There have been no studies to investigate whether glycemic control is related to tuberculosis infection. In a population-based study with more than 4000 participants and 700 diabetics, several biomarkers for glycemic control modified the relationship between tuberculosis infection and diabetes.
Keywords: Mycobacterium tuberculosis, diabetes, glycemic control, tuberculosis
Abstract
Background
Several cohort studies demonstrate that diabetics are at increased risk for active tuberculosis, and poor glycemic control may exacerbate this risk. A higher prevalence of tuberculosis infection at baseline among diabetics may partially explain these results; however, no population-based studies have investigated this association. Furthermore, whether glycemic control modifies the relationship between diabetes and tuberculosis infection, as it does with active tuberculosis, is unknown.
Methods
Diabetics were diagnosed through physician evaluation and using 3 laboratory tests including hemoglobin A1C (HbA1C), fasting plasma glucose (FPG), or 2-hour plasma glucose (PG). Tuberculosis infection was diagnosed through tuberculin skin tests, and glycemic control was assessed linearly and categorically using recommended targets.
Results
Among 4215 participants, the prevalence of tuberculosis infection was 4.1%, 5.5%, and 7.6% in nondiabetic, prediabetic, and diabetic participants (Ptrend = .012). In multivariate analysis, diabetes was associated with tuberculosis infection (adjusted odds ratio [AOR], 1.5; 95% confidence interval [CI], 1.0–2.2). Compared to nondiabetics, diabetics who were undiagnosed (AOR, 2.2 and 1.2 in diagnosed diabetics), FPG >130 mg/dL (AOR, 2.6 and 1.3 in diabetics with FPG ≤130 mg/dL), or not on insulin (AOR, 1.7 and 0.8 in diabetics on insulin) had elevated tuberculosis infection rates. In a linear dose–response analysis, increasing values of FPG (AOR, 1.02 per 1-mg/dL; 95% CI, 1.01–1.03), PG (AOR, 1.02 per 1-mg/dL; 95% CI, 1.01–1.04), and HbA1C (AOR, 1.13 per 1%; 95% CI, 1.04–1.22) all predicted tuberculosis infection.
Conclusions
Our results suggest glycemic control may modify the relationship between tuberculosis infection and diabetes.
The World Health Organization has set an ambitious target to decrease the incidence of active tuberculosis globally by 90% from 2015 to 2035 [1]. Currently, the incidence of tuberculosis is decreasing at only 1%–2% per year [2]. Individuals with diabetes have a high risk of active tuberculosis, and this has the potential to substantially impact future tuberculosis control efforts [3, 4] because the global prevalence of diabetes is increasing, especially in low-income settings [5] where tuberculosis is endemic. Furthermore, almost half of diabetics are undiagnosed globally, suggesting that this comorbidity’s impact on tuberculosis rates may be substantially higher than is currently known [6]. Despite these ominous signs, tuberculosis control policy that deals with this coepidemic has been hampered by a lack of evidenced-based research, and only conditional recommendations, pending more evidence, have been given by global health organizations [7–9]. A further understanding of the mechanisms that allow diabetes and tuberculosis to interact is necessary in order to develop innovative interventions for preventing and controlling tuberculosis in areas where both diseases are endemic [7, 9].
Several high-quality cohort studies have shown that diabetics are at an increased risk of developing active tuberculosis compared to the general population and that those with poor diabetic control may be especially vulnerable [10–13]. One explanation for these results is that diabetics are more likely, once infected, to develop primary progressive disease or to reactivate an old infection. Alternatively, diabetics in these studies may have a higher underlying prevalence of tuberculosis infection at baseline compared to the general population. However, since tuberculosis infection was not measured in these studies, the cause of this elevated risk is unclear and has not been thoroughly investigated [14]. There have been no population-based studies to investigate whether diabetics have increased susceptibility to tuberculosis infection [15]. Furthermore, whether glycemic control modifies the relationship between diabetes and tuberculosis infection, as it does with active tuberculosis, is unknown [15].
To attempt to inform this knowledge gap we had 2 objectives. First, we investigated whether diabetics were at increased risk for tuberculosis infection in a large, population-based cohort. Second, we examined whether glycemic control modified this relationship.
METHODS
Data Collection and Study Design
The National Health and Nutrition Examination Survey (NHANES) conducts complex multistage probability surveys among a representative sample of the noninstitutionalized civilian population in the United States. We used NHANES data from 2011 through 2012 for all analyses. Data were collected from participants through standardized questionnaires, biological samples, and physical examinations. Participants were interviewed in their homes to ascertain demographic characteristics and at a mobile examination center to ascertain possible risks factors and exposures for various diseases. All participants provided written informed consent for this evaluation.
