Summary
We investigated and mitigated a 2-phase clonal outbreak of Mycobacterium abscessus linked to hospital tap water. A combination of tap water avoidance among high-risk patients, including lung transplant and cardiac surgery patients, and water engineering interventions terminated the outbreak.
Keywords: hospital outbreak, nontuberculous mycobacteria, Mycobacterium abscessus, infection control, hospital water safety
Abstract
Background.
Nontuberculous mycobacteria (NTM) commonly colonize municipal water supplies and cause healthcare-associated outbreaks. We investigated a biphasic outbreak of Mycobacterium abscessus at a tertiary care hospital.
Methods.
Case patients had recent hospital exposure and laboratory-confirmed colonization or infection with M. abscessus from January 2013 through December 2015. We conducted a multidisciplinary epidemiologic, field, and laboratory investigation.
Results.
The incidence rate of M. abscessus increased from 0.7 cases per 10000 patient-days during the baseline period (January 2013–July 2013) to 3.0 cases per 10000 patient-days during phase 1 of the outbreak (August 2013–May 2014) (incidence rate ratio, 4.6 [95% confidence interval, 2.3–8.8]; P < .001). Thirty-six of 71 (51%) phase 1 cases were lung transplant patients with positive respiratory cultures. We eliminated tap water exposure to the aerodigestive tract among high-risk patients, and the incidence rate decreased to baseline. Twelve of 24 (50%) phase 2 (December 2014–June 2015) cases occurred in cardiac surgery patients with invasive infections. Phase 2 resolved after we implemented an intensified disinfection protocol and used sterile water for heater-cooler units of cardiopulmonary bypass machines. Molecular fingerprinting of clinical isolates identified 2 clonal strains of M. abscessus; 1 clone was isolated from water sources at a new hospital addition. We made several water engineering interventions to improve water flow and increase disinfectant levels.
Conclusions.
We investigated and mitigated a 2-phase clonal outbreak of M. abscessus linked to hospital tap water. Healthcare facilities with endemic NTM should consider similar tap water avoidance and engineering strategies to decrease risk of NTM infection.
(See the Editorial Commentary by Crist and Perz on pages 912-3.)
Nontuberculous mycobacteria (NTM) are hardy environmental microorganisms found in water, dust, and soil [1]. NTM commonly colonize municipal water [2–4], including tap water at healthcare facilities [5–9], and form biofilms in water distribution systems [10].
Outbreaks of Mycobacterium abscessus and other rapidly growing mycobacteria are common and have been associated with colonized plumbing systems in commercial buildings [11, 12] and healthcare facilities [13–16]. Organisms belonging to the Mycobacterium abscessus complex (M. abscessus subspecies abscessus, M. abscessus subspecies massiliense, and M. abscessus subspecies bolletii) are intrinsically resistant to many antibiotics and disinfectants [17]. Infections due to M. abscessus are difficult to diagnose and typically require months of therapy using multiple antibiotics [18, 19].
In March 2014, we identified an increase in patients with positive cultures for M. abscessus. Most patients had positive respiratory tract cultures, including numerous positive bronchoalveolar lavage (BAL) cultures from lung transplant recipients. We undertook a multifaceted investigation to confirm cases, identify sources, and implement mitigation strategies.
METHODS
Study Setting
Duke University Hospital (DUH) is a 957-bed tertiary care hospital located in North Carolina. A new hospital addition containing 160 intensive care unit (ICU) and intermediate beds, as well as 16 operating suites, was opened for patient care in late July 2013. The original portions of DUH continued to be used for patient care, predominantly for non-ICU medical and surgical patients after July 2013. DUH utilizes the municipal water supply.
The Duke University Institutional Review Board approved this investigation and research.
Epidemiologic Investigation
We identified all patients with growth of M. abscessus from any clinical specimen obtained at the hospital from January 2013 through December 2015. All patients with a first-time positive culture were considered cases with the following exceptions: Patients with a culture obtained during the first 2 days of admission or from a DUH outpatient clinic were excluded unless they had previously been hospitalized at DUH within 30 days prior to culture collection. After retrospective case finding was completed for the period from January 2013 through February 2014, we prospectively applied the same case definition for detection of incident cases from March 2014 through December 2015. Relevant clinical data were abstracted from the electronic medical record.
Field Investigation
We investigated the environment, equipment, and practices used in the bronchoscopy suite [20, 21] and the clinical microbiology laboratory [22, 23] to exclude a pseudo-outbreak. Subsequently, based on characteristics of case patients, we investigated additional locations of the healthcare facility, including ICUs and operating rooms (ORs) in the new addition, to identify potential environmental sources of M. abscessus where exposure to this organism was considered possible.
We conducted a comprehensive survey of the existing and new hospital addition’s water systems [24]. This survey included a water flow analysis to identify potential locations with reduced or restricted water flow, reduced disinfectant levels, or cooling within the hot water distribution system.
We performed mycobacterial cultures of biofilms [10] from water outlets and equipment in the hospital, as well as from water outlets in the community surrounding the hospital (Supplementary Table). We also performed air-sampling experiments in ORs to evaluate for aerosolized particles containing NTM [25].
Laboratory Methods
Standard mycobacterial culture methods were utilized (see page 3 of the Supplementary Data) [2, 26]. All environmental and selected patient isolates of M. abscessus were identified to subspecies and typed using erythromycin ribosomal methylase [erm(41)] [27] and region V RNA polymerase subunit beta (rpoβ) [28] gene sequencing. Variable number tandem repeats (VNTRs) [29] and pulsed-field gel electrophoresis (PFGE) [30] were also performed on representative environmental and patient isolates.
