Among 81 commonly used outpatient antibiotic formulations, around one-eighth increased in price by >90% from 2013 to 2016, with the majority occurring due to fewer approved generic manufacturers. Policy solutions are needed to improve competition among manufacturers of generic antibiotics.
Keywords: generic drugs, drug prices, oral antibiotics
Abstract
Background
In recent years, the price of many older generic drugs, including numerous antibiotics, has increased substantially. We sought to analyze recent price changes and extent of generic competition within a cohort of commonly prescribed off-patent oral antibiotics.
Methods
We extracted oral antibiotics recommended for common outpatient conditions in the Infectious Diseases Society of America Practice Guidelines. We determined all US Food and Drug Administration–approved manufacturers for each formulation and strength in 2013 and 2016 and the yearly national average drug acquisition cost (NADAC) price between 2013 and 2016. Wilcoxon signed rank test was used to compare changes in drug prices and number of manufacturers from 2013 to 2016. Spearman correlation coefficient was used to assess the association between drug prices and number of manufacturers.
Results
Twenty-two antibiotics (81 formulations and strengths) were analyzed. There was no change in the median NADAC price or the number of manufacturers between 2013 and 2016. However, 11 (14%) formulations increased in price by 90% or more, and 13 (16%) had 2 or fewer manufacturers during all 4 years. Antibiotic prices were negatively associated with the number of available manufacturers.
Conclusions
While prices and the number of manufacturers for common oral antibiotics were overall stable between 2013 and 2016, reduced manufacturer competition was associated with increased prices. A subset of antibiotics exhibited substantial price increases, and most, but not all, had limited manufacturer competition. Policy solutions are needed to ensure availability of low-cost, essential generic antibiotics.
Prescription drug spending in the United States continues to increase at a pace beyond that in many other sectors of the healthcare marketplace, with brand-name drugs accounting for the majority of this spending [1]. Manufacturers of brand-name drugs set prices as high as the “market will bear” during their limited period of market exclusivity—usually about 12–15 years—after which interchangeable generic versions reach the market and lead to reduced prices from competition [2]. If insufficient numbers of generic manufacturers exist to provide effective competition, however, prices can rise back to brand-name levels, as occurred with 3 antiparasitic drugs—albendazole (Albenza), praziquantel (Biltricide), and pyrimethamine (Daraprim). These drugs increased in price by 1920%, 356%, and 5433%, respectively, in recent years [3, 4].
Limited generic competition can also leave drugs susceptible to shortages [5]. Recently, the sole manufacturer of penicillin G benzathine (Bicillin L-A), the first-line treatment for syphilis and the only recommended treatment for syphilis in pregnant women, experienced manufacturing problems that resulted in a drug shortage [6]. Between 2001 and 2013, there were 148 shortages of antibiotics, including many that were intravenous, broad spectrum, and used to treat serious infections [7]. Antibiotic shortages can have substantial impacts on patient care, including the need to use alternative antibiotics that may be less effective, toxic, or more costly [8]. Drug shortages can also lead to price increases. For example, a shortage of doxycycline hyclate and doxycycline monohydrate in 2013 led to a 5500% price increase for some formulations [9].
Because the antibiotic market has been particularly affected by generic drug price hikes and drug shortages, we sought to analyze changes in the prices and the number of manufacturers, over time, among a cohort of essential oral antibiotics.
METHODS
Sample Identifications
We searched for infectious disease conditions commonly managed in the outpatient setting listed in the Infectious Diseases Society of America Practice Guidelines. These conditions included cystitis/pyelonephritis, community-acquired pneumonia, rhinosinusitis, streptococcus pharyngitis, skin and soft tissue infection, and diabetic foot infection. Within these guidelines, we extracted all oral antibiotics recommended for treatment of these conditions, excluding antibiotics only available by intravenous and intramuscular routes (N = 22) and drugs not approved for use in the United States (N = 1). If a guideline specified that a condition is managed primarily in the inpatient or outpatient setting, we included only those antibiotics recommended within the outpatient setting. We then used the publicly available US Food and Drug Administration (FDA) Orange Book [10] to identify all of the distinct formulations and strengths available for each antibiotic in our final cohort by searching for the antibiotic’s generic name and excluding discontinued products.
