenofovir/lamivudine/efavirenz among individuals with HIV. AIDS 2016; 30:1935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Havens PL, Kiser JJ, Stephensen CB et al. ; Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 063 Study Team Association of higher plasma vitamin D binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: cause of a functional vitamin D deficiency?Antimicrob Agents Chemother 2013; 57:5619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Post FA, Hamzah L, Fox J. Tenofovir disoproxil fumarate-associated bone loss: does vitamin D-binding protein play a role?AIDS 2017; 31:178–9. [DOI] [PubMed] [Google Scholar]
- 32. Young J, Mucsi I, Rollet-Kurhajec KC, Klein MB; Canadian Co-infection Cohort Fibroblast growth factor 23: associations with antiretroviral therapy in patients co-infected with HIV and hepatitis C. HIV Med 2016; 17:373–9. [DOI] [PubMed] [Google Scholar]
- 33. Uzum AK, Salman S, Telci A et al. . Effects of vitamin D replacement therapy on serum FGF23 concentrations in vitamin D-deficient women in short term. Eur J Endocrinol 2010; 163:825–31. [DOI] [PubMed] [Google Scholar]
- 34. Alshayeb H, Showkat A, Wall BM, Gyamlani GG, David V, Quarles LD. Activation of FGF-23 mediated vitamin D degradative pathways by cholecalciferol. J Clin Endocrinol Metab 2014; 99:E1830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Havens PL, Hazra R, Stephensen CB et al. ; Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 063 Study Team Vitamin D3 supplementation increases fibroblast growth factor-23 in HIV-infected youths treated with tenofovir disoproxil fumarate. Antivir Ther 2014; 19:613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.