Statistical Analysis
Incidence rate ratios (IRRs) were estimated using maximum likelihood, assuming the number of cases followed the Poisson distribution. Wald 95% confidence intervals (CIs) were calculated, and likelihood ratio χ2 tests were used to compare incidence rates. Calculations were performed in SAS software, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Outbreak Investigation, Phase 1: August 2013–May 2014
Epidemiologic Investigation
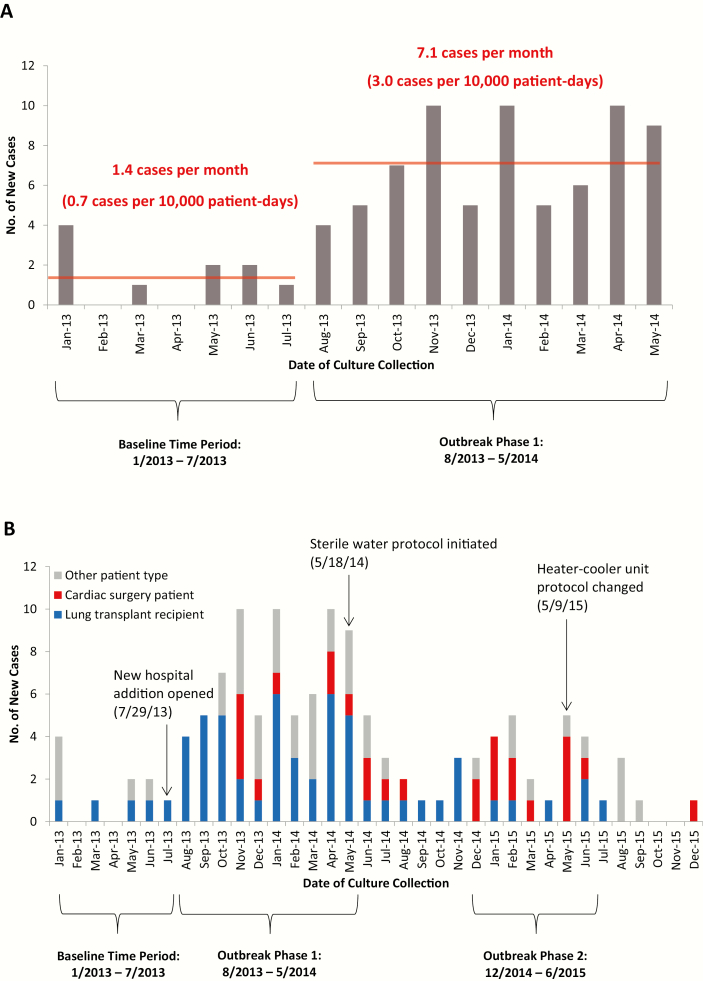
Ten cases of M. abscessus were detected over 150 651 patient-days during the preoutbreak baseline period from January 2013 through July 2013; 71 cases were identified over 234956 patient-days from August 2013 through May 2014 (phase 1) (Figure 1A). Thus, the incidence rate increased from a baseline of 0.7 cases per 10000 patient-days to 3.0 cases per 10000 patient-days (IRR, 4.6 [95% CI, 2.3–8.8]; P < .001).
Figure 1.
Epidemic curve of Mycobacterium abscessus colonization or infection. A, Initial epidemic curve including the baseline time period (January 2013–July 2013) and phase 1 of the outbreak (August 2013–May 2014). B, Final epidemic curve portraying key events and incident cases over the full 3-year study period, including phase 2 of the outbreak (December 2014–June 2015). Cases were stratified by patient type. The timeline of both epidemic curves was constructed from dates of culture collection, which in some cases occurred months after date of suspected patient inoculation. In particular, cardiac surgery cases that were identified after the 9 May 2015 heater-cooler unit disinfection protocol changes were either linked to cardiac surgeries performed before these protocol changes (n = 3) or were thought to be unrelated to the cardiac surgery (n = 1).
Lung transplant recipients represented 39 of 71 (55%) cases during phase 1 (Table 1). Other cases included patients with recent cardiac surgery (n = 9 [13%]), cancer (n = 5 [7%]), and hematopoietic stem cell transplantation (n = 5 [7%]), as well as multiple other patient types (n = 13 [18%]). Sixty-nine (97%) patients had exposure to the new hospital addition within 30 days prior to the first positive culture.
Table 1.
Characteristics of Patients Who Developed Incident Colonization or Infection With Mycobacterium abscessus During or up to 30 Days After Hospitalization, 2013–2015
| Characteristic | Overall (January 2013– December 2015) (n = 126) |
Phase 1 (August 2013– May 2014) (n = 71) |
Phase 2 (December 2014– June 2015) (n = 24) |
|||
|---|---|---|---|---|---|---|
| Median age, y (IQR) | 63 (50–68) | 63 (50–67) | 59 (49–68) | |||
| Male sex, No. (%) | 92 (73) | 52 (73) | 15 (63) | |||
| Patient type, No. (%) | ||||||
| Lung transplant | 58 (46) | 39 (55) | 5 (21) | |||
| Cardiac surgery | 27 (21) | 9 (13) | 13 (54) | |||
| VAD (no heart transplant) | 9 (7) | 2 (3) | 6 (25) | |||
| Heart transplant | 8 (6) | 4 (6) | 4 (17) | |||
| Valve surgery (no VAD) | 6 (5) | 2 (3) | 2 (8) | |||
| Coronary artery bypass grafting (no valve surgery) | 3 (2) | 1 (1) | 1 (4) | |||
| Ventricular septal defect repair | 1 (1) | 0 (0) | 0 (0) | |||
| Malignancy (no hematopoietic stem cell transplant) | 10 (8) | 5 (7) | 1 (4) | |||
| Chronic lung disease (no lung transplant) | 8 (6) | 4 (6) | 2 (8) | |||
| Hematopoietic stem cell transplant | 5 (4) | 5 (7) | 0 (0) | |||
| Esophagectomy | 5 (4) | 2 (3) | 1 (4) | |||
| Other solid organ transplant (no lung or heart transplant) | 4 (3) | 2 (3) | 1 (4) | |||
| Orthopedic surgery | 3 (2) | 2 (3) | 0 (0) | |||
| Other patient type | 6 (5) | 3 (4) | 1 (4) | |||
| Site of first positive culture, No. (%) | ||||||
| Respiratory | 86 (68) | 56 (79) | 9 (38) | |||
| Bronchoalveolar lavage | 67 (53) | 45 (63) | 6 (25) | |||
| Blood | 15 (12) | 4 (6) | 6 (25) | |||
| Pleural fluid | 8 (6) | 4 (6) | 2 (8) | |||
| Sternal wound | 5 (4) | 0 (0) | 5 (21) | |||
| Bone | 3 (2) | 2 (3) | 0 (0) | |||
| VAD driveline site | 3 (2) | 0 (0) | 2 (8) | |||
| Peritoneal fluid | 2 (2) | 2 (3) | 0 (0) | |||
| Other | 4 (3) | 3 (4) | 0 (0) | |||
| Time of diagnosis in solid organ transplant recipients | ||||||
| Median days from transplant surgery to positive culture (IQR) | ||||||
| All solid organ transplant recipients (n = 70) | 41 (12–114) | 18 (8–73) | 105 (59–352) | |||
| Lung transplant (n = 58) | 34 (11–94) | 13 (6–47) | 80 … | |||
| Heart transplant (n = 8) | 43 (18–106) | 18 … | 95 … | |||
| Other solid organ transplant (n = 4) | 169 … | 483 … | 144 … | |||
| Positive culture during index transplant surgery hospitalization, no./total No. (%) | ||||||
| All solid organ transplant recipients | 43/70 (61) | 33/45 (73) | 3/10 (30) | |||
| Lung transplant | 36/58 (62) | 29/39 (74) | 1/5 (20) | |||
| Heart transplant | 6/8 (75) | 4/4 (100) | 2/4 (50) | |||
| Other solid organ transplant | 1/4 (25) | 0/2 (0) | 0/1 (0) | |||
Patients with a positive culture obtained on day 1 or day 2 of admission were excluded unless they had previously been hospitalized within 30 days prior to the date of the first positive culture.