Data Extraction
We sought 2 primary pieces of data for each antibiotic in our sample. First, we identified the total number of FDA-approved manufacturers for each antibiotic in 2013 and 2016 by calculating the total number of distinct manufacturer applicants for each formulation and strength listed in the FDA Orange Book, 33rd edition (2013), and in the online Orange Book database as of October 2016. The FDA Orange Book lists drug applicants that are approved to sell a particular drug in the United States.
Next, we extracted the national average drug acquisition cost (NADAC) price per unit of each antibiotic formulation from the publicly available NADAC database. NADAC is the pricing benchmark used by the Centers for Medicare and Medicaid and represents acquisition costs for covered outpatient drugs purchased by retail community pharmacies; it does not include rebates [11]. We collected yearly antibiotic prices from 2013 to 2016, using the latest date for which pricing was available between September and November of each year. We excluded antibiotics that did not have NADAC pricing information available for all 4 years (3 antibiotics; 72 total formulations and strengths). To estimate the daily cost of each antibiotic, we multiplied the NADAC price per unit by the usual number of doses per day for each antibiotic formulation. If the dosing of a particular antibiotic varied depending on the disease or organism, we chose the less frequent dosing schedule. We labeled this value as the “price per day.”
Then, we examined the effect of 2 variables on the degree of competition among manufacturers and antibiotic prices: the year of the new drug application (NDA) and the type of antibiotic formulation. We extracted the year of the NDA from the publicly available Drugs @FDA database for each formulation and strength and categorized each into 2 groups: “approved in 1982 or earlier” or “approved after 1982.” The NDA approval year was not available for 2 formulations and strengths. We also categorized each antibiotic by formulation, as either a “tablet or capsule” or “non-tablet/capsule.”
Statistical Analyses
Descriptive statistics are shown as median (min, max). The comparison between 2013 and 2016 drug prices and number of manufacturers was obtained using Wilcoxon signed rank test. The association between the change in drug price and the change in number of manufacturers from 2013 to 2016 was assessed using Spearman correlation coefficient. A subgroup analysis was performed for the year of the NDA and the type of drug formulation. All analyses were carried out using the SAS system (v. 9.3; SAS Institute, Cary, North Carolina). All P values are 2-sided and a value <0.05 was considered statistically significant.
RESULTS
Twenty-two antibiotics (81 formulations and strengths) were included in the analysis (see Supplementary Materials). About three-quarters (58/81, 72%) were tablets and capsules, with the remaining 23 formulated in packets, suspensions, and solutions. About half were FDA approved after 1982. The median price per unit, price per day, and number of manufacturers between 2013 and 2016 are shown in Table 1.
Table 1.
Pricing and Manufacturer Trends Among a Sample of First-Line Oral Antibiotics Used to Treat Common Infections, 2013–2016
| Antibiotics by Formulation and Year of FDA Approval | 2013 | 2016 | P Value |
|---|---|---|---|
| All antibiotics | |||
| Price per unit | 0.43 (0.02, 113) | 0.43 (0.02, 63.7) | .58 |
| Price per day | 0.77 (0.06, 227) | 0.86 (0.06, 63.72) | .47 |
| Number of manufacturers | 5 (1, 15) | 5 (1, 18) | .09 |
| Tablet/capsule formulations | |||
| Price per unit | 0.51 (0.06, 113) | 0.55 (0.07, 19.1) | .83 |
| Price per day | 1.00 (0.12, 227) | 1.16 (0.17, 43.95) | .97 |
| Number of manufacturers | 5 (1, 15) | 6 (1, 18) | .07 |
| Nontablet/capsule formulations | |||
| Price per unit | 0.36 (0.02, 49.2) | 0.33 (0.02, 63.7) | .19 |
| Price per day | 0.64 (0.06, 49.2) | 0.60 (0.06, 63.7) | .15 |
| Number of manufacturers | 3 (1, 7) | 4 (1,6) | .84 |
| Approved 1982 or earlier | |||
| Price per unit | 0.24 (0.02, 3.51) | 0.29 (0.02, 14.7) | .16 |
| Price per day | 0.60 (0.06, 9.80) | 0.72 (0.06, 44.0) | .11 |
| Number of manufacturers | 4.50 (1, 13) | 5 (1, 10) | .78 |
| Approved after 1982 | |||
| Price per unit | 0.53 (0.03, 113) | 0.62 (0.03, 63.7) | .84 |
| Price per day | 0.98 (0.09, 227) | 0.99 (0.08, 63.7) | .84 |
| Number of manufacturers | 5 (1, 15) | 6 (1, 18) | .01 |
All data are presented as median (min, max).