We included demographic and clinical characteristics from various questionnaires as well as information from tuberculosis and diabetes surveys. Tuberculosis infection and diabetes were evaluated through several laboratory assessments. In 2011–2012, NHANES measured tuberculosis infection using 2 tests: the tuberculin skin test (TST) and the QuantiFERON Gold In-Tube test (QFT-GIT). The TST was administered on the forearm using purified protein derivative (Tubersol, Sanofi, Bridgewater, New Jersey). Trained health workers measured the TST induration reaction 46–76 hours after administration. The QFT-GIT was administered on the same day as the TST and was performed following manufacturer guidelines.
Several diabetes diagnostic tests were used. During the home visit, participants reported whether they had a physician-based diabetes diagnosis. During laboratory testing, hemoglobin A1C (HbA1c) was administered and measured on all eligible participants. Fasting plasma glucose (FPG) and 2-hour plasma glucose (PG) tests were performed on all participants examined in a morning session after a 9-hour fast. Participants were randomly assigned to a morning examination session (or an afternoon or evening session); therefore, only a random subset of eligible participants took these 2 tests. The oral glucose tolerance test was administered using a calibrated dose of 75 g of glucose and a venipuncture 2 hours later.
Definitions
Diabetes and Prediabetes
We defined diabetes through criteria given by the American Diabetes Association [16] and the World Health Organization [17, 18] and using methods from recent diabetes prevalence surveys [19, 20]. During the home visit, participants self-reported whether they had a physician-based diabetes diagnosis. Participants who responded positively to this question were classified as diagnosed diabetes; undiagnosed diabetes was defined as participants reporting never previously diagnosed with diabetes but with 1 of the following test results: HbA1C ≥6.5%, FPG ≥126 mg/dL, or PG ≥200 mg/dL.
Using the same guidelines, we performed a similar process to diagnose prediabetes for all individuals who did not meet the diabetes criteria listed above. Prediabetics had 1 of the following: previously told by a physician they had prediabetes, HbA1C 5.7%–6.4%, FPG 100–125 mg/dL, or PG 140–199 mg/dL.
Glycemic Control and Diabetes Severity
Glycemic control was assessed based on glycemic targets for diabetic patients set by the American Diabetes Association in 2016 [21]. Cutoffs used to classify poor glycemic control were HbA1c ≥7% and FPG >130 mg/dL. We also examined the dose–response relationship between tuberculosis infection and each of the 3 laboratory diagnostic methods (HbA1c, FPG, PG) linearly. We fit a logistic regression model testing the probability of tuberculosis infection for every 1-mg/dL increase in FPG or PG and for every 1% increase of HbA1c.
Diabetes severity was evaluated using answers from several sociodemographic questionnaires administered by NHANES. Survey-based methods used as proxies for disease severity included undiagnosed diabetes, time since diabetes diagnosis, current insulin use, and use of diabetic pills to lower blood sugar.
Tuberculosis Infection
In NHANES 2011–2012, both TST and QFT-GIT were administered to participants in order to assess tuberculosis infection. The sensitivity of the QFT-GIT in diabetic patients is debated, and studies have been inconsistent [22–24]. Because of this, we used the TST in our primary analysis to diagnose tuberculosis infection. Tuberculosis infection was defined as an induration ≥10 mm, as seen in prior NHANES studies [25, 26]. To assess the robustness of our results, we conducted a sensitivity analyses using the QFT-GIT. To provide proper validation, we included only participants who took both the TST and QFT-GIT in all analyses. A QFT-GIT was considered positive when the tuberculosis antigen minus nil value was ≥0.35 IU/mL, with the tuberculosis response ≥25% of the nil value and the nil value ≤8.0 IU/mL, per guidelines [27].
Statistical Analytical Plan
Statistical weights were used in all prevalence and modeling analyses to account for unequal probabilities of selection and nonresponse and thus provide estimates representative of the civilian, noninstitutionalized US population. The weighted prevalence of tuberculosis infection was estimated using standard 2 × 2 contingency tables and was stratified by demographic and clinical participant characteristics. Proportions were compared with Pearson χ2 statistics with a Rao and Scott second-order correction using an F statistic with noninteger degrees of freedom, accounting for the survey design [28, 29]. A binary logistic regression model was used to calculate odds ratios (ORs), and these were compared by demographic and risk factors in univariate and multivariate analyses. Variables suggestive of an association with tuberculosis infection (P < .2) and those shown to have an association in prior studies were included in multivariate analyses. Cochran-Armitage tests were used to test for trends between multiple categories in 1 variable.