Abbreviations: IQR, interquartile range; VAD, ventricular assist device.
Mycobacterium abscessus was initially recovered from the respiratory tract in 56 (79%) phase 1 cases, including 36 of 39 (92%) lung transplant recipients. Thirty-five of 36 (97%) respiratory isolates from lung transplant recipients were recovered from BAL cultures. The median time from lung transplantation to first positive M. abscessus culture was 13 days (interquartile range [IQR], 6–47 days; range, 0–4716 days).
Thirty-six of 71 (51%) patients who met the case definition during phase 1 received antimicrobial therapy for infections, and 17 (24%) patients died within 60 days of the first positive culture.
Field Investigation
Phase 1 of the outbreak temporally correlated with the opening of the new hospital addition (Figure 1B). The predominance of respiratory isolates suggested that colonization or infection occurred via exposure through the aerodigestive tract, likely from routine care practices using tap water. Investigation of bronchoscopy equipment, the endoscope reprocessing suite, microbiology laboratory, and lung and cardiac surgery ORs revealed no source of the outbreak.
Environmental cultures obtained in April and May 2014 from biofilms of water sources were positive for NTM in 19 of 24 (79%) locations at the new hospital addition, 14 of 25 (56%) sites at the existing hospital, and 5 of 12 (42%) locations in the community surrounding the hospital (Supplementary Table). Only cultures taken from biofilms of water sources at the hospital addition were positive for M. abscessus, with 12 of 24 (50%) sites positive for M. abscessus subspecies abscessus. Sites testing positive for M. abscessus included patient room faucets, patient care ice machines, ICU hallway water faucets, a patient room shower head, a utility room water basin, and an OR scrub sink faucet.
Interventions and Subsequent Surveillance
Based on data from the field investigation, we developed and implemented a sterile water protocol in May 2014 that ultimately included all lung and heart transplant recipients, ICU patients, and patients with disrupted gastrointestinal tracts. These patients received sterile water instead of tap water for oral care, speech therapy assessments, enteral tube flushes, respiratory therapy, consumption, and, until surgical sites were well-healed, bathing. New lung and heart transplant recipients also continued tap water avoidance after hospital discharge in the early postoperative period.
We noted an overall decrease in M. abscessus cases, including cases among lung transplant recipients, during the period from June 2014 through November 2014 (Figure 1B). The overall incidence rate decreased from 3.0 to 1.0 cases per 10000 patient-days (IRR, 0.3 [95% CI, .2–.6]; P < .001), and the incidence rate among lung transplant recipients decreased from 1.7 to 0.6 cases per 10000 patient-days (IRR, 0.3 [95% CI, .2–.7]; P = .005).
Outbreak Investigation, Phase 2: December 2014–June 2015
Epidemiologic Investigation
Two patients who had recently undergone cardiac surgery had cultures collected in December 2014 that returned positive for M. abscessus. These cases led to a second epidemiological investigation that ultimately included a cluster of 13 cases among patients who had undergone recent cardiac surgery at the hospital and had positive cultures from December 2014 through June 2015 (phase 2) (Figure 1B). One case reflected respiratory colonization, but the remaining 12 (92%) patients developed extrapulmonary invasive disease.
We analyzed the cohort of all cardiac surgery patients who met the case definition over the entire 3-year study period. Twenty-two cases of extrapulmonary invasive M. abscessus infection occurred after cardiac surgery. The first invasive infection was identified in November 2013. Infections followed heart transplant (n = 8 [36%]), ventricular assist device (VAD) insertion (n = 8 [36%]), valve replacement (n = 4 [18%]), and coronary artery bypass grafting (CABG) (n = 2 [9%]) surgeries. Sites of initial positive cultures included bloodstream (n = 10 [45%]), sternal wound (n = 5 [23%]), VAD driveline site (n = 2 [9%]), pleural fluid (n = 2 [9%]), respiratory tract (n = 2 [9%]), and pelvic ascites (n = 1 [5%]). Three additional cardiac surgery patients with invasive infections did not meet the case definition because they had not been hospitalized within 30 days prior to initial culture collection.
Twenty-one of 22 (95%) patients from this cardiac surgery cohort required cardiopulmonary bypass (CPB) during surgery; the remaining patient underwent chest exploration with a CPB machine on standby in the OR. Median incubation time from possible inoculation in the OR to first positive nonrespiratory culture was 42 days (IQR, 20–94 days; range, 2–366 days). Nearly all cardiac surgeries performed after July 2013, including all cardiac surgeries performed in this cohort, occurred in ORs in the hospital addition.
All 22 cardiac surgery patients had extensive perioperative comorbidities and complicated postoperative courses in addition to M. abscessus infection. Nineteen (86%) patients received antimicrobial therapy, and 9 (41%) died within 60 days of the first positive culture.