Changes in Price
There was no overall change in the median NADAC price per unit or price per day between 2013 and 2016. We identified 11/81 (14%) antibiotic formulations that increased in price by 90% or more and 5/81 (6%) that increased in price by 200% or more (Table 2). Of the 11 drugs with substantial price increases, 6 (55%) were produced by 2 or fewer manufacturers during all 4 years and 5 (45%) were produced by 4 or more manufacturers. By contrast, among the antibiotics that did not have substantial price increases, only 7/70 (10%) were produced by 2 or fewer manufacturers. We identified 1 antibiotic formulation that decreased in price by 90% or more in our study period: linezolid (Zyvox) 600-mg tablet decreased in price by 94% and had an increase in the number of manufacturers from 1 to 10 (brand-name linezolid lost its market exclusivity in 2015).
Table 2.
Antibiotic Formulations in the Sample With Substantial Price Increases from 2013 to 2016
| Drug | Number of Manufacturers, 2013 | Number of Manufacturers, 2016 | Percentage Price Increase | Year of US Food and Drug Administration Approval |
|---|---|---|---|---|
| Erythromycin 500-mg tablet | 1 | 1 | 348.3 | 1972 |
| TMP-SMX 200-mg/5 mL; 40-mg/5 mL suspension | 4 | 4 | 298.1 | 1975 |
| Erythromycin ethylsuccinate 400-mg tablet | 1 | 1 | 270.7 | 1975 |
| Erythromycin 250-mg tablet | 1 | 1 | 241.3 | 1972 |
| Cephalexin 125-mg/5 mL suspension | 7 | 3 | 206.4 | 1971 |
| Cephalexin 250-mg/5 mL suspension | 7 | 3 | 165.1 | 1971 |
| Cefuroxime 250-mg tablet | 9 | 8 | 138 | 1987 |
| Cefuroxime 500-mg tablet | 9 | 8 | 137 | 1987 |
| Penicillin VK 125-mg/5 mL solution | 2 | 2 | 99.6 | 1968 |
| Dicloxacillin 250-mg capsule | 2 | 2 | 97 | 1968 |
| Dicloxacillin 500-mg capsule | 2 | 2 | 91.1 | 1968 |
Abbreviation: TMP-SMX, trimethroprim/sulfamethoxazole.
Manufacturing Trends
Among all antibiotics, there was no change in the median number of FDA-approved manufacturers per antibiotic between 2013 and 2016. We identified 13/81 (16%) antibiotic formulations with 2 or fewer FDA-approved manufacturers listed for all 4 years (Table 3). Most of these drugs were FDA-approved prior to 1982 (9/13, 69%), and 6/13 (46%) sustained price increases of 90% or more. By contrast, 68/81 (84%) drugs had 3 or more manufacturers, of which 29 (43%) were FDA approved before 1982 and only 5 (7%) sustained price increases of 90% or more.
Table 3.