We calculated the prevalence of tuberculosis infection for each measurement method of glycemic control and disease severity and then compared the corresponding prevalence of tuberculosis infection in nondiabetic participants. Univariate and multivariate logistic regression models were then constructed to determine whether effect modification was present. Statistical significance was assessed using 95% confidence intervals (CIs) in all models. Data were analyzed using Stata version 14.1 statistical software.
RESULTS
Study Population and Diabetes Prevalence
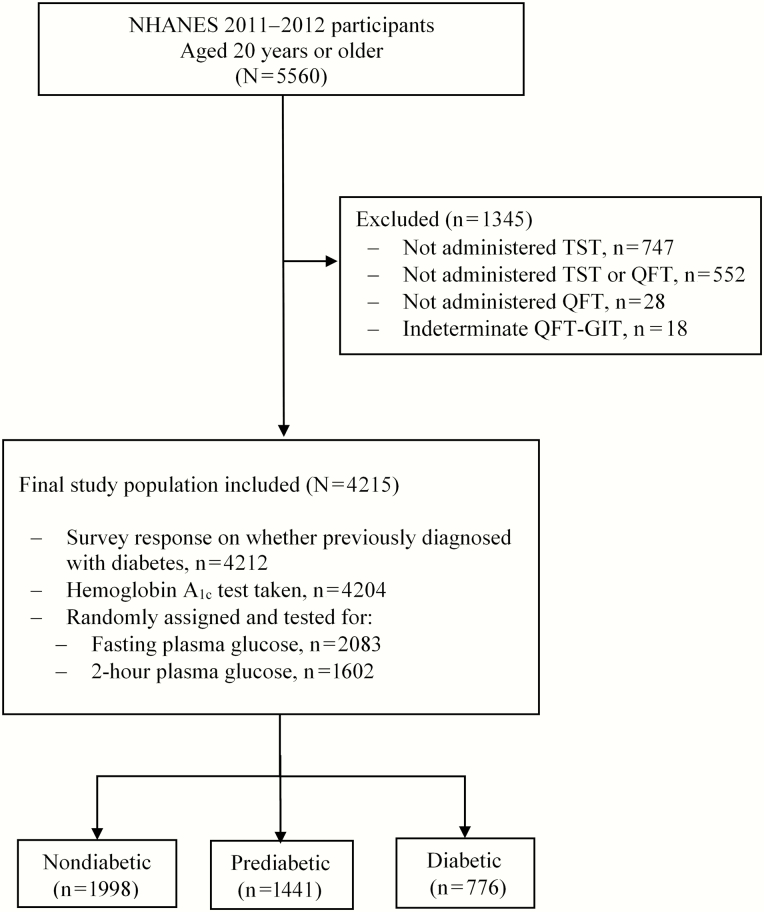
Participants aged ≥20 years were included (n = 5560). We excluded participants who only took the TST (n = 28), only took the QFT-GIT (n = 747), or did not take either test (n = 552). We also excluded participants with an indeterminate QFT-GIT (n = 18). This left 4215 participants for the final analysis (Figure 1).
Figure 1.
Eligibility and enrollment of included participants. Abbreviations: NHANES, National Health and Nutrition Examination Survey; QFT-GIT, QuantiFERON Gold In-Tube test TST, tuberculin skin test.
The prevalence of diagnosed diabetes in our study population was 9.2% (95% CI, 7.9–10.7; N = 535), 4.0% (95% CI, 3.3–4.9; N = 241) for undiagnosed diabetes, and 33.7% (95% CI, 31.0–36.4; N = 1441) for prediabetes (Table 1). Among diabetic patients, 30.4% (95% CI, 25.6–35.6) were undiagnosed. Diabetics were more likely to be older, have a lower education level, and have a high body mass index (P < .01). There was a statistically increasing trend between nondiabetic, prediabetic, and diabetic patients in regard to their median PG, FPG, and HbA1c (all Ptrend < .01).
Table 1.