Field Investigation
We reinvestigated lung and cardiac surgery OR suites and surgical practices. Mycobacterial cultures from cardiothoracic surgery OR air samples were negative. We also reviewed our maintenance and disinfection protocol for heater-cooler units (HCUs) of CPB machines. Our hospital used Stöckert 3T HCUs, and the manufacturer’s instructions for use changed in 2010, recommending filtered rather than unfiltered tap water for use in these machines; however, we found that water changes were performed with unfiltered tap water and that the disinfection procedure was not performed according to the instructions for use. Cultures from biofilms of HCUs were negative for M. abscessus, but a culture from a biofilm of the faucet used to fill HCUs was positive for M. abscessus. Only a single series of cultures from biofilms of external HCU components, including tubing and overflow water canisters, was performed before we instituted a new water change and disinfection protocol for HCUs, as described below.
Our survey of the hospital water system identified several factors that may have contributed to increased concentrations of M. abscessus within the new addition’s water distribution system. The addition utilized Leadership in Energy and Environmental Design (LEED) standards to reduce water usage [31]. The water system had low flow rates and low residual disinfectant (chloramine) levels at multiple water outlets. The hot water system was a recirculating loop, and hot water stored in reservoirs required prolonged flow times to reach outlets. Relatively small amounts of water from the municipal water supply entered the recirculating hot water loop each day, which also contributed to low chloramine levels.
Interventions and Subsequent Surveillance
We made several process, patient care, and water engineering changes to terminate the outbreak among cardiac surgery patients and prevent new clusters among other patient types (Supplementary Figure 2).
A study published in the midst of our investigation demonstrated the potential for aerosolized NTM from CPB machine HCUs to contaminate patients during cardiac surgery [25]. In early May 2015, based on the results of this study and our epidemiologic investigation, we changed our protocol for maintenance and disinfection of HCUs. The new protocol included daily water changes with sterile water and daily disinfection with hydrogen peroxide, in addition to intermittent bleach-based disinfection. We also directed HCU exhaust away from the surgical field. We replaced all existing HCUs with new HCUs by early June 2015 and used only sterile water in these machines. Also in early June, we notified at-risk patients and healthcare providers of the potential for NTM infection after cardiac surgery and described associated signs and symptoms to facilitate case detection. At the same time, we notified the US Food and Drug Administration (FDA) about the potential causal link between HCU use and NTM infection.
We implemented 3 primary water engineering–related interventions at the new addition to reduce microbial proliferation and burden: we flushed water throughout both the cold water and recirculating hot water systems; removed or adjusted water flow restrictors, aerators, and a redundant hot water tank; and decreased the percentage of recirculating hot water that bypassed heat exchangers. These changes led to higher and more consistent flow rates, increased mixing of municipal water within the hot water loop, faster delivery of hot water to distal outlets, and increased chloramine levels throughout the plumbing system. Additionally, we installed point-of-use 0.2-µm water filters [32] at OR scrub sink faucets.
A single case of M. abscessus infection occurred in a cardiac surgery patient who underwent CPB after implementation of HCU protocol changes and engineering interventions (Figure 1B). This patient developed M. abscessus infection of pelvic ascites after CABG, and we suspected that inoculation did not occur at the time of CABG.
Laboratory Investigation, Phase 1 and Phase 2
Seventy case patient and 17 environmental isolates of M. abscessus recovered during the study period were submitted for erm(41) gene sequencing, region V rpoβ gene sequencing, and/or VNTR. All but 2 patient isolates and all environmental isolates analyzed belonged to 1 of 2 clones (see page 5 of the Supplementary Data).
Fifty-three of 70 (76%) patient isolates were consistent with clone A; 15 (21%) isolates were consistent with clone B; and only 2 (3%) isolates were inconsistent with either clone (Table 2). All 18 patient isolates analyzed from cardiac surgery patients with invasive infections and all 17 environmental isolates of M. abscessus exhibited clone A fingerprinting patterns.
Table 2.
Summary of Gene Sequencing and Variable Number Tandem Repeats of Case Patients and Environmental Isolates of Mycobacterium abscessus Obtained From 2013 Through 2015
| Characteristic or Isolate Type | M. abscessus Clone Aa | M. abscessus Clone Bb | Other M. abscessus Isolatesc |
|---|---|---|---|
| Gene sequencing | |||
| erm(41) gene | Subspecies abscessus Type VI |
Subspecies massiliense | Miscellaneous |
| rpoβ gene | Subspecies abscessus C→T mutation, base pair 207 |
Subspecies abscessus | Miscellaneous |
| VNTR | |||
| Primer TR155 | 5 copies | … | … |
| Type of isolate | |||
| Patient isolatesd | |||
| All cases analyzed (n = 70) | 53 (76) | 15 (21) | 2 (3) |
| Phase 1 cases (n = 38)e | 31 (82) | 6 (16) | 1 (3) |
| Phase 2 cases (n = 15)e | 13 (87) | 2 (13) | 0 (0) |
| First positive culture was from respiratory tract (n = 39) | 29 (74) | 9 (23) | 1 (3) |
| First positive culture was not from respiratory tract (n = 31) | 24 (77) | 6 (19) | 1 (3) |
| Lung transplant cases (n = 33) | 25 (76) | 8 (24) | 0 (0) |
| Invasive cardiac surgery infections (n = 18) | 18 (100) | 0 (0) | 0 (0) |
| Environmental isolates (n = 17) | 17 (100) | 0 (0) | 0 (0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: VNTR, variable number tandem repeat.
aAn isolate was considered consistent with clone A if it had a C→T mutation at base pair 207 of the rpoβ gene or VNTR with 5 copies for primer TR155. All isolates from cases meeting 1 of these criteria that underwent erm(41) gene sequencing had the M. abscessus subspecies abscessus type VI erm(41) gene.
bAn isolate was considered consistent with clone B if it had an M. abscessus subspecies M. massiliense erm(41) gene. All isolates from cases meeting this criterion that underwent rpoβ gene sequencing had the M. abscessus subspecies abscessus rpoβ gene.
cAll isolates with gene sequencing not consistent with clone A or clone B were considered “other” M. abscessus isolates.
dPatient isolates included met the outbreak case definition and represented either M. abscessus colonization or infection. Only selected case patient isolates underwent molecular fingerprinting.
ePhase 1 of the outbreak occurred from August 2013 through May 2014. Phase 2 occurred from December 2014 through June 2015.