Antibiotic Formulations in the Sample With 2 or Fewer Manufacturers
| Drug | Number of Manufacturers, 2013 | Number of Manufacturers, 2016 | Year of US Food and Drug Administration Approval |
|---|---|---|---|
| Fosfomycin trometamol 3 g/ packet | 1 | 1 | 1996 |
| Erythromycin 250-mg tablet | 1 | 1 | 1972 |
| Erythromycin 500-mg tablet | 1 | 1 | 1972 |
| Erythromycin ethylsuccinate 400-mg tablet | 1 | 1 | 1975 |
| Dicloxacillin 250-mg capsule | 2 | 2 | 1968 |
| Dicloxacillin 500-mg capsule | 2 | 2 | 1968 |
| Cefaclor 250-mg capsule | 2 | 2 | 1979 |
| Cefaclor 500-mg capsule | 2 | 2 | 1979 |
| Penicillin VK 125-mg/5 mL solution | 2 | 2 | 1968 |
| Penicillin VK 250-mg/5 mL solution | 2 | 2 | 1968 |
| Azithromycin 1-g powder packet | 1 | 1 | 1994 |
| Linezolid 100-mg/5 mL suspension | 1 | 2 | 2000 |
| Doxycycline 40-mg capsule | 2 | 1 | 2006 |
Association Between Antibiotic Characteristics and Price Changes
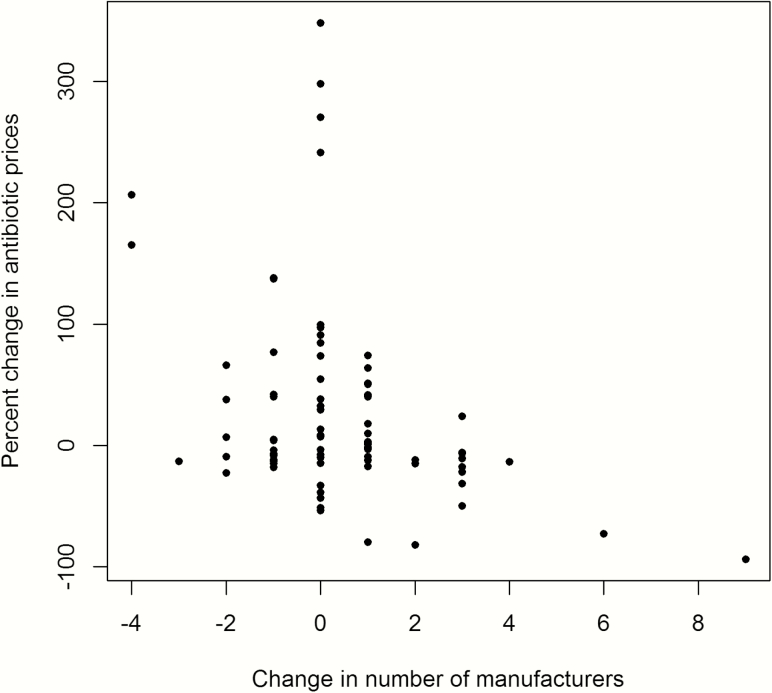
There was no association between antibiotic formulation (tablet/capsule vs other) or year of approval (pre-1982 vs post-1982) and changes in price. We found that the change in the antibiotic price per unit was negatively associated with the change in the number of manufacturers (Figure 1). The strength of this association varied depending on the antibiotic category.
Figure 1.
Association between the change in number of manufacturers and the change in antibiotic prices.
In examining the number of manufacturers and price of all antibiotics in our sample, the Spearman correlation coefficient was –0.31 (P = .0046), indicating that an increase in the number of manufacturers was associated with a decrease in drug prices. Antibiotics in a tablet/capsule formulation and with an NDA approval year after 1982 exhibited slightly stronger associations (Spearman coefficient, –0.39 and P = .003 and Spearman coefficient, –0.44 and P = .005).