We additionally performed gene sequencing and VNTR on patient isolates collected prior to the study period. Isolates with clone A and clone B fingerprinting were identified as early as 2010 and 2007, respectively.
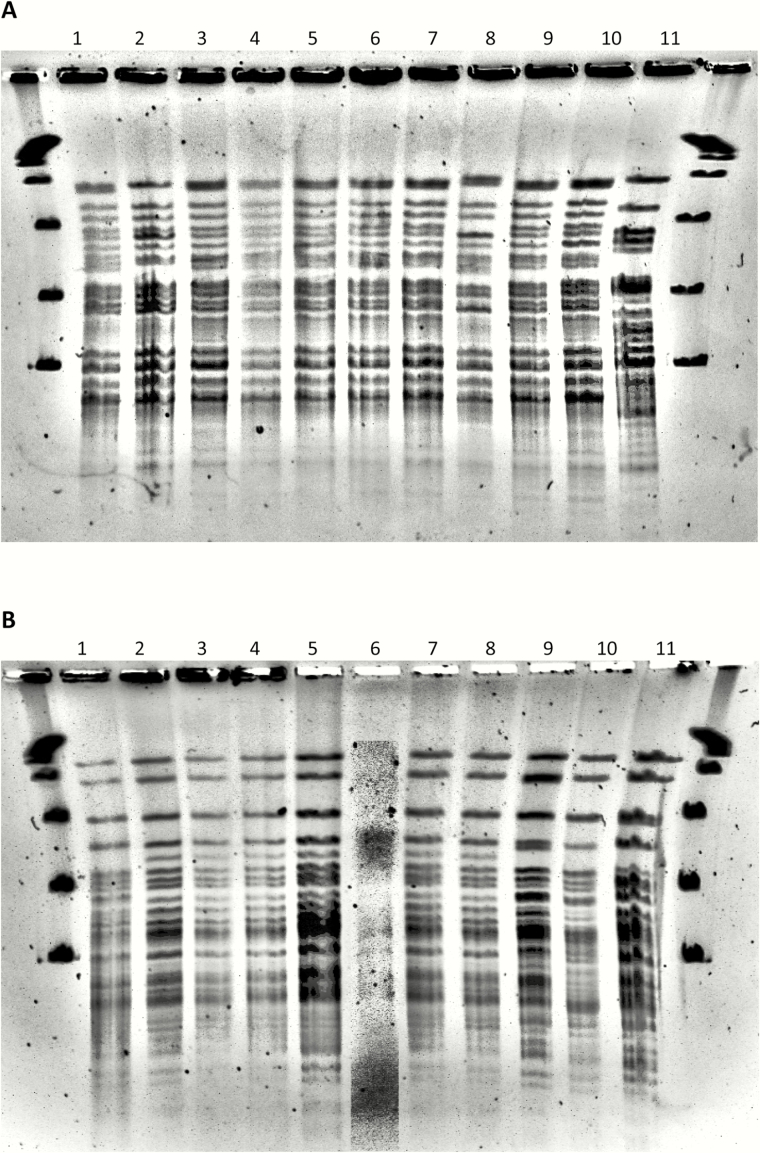
PFGE was performed on 9 clone A patient isolates collected from 2010 through 2015 and 8 clone A environmental isolates from phase 1 of this investigation; all isolates were clonal (Figure 2A) [30]. PFGE was also performed on 10 clone B patient isolates collected from 2007 through 2015, and 9 isolates were clonal (Figure 2B).
Figure 2.
XbaI pulsed-field gel electrophoresis (PFGE) of patient and environmental isolates of Mycobacterium abscessus obtained from 2007 through 2015. A, Isolates selected for this panel demonstrated gene sequencing and/or variable number tandem repeats (VNTRs) consistent with outbreak clone A [erm(41) gene: subspecies abscessus, type VI; rpoβ gene: subspecies abscessus, C→T mutation at base pair 207; VNTR: primer TR155, 5 copies]. The panel includes 2 preoutbreak patient isolates from 2010–2012 (lanes 1, 2), 4 case patient isolates from phase 1 of the outbreak period (lanes 3, 4, 5, 6), 2 case patient isolates from phase 2 of the outbreak period (lanes 7, 9), 1 patient isolate from the outbreak period that did not meet the case definition (lane 8), 1 environmental isolate from phase 1 (lane 10), and the M. abscessus subspecies abscessus type strain—ATCC19977T (lane 11). The 9 patient isolates included samples from bronchoalveolar lavage (n = 5), blood (n = 1), pleural fluid (n = 1), sputum (n = 1), and sternal wound (n = 1). All clinical and environmental isolates (lanes 1–10) were clonal [30]. Isolates were indistinguishable or closely related except isolate 8 (possibly related), and all 10 isolates were unrelated to the M. abscessus subspecies abscessus type strain (lane 11). Seven additional environmental isolates from phase 1 were also indistinguishable (data not shown). B, Isolates selected for this panel demonstrated gene sequencing consistent with outbreak clone B [erm(41) gene: subspecies massiliense; rpoβ gene: subspecies abscessus]. The panel includes 4 preoutbreak patient isolates from 2007 through 2013 (lanes 1, 2, 3, 4), 3 case patient isolates from phase 1 of the outbreak period (lanes 5, 6, 7), 1 case patient isolate from phase 2 of the outbreak period (lane 9), 2 patient isolates from the outbreak period that did not meet the case definition (lanes 8, 10), and the M. abscessus subspecies massiliense type strain—CIP108297T (lane 11). The 10 patient isolates included samples from bronchoalveolar lavage (n = 4), blood (n = 2), pleural fluid (n = 2), soft tissue (n = 1), and sputum (n = 1). All patient isolates (lanes 1–10) except isolate 10 were clonal [30]. Patient isolates other than isolate 10 were indistinguishable or closely related except isolates 1 and 9 (possibly related). Isolate 6 had poor image resolution but appeared related. All 10 isolates were unrelated to the M. abscessus subspecies massiliense type strain (lane 11).
DISCUSSION
We report a biphasic outbreak of M. abscessus in patients hospitalized at a tertiary care hospital.
Cases from phase 1 of the outbreak predominantly involved lung transplant recipients with colonization or infection of the respiratory tract. We hypothesized that micro-aspiration of M. abscessus from tap water used for patient care activities led to subsequent pulmonary colonization or infection [33, 34].
The second phase of the outbreak primarily involved cardiac surgery patients with invasive infections. Based on reports of NTM infections in cardiac surgery patients and the prevalence of wound and bloodstream infections, we hypothesized that patients in this second cluster acquired infection via aerosols generated from colonized HCUs [25].
One of the 2 distinct molecular clones of M. abscessus involved in this outbreak was recovered from tap water sources at the new hospital addition; however, both clones were present in clinical cases that occurred several years before the addition opened. Also, both M. abscessus clones were isolated from patients who had never been admitted to the hospital. Thus, isolates of M. abscessus obtained from this investigation were not unique to the new addition, the existing hospital, or the study time period and likely represent colonization of the local municipal water supply.
Low flow rates within the hospital addition’s water circuit and a redundant hot water circulation system may have led to amplification of NTM in the addition’s water supply over time. These conditions contributed to low chloramine levels and water temperatures favorable for M. abscessus growth.
Our initial mitigation strategy eliminated exposure to tap water for high-risk patients via a protocol that used only sterile water for clinical care practices. We observed a sustained decrease in the incidence rate of M. abscessus obtained from pulmonary sources after this intervention. Then, after a second cluster of cases occurred in patients after cardiac surgery, we purchased new HCUs, intensified the HCU disinfection protocol, and began using only sterile water for HCU water changes. Subsequently, the outbreak among cardiac surgery patients resolved.
We also implemented water engineering–related mitigation strategies designed to decrease the burden of M. abscessus in the hospital water supply (Table 3). Complete eradication of M. abscessus and associated biofilms from hospital tap water and plumbing infrastructure was not realistic given the environmental persistence of NTM [32]. Nonetheless, engineering interventions should decrease risk of patient colonization if tap water exposure occurs despite avoidance strategies.
Table 3.
Selected Interventions Made and Recommendations for Other Healthcare Facilities to Decrease Risk of Healthcare-Associated Nontuberculous Mycobacterial Infection
| Intervention Made at Our Hospital | Recommendations for Other Healthcare Facilities |
|---|---|
| Clinical practice and equipment-related interventions | |
| Sterile water use for direct patient-care activities of patients at risk |
• Healthcare facilities with infections from NTM should consider avoidance of tap water [35] and use of sterile water for patient care activities, such as oral care, enteral tube flushing, speech assessment, consumption, and bathing. • Local epidemiology should determine target patient groups, potentially including critically ill and immunosuppressed patients, those with disrupted gastrointestinal tracts, and patients with early postoperative wounds. Continuing to avoid tap water after hospital discharge may also reduce risk for recent lung or heart transplant recipients. |
| HCU sterile water use and disinfection protocol |
• Hospitals should adhere to FDA and manufacturer recommendations for HCU use, water changes, and disinfection practices [36, 37]. |
| Epidemiologic and clinical surveillance for NTM infection | • Hospital epidemiologists should perform retrospective and prospective surveillance for invasive NTM infections. • Clinicians should consider NTM infection when evaluating patients with infection after cardiac surgery, especially for atypical clinical manifestations and prolonged incubation periods. • Clinicians should request mycobacterial cultures, particularly for surgical specimens. |
| Environmental and engineering-based interventions | |
| Periodic flushing of both cold water and recirculating hot water; removal of flow restrictors and redundancies in plumbing system |
• Facilities with high-efficiency and/or recirculating water systems should consult with water engineering experts about periodically flushing stagnant or recirculating water from the circulation, avoiding or removing flow restrictors, and minimizing redundancies in the plumbing system. |
| Monitoring of hot water temperatures and flow times to water outlets | • Water engineering personnel should perform surveillance of hot water temperatures throughout the hot water distribution system [38]. |
| Monitoring of chloramine levels in hospital water supply | • Water engineering personnel should measure disinfectant (eg, chloramine or chorine) levels at entry to the facility and at point of use. If proximal disinfectant levels are low, healthcare facilities may need to contract with municipal water authorities. |
| Installation of point-of-use water filters in clinical locations | • Facilities with endemic NTM should consider use of 0.2-µm point-of-use water filters [32]. |
| Mycobacterial cultures of biofilms obtained from hospital water outlets | • If facilities experience outbreaks of NTM, they should evaluate the water system and associated equipment for NTM colonization. |
Abbreviations: FDA, US Food and Drug Administration; HCU, heater-cooler unit; NTM, nontuberculous mycobacteria.
Other recent outbreaks of postoperative invasive NTM infections traced to the use of CPB and Stöckert 3T HCUs have received widespread attention. Sax et al investigated an outbreak of Mycobacterium chimaera at a Swiss hospital among cardiac surgery patients that was molecularly linked to colonized HCUs [25, 39]. Hospitals in Europe, Pennsylvania, and Iowa have also reported M. chimaera infections following cardiac surgery [40–43]. Whole genome sequencing performed on M. chimaera isolates from 11 patients and 5 Stöckert 3T HCUs from hospitals in Pennsylvania and Iowa suggested point-source contamination [44], which may have occurred at the manufacturing site in Germany [45]. The FDA and Centers for Disease Control and Prevention released updated safety recommendations in October 2016 that advised avoidance of 3T HCU devices manufactured prior to September 2014 and notification of patients who had been exposed to these devices [36, 46].
Data from our investigation do not conclusively prove that aerosolization of M. abscessus from colonized HCUs caused invasive infections in cardiac surgery patients; however, we believe this mechanism provides the most likely explanation for these infections (see page 5 of the Supplementary Data). Infections in cardiac surgery patients at our hospital most likely arose from M. abscessus present in the hospital water supply that in turn colonized HCUs. In contrast, outbreaks of M. chimaera in cardiac surgery patients at multiple other hospitals worldwide likely stemmed from point-source contamination in Germany [44, 45].