DISCUSSION
Among a cohort of widely used oral antibiotics recommended for treatment of common infections, the median price and number of manufacturers were stable between 2013 and 2016. However, 14% of antibiotics sustained large price increases—90% or more—during that time, and a substantial minority of antibiotics had limited competition. Antibiotic prices were negatively associated with manufacturer competition.
Our results are consistent with a recent Government Accountability Office (GAO) analysis of 1441 generic drugs under Medicare Part D. The GAO found an overall 59% price decline from the first quarter of 2010 to the second quarter of 2015, with 315 (22%) demonstrating a price increase of at least 100% [12]. A report by the American Association of Retired Persons similarly found that while overall generic drug prices are decreasing, particular generic drugs have exhibited steep price increases [13].
Sufficient generic competition is a primary reason why prices for most of the antibiotics in our sample were stable [14]. Nearly all of the antibiotics with stable prices were produced by 3 or more manufacturers, and antibiotic prices were negatively associated with the number of manufacturers. This is consistent with data from the FDA, which has shown that while entry of the first generic competitor into a drug market does not significantly decrease the original price, entry of a second generic competitor will decrease the price by one-half, with incremental price decreases resulting from the third (44%), fourth (39%), and fifth (33%) generic competitor [15].
By contrast, 16% of the antibiotics in our sample increased in price by more than 90% and had limited competition. For example, cephalexin 125-mg/5 mL suspension and cephalexin 250-mg/5 mL suspension exhibited price increases of 206% and 165% respectively, while the number of manufacturers for each formulation decreased from 7 to 3. Six of the other antibiotic formulations with substantial price increases were produced by 2 or fewer FDA-approved manufacturers during the entire study period.
Notably, a few drugs sustained price increases despite seemingly sufficient generic competition, as occurred with trimethroprim/sulfamethoxazole (TMP-SMX) 200 mg/5 mL and 40 mg/5 mL suspension and cefuroxime 250-mg and 500-mg tablets. One possible explanation is that despite having multiple FDA-approved manufacturers listed in FDA’s Orange Book, manufacturers exited the market without unregistering their drug. Alternative explanations include higher costs of manufacture due to price increases of key ingredients. Anticompetitive manufacturer practices can also lead to high drug prices. On 14 December 2016, 20 states filed a complaint accusing multiple generic manufacturers of price collusion, which included doxycycline hyclate delayed-release tablet (Doryx) [16].
Substantial price increases that affect commonly used drugs can be harmful to patients, particularly the uninsured and underinsured [17]. For example, it is well known that patients prescribed oral antibiotics are vulnerable to nonadherence [18]. One study found that patients who were prescribed oral antibiotics at hospital discharge for Staphylococcus aureus skin and soft tissue infection had limited adherence, leading to worse clinical outcomes [19]. The price increases of dicloxacillin, cephalexin, and TMP-SMX are concerning given the frequency with which these antibiotics are prescribed for the treatment of suspected methicillin-susceptible S. aureus and methicillin-resistant S. aureus skin and soft tissue infection, and the clinical implications of nonadherence in this patient population.
Policymakers need to help guard against price increases that lead to antibiotic non-adherence, which can be damaging to patients and the public health through the promotion of multidrug-resistant bacteria. One way to do that would be to stabilize the market for generic antibiotics with limited competition. The FDA already expedites generic applications from manufacturers that enter a market with no generic competitors, and this practice was recently extended to markets with 3 or fewer manufacturers [20]. Strategies for accomplishing this goal also include temporary importation of off-patent antibiotics from other well-regulated settings outside the United States in response to price increases [21] and the establishment of formal systems for expediting access to generic manufacturers approved in such settings [22]. The government might also offer long-term purchasing contracts for certain antibiotics, in the same way that it does for pediatric vaccines [23]. The Federal Trade Commission should also closely scrutinize further consolidation among generic drug manufacturers to ensure that sufficient competitors exist to produce essential antibiotics [24]. However, our results show that a focus on increasing the number of generic antibiotic manufacturers will not be sufficient, and additional steps will be needed to ensure that older drugs do not increase in price without a valid reason, such as increased supply chain costs. For example, the state of Maryland recently passed a law intended to prevent large jumps in the price of older, generic drugs by requiring review by the state attorney general and granting judicial authority to reverse the price hike [25].