This outbreak and investigation has important implications for other medical centers (Table 3). First, NTM are often present in hospital water, and factors that increase their concentration or promote aerosolization onto vulnerable patients can produce outbreaks. Second, long-term surveillance for NTM by healthcare facilities is critical for outbreak detection. NTM surveillance is difficult and can be complicated by preexisting endemic disease in the community with the same NTM strains, atypical clinical manifestations, and prolonged incubation periods. Third, strategies that minimize exposure of vulnerable patients to waterborne NTM can successfully mitigate outbreaks; however, multiple or unexpected types of exposures may occur. Therefore, in response to outbreaks, we recommend combining water avoidance strategies with water engineering–based interventions designed to decrease the concentration of NTM colonizing healthcare facility water or equipment. We found that redundant water systems designed to conserve water may create low-flow states and contribute to NTM proliferation within hospital plumbing systems. Indications and methods for screening healthcare facility water systems for NTM to help prevent outbreaks need to be developed.
This investigation had limitations. The case definition did not differentiate colonization from invasive infection because of the inherent difficulties in making this clinical distinction, especially in lung transplant patients. Also, our investigation did not capture infected patients with negative cultures, no cultures, or those who did not return to our hospital for follow-up. In addition, while many patients died, we cannot state that these deaths were attributable to M. abscessus infection, in part because many patients were critically ill with multiple comorbidities in addition to M. abscessus infection. Finally, to protect subsequent patients, we implemented an HCU disinfection protocol prior to confirming a microbiological link between colonized HCUs and invasive M. abscessus infections following cardiac surgery. The limited number of HCU cultures and low sensitivity of environmental culture techniques for NTM [2] could explain our inability to microbiologically confirm HCU colonization with M. abscessus.
In summary, we utilized a multidisciplinary team to investigate and mitigate a hospital-associated outbreak of clonally related M. abscessus that was epidemiologically linked to colonized tap water. We made multiple patient care and water engineering interventions. Primary interventions included institution of an inpatient sterile water protocol for high-risk patients, implementation of a protocol for enhanced disinfection and sterile water use for HCUs of CPB machines, and water engineering changes designed to decrease NTM burden in the plumbing system. Other healthcare facilities, particularly those with endemic NTM or newly constructed patient care facilities, should consider similar multifaceted strategies to improve water safety and decrease risk of healthcare-associated infection from NTM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the Transplant Infectious Disease Interdisciplinary Research Training Grant of the National Institutes of Health (grant number 5T32AI100851-02); the Cardiothoracic Surgical Trials Network of the National Institutes of Health (grant number U01-HL088953); and the Amon G. Carter Foundation.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Primm TP, Lucero CA, Falkinham JO., 3rd Health impacts of environmental mycobacteria. Clin Microbiol Rev 2004; 17:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falkinham JO, 3rd, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl Environ Microbiol 2001; 67:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomson R, Tolson C, Sidjabat H, Huygens F, Hargreaves M. Mycobacterium abscessus isolated from municipal water—a potential source of human infection. BMC Infect Dis 2013; 13:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomson R, Tolson C, Carter R, Coulter C, Huygens F, Hargreaves M. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J Clin Microbiol 2013; 51:3006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams MM, Armbruster CR, Arduino MJ. Plumbing of hospital premises is a reservoir for opportunistically pathogenic microorganisms: a review. Biofouling 2013; 29:147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin JH, Lee EJ, Lee HR, et al. Prevalence of non-tuberculous mycobacteria in a hospital environment. J Hosp Infect 2007; 65:143–8. [DOI] [PubMed] [Google Scholar]
- 7. du Moulin GC, Stottmeier KD, Pelletier PA, Tsang AY, Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 1988; 260:1599–601. [DOI] [PubMed] [Google Scholar]
- 8. El Sahly HM, Septimus E, Soini H, et al. Mycobacterium simiae pseudo-outbreak resulting from a contaminated hospital water supply in Houston, Texas. Clin Infect Dis 2002; 35:802–7. [DOI] [PubMed] [Google Scholar]
- 9. Prabaker K, Muthiah C, Hayden MK, et al. Pseudo-outbreak of Mycobacterium gordonae following the opening of a newly constructed hospital at a Chicago Medical Center. Infect Control Hosp Epidemiol 2015; 36:198–203. [DOI] [PubMed] [Google Scholar]
- 10. Wallace RJ, Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998; 52:453–90. [DOI] [PubMed] [Google Scholar]
- 11. Stout JE, Gadkowski LB, Rath S, Alspaugh JA, Miller MB, Cox GM. Pedicure-associated rapidly growing mycobacterial infection: an endemic disease. Clin Infect Dis 2011; 53:787–92. [DOI] [PubMed] [Google Scholar]
- 12. Winthrop KL, Abrams M, Yakrus M, et al. An outbreak of mycobacterial furunculosis associated with footbaths at a nail salon. N Engl J Med 2002; 346:1366–71. [DOI] [PubMed] [Google Scholar]
- 13. Chadha R, Grover M, Sharma A, et al. An outbreak of post-surgical wound infections due to Mycobacterium abscessus. Pediatr Surg Int 1998; 13:406–10. [DOI] [PubMed] [Google Scholar]
- 14. Iroh Tam PY, Kline S, Wagner JE, et al. Rapidly growing mycobacteria among pediatric hematopoietic cell transplant patients traced to the hospital water supply. Pediatr Infect Dis J 2014; 33:1043–6. [DOI] [PubMed] [Google Scholar]
- 15. Agarwal A, Maloney RW. Mycobacterium abscessus outbreak after rhytidectomies performed in an outpatient surgery center. Plast Reconstr Surg 2011; 128:85–6e. [DOI] [PubMed] [Google Scholar]
- 16. Tagashira Y, Kozai Y, Yamasa H, Sakurada M, Kashiyama T, Honda H. A cluster of central line-associated bloodstream infections due to rapidly growing nontuberculous mycobacteria in patients with hematologic disorders at a Japanese tertiary care center: an outbreak investigation and review of the literature. Infect Control Hosp Epidemiol 2015; 36:76–80. [DOI] [PubMed] [Google Scholar]
- 17. Cortesia C, Lopez GJ, de Waard JH, Takiff HE. The use of quaternary ammonium disinfectants selects for persisters at high frequency from some species of non-tuberculous mycobacteria and may be associated with outbreaks of soft tissue infections. J Antimicrob Chemother 2010; 65:2574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novosad SA, Beekmann SE, Polgreen PM, Mackey K, Winthrop KL; M. abscessus Study Team. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis 2016; 22:511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011; 52:565–71. [DOI] [PubMed] [Google Scholar]
- 20. Maloney S, Welbel S, Daves B, et al. Mycobacterium abscessus pseudoinfection traced to an automated endoscope washer: utility of epidemiologic and laboratory investigation. J Infect Dis 1994; 169:1166–9. [DOI] [PubMed] [Google Scholar]
- 21. Cox R, deBorja K, Bach MC. A pseudo-outbreak of Mycobacterium chelonae infections related to bronchoscopy. Infect Control Hosp Epidemiol 1997; 18:136–7. [DOI] [PubMed] [Google Scholar]
- 22. Blossom DB, Alelis KA, Chang DC, et al. Pseudo-outbreak of Mycobacterium abscessus infection caused by laboratory contamination. Infect Control Hosp Epidemiol 2008; 29:57–62. [DOI] [PubMed] [Google Scholar]
- 23. Ashford DA, Kellerman S, Yakrus M, et al. Pseudo-outbreak of septicemia due to rapidly growing mycobacteria associated with extrinsic contamination of culture supplement. J Clin Microbiol 1997; 35:2040–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Society for Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE). ANSI/ASHRAE Standard 188–2015, Legionellosis: risk management for building water systems. Atlanta, Georgia: ASHRAE, 2015. [Google Scholar]
- 25. Sax H, Bloemberg G, Hasse B, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis 2015; 61:67–75. [DOI] [PubMed] [Google Scholar]
- 26. Kent P, Kubica GP. Public health mycobacteriology—a guide for the level III laboratory. Atlanta, GA: US Department of Health and Human Services. Public Health Service. Centers for Disease Control, 1985. [Google Scholar]
- 27. Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol 2015; 53:1211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adékambi T, Colson P, Drancourt M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol 2003; 41:5699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong YL, Ong CS, Ngeow YF. Molecular typing of Mycobacterium abscessus based on tandem-repeat polymorphism. J Clin Microbiol 2012; 50:3084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Yakrus MA, Graviss EA, et al. Pulsed-field gel electrophoresis study of Mycobacterium abscessus isolates previously affected by DNA degradation. J Clin Microbiol 2004; 42:5582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzer O. A comparative review of environmental concern prioritization: LEED vs other major certification systems. J Environ Manage 2015; 154:266–83. [DOI] [PubMed] [Google Scholar]
- 32. Williams MM, Chen TH, Keane T, et al. Point-of-use membrane filtration and hyperchlorination to prevent patient exposure to rapidly growing mycobacteria in the potable water supply of a skilled nursing facility. Infect Control Hosp Epidemiol 2011; 32:837–44. [DOI] [PubMed] [Google Scholar]
- 33. Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 2007; 131:1166–72. [DOI] [PubMed] [Google Scholar]
- 34. Griffin SM, Robertson AG, Bredenoord AJ, et al. Aspiration and allograft injury secondary to gastroesophageal reflux occur in the immediate post-lung transplantation period (prospective clinical trial). Ann Surg 2013; 258: 705–11; discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 35. Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis 2016; 62:1423–35. [DOI] [PubMed] [Google Scholar]
- 36. US Food and Drug Administration. Update: Mycobacterium chimaera infections associated with LivaNova PLC (formerly Sorin Group Deutschland GmbH) Stӧckert 3T Heater-Cooler System: FDA safety communication Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm520191.htm. Accessed 13 October 2016.
- 37. Sorin Group Deutschland GMBH. Heater-Cooler System 3T. Operating instructions. Version 09/2015 Available at: http://www.livanova.sorin.com/products/cardiac-surgery/perfusion/hlm/3t. Accessed 31 October 2016.
- 38. Sehulster L, Chinn RY; CDC; HICPAC Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–42. [PubMed] [Google Scholar]
- 39. Kohler P, Kuster SP, Bloemberg G, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J 2015; 36:2745–53. [DOI] [PubMed] [Google Scholar]
- 40. European Centre for Disease Prevention and Control. Invasive cardiovascular infection by Mycobacterium chimaera potentially associated with heater-cooler units used during cardiac surgery Available at: http://ecdc.europa.eu/en/publications/Publications/mycobacterium-chimaera-infection-associated-with-heater-cooler-units-rapid-risk-assessment-30-April-2015.pdf. Accessed 7 May 2015.
- 41. WellSpan York Hospital Open Heart Surgery Infections. Frequently asked questions (FAQ) for physicians and APCs Available at: https://www.wellspan.org/media/1243364/WYH-OpenHeart-FAQ-Providers.pdf. Accessed 4 March 2016.
- 42. Milton S. Hershey Medical Center. Open-heart surgery: important information for medical professionals Available at: http://www.pennstatehershey.org/web/guest/patientcare/open-heart/medical-professionals. Accessed 4 March 2016.
- 43. University of Iowa Hospitals and Clinics. Potential infection risk in major heart and lung surgeries Available at https://uihc.org/news/potential-infection-risk-major-heart-and-lung-surgeries. Accessed 13 October 2016.
- 44. Perkins KM, Lawsin A, Hasan NA, et al. Notes from the field: Mycobacterium chimaera contamination of heater-cooler devices used in cardiac surgery—United States. MMWR Morb Mortal Wkly Rep 2016; 65:1117–8. [DOI] [PubMed] [Google Scholar]
- 45. Haller S, Holler C, Jacobshagen A, et al. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: results of an outbreak investigation in Germany, April 2015 to February 2016. Euro Surveill 2016; 21. [DOI] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention. CDC advises hospitals to alert patients at risk from contaminated heater-cooler devices used during cardiac surgery Available at: https://emergency.cdc.gov/han/han00397.asp. Accessed 13 October 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.