Our study has several limitations. While we attempted to characterize all oral drugs within the US antibiotic market recommended for use for common conditions, a small number of antibiotics in this category were excluded from our sample due to insufficient pricing data. The NADAC pricing database that we used is only one pricing benchmark, and it is based on voluntary national surveys and may not be representative of all retail pharmacies. Finally, the FDA’s Orange Book manufacturer database only represents FDA approvals and not manufacturer sales. The database may therefore include approved manufacturers that are not actively selling a drug in the United States or manufacturers that have discontinued a drug but did not notify the FDA, which would have led to overestimation of the number of manufacturers.
CONCLUSIONS
Antibiotic prices and the number of manufacturers for commonly prescribed oral, off-patent antibiotics have, overall, been stable in the past few years. However, reduced generic competition was associated with an increase in antibiotic prices, which was particularly noticeable in a subset of these important antibiotics. Unless policymakers take steps to ensure effective competition among generic antibiotic manufacturers, patients will face greater costs, leading to nonadherence, worse patient outcomes, and the possibility of further antibiotic resistance, to the detriment of the public health.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the Laura and John Arnold Foundation, with additional support from the Engelberg Foundation and Harvard Program in Therapeutic Science (to A. S. K.) and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000114 to L. Z.).
Potential conflicts of interest. A. S. K. reports serving as an expert witness on behalf of a class of plaintiffs against Warner-Chilcott for anticompetitive conduct related to marketing of doxycycline hyclate delayed release (2013–2014; case now closed). All remaining authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Department of Health & Human Services. ASPE issue brief: observations on trends in prescription drug spending Available at: https://aspe.hhs.gov/system/files/pdf/187586/Drugspending.pdf. Accessed 8 March 2017.
- 2. Department of Health & Human Services. Understanding recent trends in generic drug prices Available at: https://aspe.hhs.gov/system/files/pdf/175071/GenericsDrugpaperr.pdf. Accessed 10 March 2017.
- 3. Alpern JD, Song J, Stauffer WM. Essential medicines in the United States—why access is diminishing. N Engl J Med 2016; 374:1904–7. [DOI] [PubMed] [Google Scholar]
- 4. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA 2016; 316:858–71. [DOI] [PubMed] [Google Scholar]
- 5. US Food and Drug Administration. Frequently asked questions about drug shortages Available at: https://www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050796.htm - q3. Accessed 21 March 2017.
- 6. Centers for Disease Control and Prevention. Penicillin G benzathine (Bicillin-LA) shortage: message from CDC regarding the recent shortage of benzathine in the United States Available at: https://www.cdc.gov/std/treatment/drugnotices/bicillinshortage.htm. Accessed 20 March 2017.
- 7. Quadri F, Mazer-Amirshahi M, Fox ER et al. Antibacterial drug shortages from 2001 to 2013: implications for clinical practice. Clin Infect Dis 2015; 60:1737–42. [DOI] [PubMed] [Google Scholar]
- 8. Gundlapalli AV, Beekmann SE, Graham DR, Polgreen PM; Infectious Diseases Society of America’s Emerging Infections Network Perspectives and concerns regarding antimicrobial agent shortages among infectious disease specialists. Diagn Microbiol Infect Dis 2013; 75:256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alpern JD, Stauffer WM, Kesselheim AS. High-cost generic drugs—implications for patients and policymakers. N Engl J Med 2014; 371:1859–62. [DOI] [PubMed] [Google Scholar]
- 10. Orange Book. US Food and Drug Adminstration website Available at: https://www.fda.gov/drugs/informationondrugs/ucm129662.htm. Accessed 11 December 2016.
- 11. Centers for Medicare & Medicaid Services. Methodology for calculating the national average drug acquisition cost (NADAC) for Medicaid covered outpatient drugs Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/ful-nadac-downloads/nadacmethodology.pdf. Accessed 10 March 2017.
- 12. Dicken JE. Report to Congressional requesters: generic drugs under Medicare Available at: http://www.gao.gov/assets/680/679022.pdf. Accessed 10 April 2017.
- 13. Schondelmeyer S, Purvis L. Trends in retail prices of generic prescription drugs widely used by older Americans, 2006–2013 AARP. Available at: http://www.aarp.org/content/dam/aarp/ppi/2015/trends-in-retail-prices-of-generic-prescription-drugs-widely-used-by-older-americans.pdf. Accessed 5 June 2017.
- 14. Dave C, Kesselheim AS, Fox E, Hartzema AG. High generic drug prices and market competition levels: a retrospective cohort study Annals of Internal Medicine. 2017; 167(3):145–51. [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration. Generic competition and drug prices Available at: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm129385.htm. Accessed 20 January 2017.
- 16. Thomas K. 20 states accuse generic drug companies of price fixing Available at: https://www.nytimes.com/2016/12/15/business/generic-drug-price-lawsuit-teva-mylan.html. Accessed 20 April 2017.
- 17. Mojtabai R, Olfson M. Medication costs, adherence, and health outcomes among Medicare beneficiaries. Health Aff (Millwood) 2003; 22:220–9. [DOI] [PubMed] [Google Scholar]
- 18. Faure H, Leguelinel-Blache G, Salomon L, Poujol H, Kinowski JM, Sotto A. Assessment of patient adherence to anti-infective treatment after returning home. Med Mal Infect 2014; 44:417–22. [DOI] [PubMed] [Google Scholar]
- 19. Eells SJ, Nguyen M, Jung J, Macias-Gil R, May L, Miller LG. Relationship between adherence to oral antibiotics and postdischarge clinical outcomes among patients hospitalized with Staphylococcus aureus skin infections. Antimicrob Agents Chemother 2016; 60:2941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Food and Drug Administration. Manual of policies and procedures, June 27, 2017 Available at: https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ManualofPoliciesProcedures/UCM407849.pdf.
- 21. Greene JA, Anderson G, Sharfstein JM. Role of the FDA in affordability of off-patent pharmaceuticals. JAMA 2016; 315:461–2. [DOI] [PubMed] [Google Scholar]
- 22. Kesselheim AS, Bollyky TJ. Can Drug Importation Address High Generic Drug Prices? Center for Health Policy at Brookings May 2017. Available at: https://www.brookings.edu/wp-content/uploads/2017/05/wp29_bollykykesselheim_drugimportation.pdf. [Google Scholar]
- 23. Peter G, Marcuse EK, Breiman RF et al. ; The National Vaccine Advisory Committee. Strategies to sustain success in childhood immunizations. JAMA 1999; 282:363–70. [DOI] [PubMed] [Google Scholar]
- 24. Sagonowsky E. The top 15 generic drug makers by 2016 revenue Available at: http://www.fiercepharma.com/special-report/top-15-generic-drugmakers-2016?utm_ medium=nl&utm_source=internal&mrkid=4651002&mkt_tok=eyJpIjoiWVdFNFpEQTFaR001TmpVMiIsInQiOiJEWG9KMmcrUmtz VGJpK21lc09LV3NpTmI2dHBxM1VVYjliWkdmbU8zUDlwcjhJbHF lSStkS3lVV091RVhoMFhwZDJtSlUxUGcyZVZJWDJzUklsTEFRdzdxY21Ldzl1MFVkUTRLU0UwbzQ1T2F5RXBab2M4U2lIa0k1YzdOXC9VUncifQ%3D%3D. Accessed 5 June 2017.
- 25. Duncan I. Maryland General Assembly passes bill aimed at drug ‘price gouging’ Available at: http://www.baltimoresun.com/news/maryland/politics/bs-md-drug-price-gouging-20170410-story.html. Accessed 7 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.