Demographic and Clinical Characteristics of Adults Aged ≥ 20 Years in the United States, 2011–2012, Stratified by Diabetes Status
| Variable | Nondiabetic, % (n) | Prediabetic, % (n) | Diabetic, % (n) | All Participants, % (n) | P Value a |
|---|---|---|---|---|---|
| N | 53.1 (1998) | 33.7 (1441) | 13.2 (776) | 100 (4215) | |
| Demographic characteristics | |||||
| Age group, y | |||||
| 20–29 | 27.6 (586) | 9.4 (141) | 1.7 (16) | 18.0 (743) | <.0001 |
| 30–39 | 21.7 (463) | 14.3 (228) | 7.4 (49) | 17.3 (740) | |
| 40–49 | 20.1 (343) | 20.1 (258) | 16.0 (107) | 19.6 (708) | |
| ≥50 | 30.6 (606) | 56.2 (814) | 74.9 (604) | 45.1 (2024) | |
| Sex | |||||
| Male | 44.1 (904) | 52.4 (777) | 49.8 (394) | 47.6 (2075) | .002 |
| Female | 55.9 (1094) | 47.6 (664) | 50.1 (382) | 52.4 (2140) | |
| Birthplace | |||||
| United States | 83.4 (1437) | 84.4 (1029) | 79.9 (528) | 83.3 (2994) | .124 |
| Foreign | 16.6 (560) | 15.7 (411) | 20.1 (248) | 16.7 (1219) | |
| Education | |||||
| Beyond high school | 69.6 (1258) | 60.2 (739) | 46.6 (327) | 63.4 (2324) | <.0001 |
| High school | 17.7 (378) | 22.1 (350) | 26.0 (177) | 20.3 (905) | |
| Less than high school | 12.7 (362) | 17.7 (351) | 27.4 (271) | 16.3 (984) | |
| Missing | |||||
| Household tuberculosis contact | |||||
| Yes | 2.1 (54) | 3.5 (56) | 3.4 (35) | 2.8 (145) | .1168 |
| No | 97.9 (1933) | 96.5 (1374) | 96.6 (738) | 97.3 (4045) | |
| Smoking | |||||
| Current | 17.9 (392) | 23.1 (326) | 17.4 (133) | 19.6 (851) | .0067 |
| Former | 22.6 (375) | 24.2 (354) | 32.3 (248) | 24.4 (977) | |
| Never | 59.5 (1231) | 52.3 (761) | 50.3 (395) | 55.6 (2387) | |
| Family size | |||||
| Fewer than 3 | 51.2 (943) | 54.7 (714) | 60.1 (438) | 53.5 (2095) | .045 |
| 3–5 | 42.7 (871) | 38.9 (604) | 32.9 (259) | 40.1 (1734) | |
| 6 or more | 6.2 (184) | 6.5 (123) | 7.0 (79) | 6.4 (386) | |
| Clinical characteristics | |||||
| Median BMI (IQR), kg/m2 | 26.3 (23.0–30.2) | 28.6 (24.9–33.1) | 31.1 (26.8–36.4) | 27.8 (24.1–32.5) | <.0001 |
| BMI, kg/m2 | |||||
| <18.5 | 2.3 (57) | 0.9 (18) | 0.6 (3) | 1.6 (78) | <.0001 |
| 18.5–24.9 | 35.5 (745) | 22.5 (340) | 12.6 (113) | 28.1 (1198) | |
| 25.0–29.9 | 35.6 (649) | 35.3 (488) | 25.1 (216) | 34.2 (1353) | |
| ≥30.0 | 26.6 (523) | 41.2 (583) | 61.8 (431) | 36.1 (1537) | |
| Median 2-hour plasma glucose (IQR), mg/dLb | 94 (78–108) | 117 (96–141) | 223 (201–261) | 108 (88–135) | <.0001 |
| Median fasting plasma glucose (IQR), mg/dLb | 91.5 (87–95) | 103 (99–108) | 129 (113–163) | 100 (92–110) | <.0001 |
| Median hemoglobin A1c (IQR), %b | 5.3 (5.1–5.5) | 5.8 (5.6–6.0) | 6.7 (6.2–7.8) | 5.5 (5.2–5.9) | <.0001 |
| Hepatitis B, surface antigen | |||||
| Positive | 0.3 (14) | 0.4 (14) | 0.2 (4) | 0.3 (32) | .5246 |
| Negative | 99.7 (1965) | 99.6 (1414) | 99.8 (755) | 99.7 (4134) | |
| Hepatitis C, antibody | |||||
| Positive | 1.1 (29) | 1.9 (28) | 3.4 (24) | 1.7 (18) | .168 |
| Negative | 98.9 (1943) | 98.1 (1395) | 96.6 (734) | 98.3 (4072) | |
Abbreviations: BMI, body mass index; IQR, interquartile range.
Data are weighted percentages of participants in each subsample. The absolute number of participants is shown in parentheses. The absolute number of participants does not perfectly correspond to percentages because the percentages are weighted, adjusting for the survey design. Percentages may not total 100% because within column percentages were rounded to the nearest integer. Column totals vary across different characteristics because of missing values for some participants.
a P value is an F statistic with noninteger degrees of freedom by using a second-order Rao and Scott correction, taking into account the survey design. This P value is based on Pearson χ2 statistic for 2-way contingency tables.
bNot every participant took all 3 tests.
Prevalence of Tuberculosis Infection
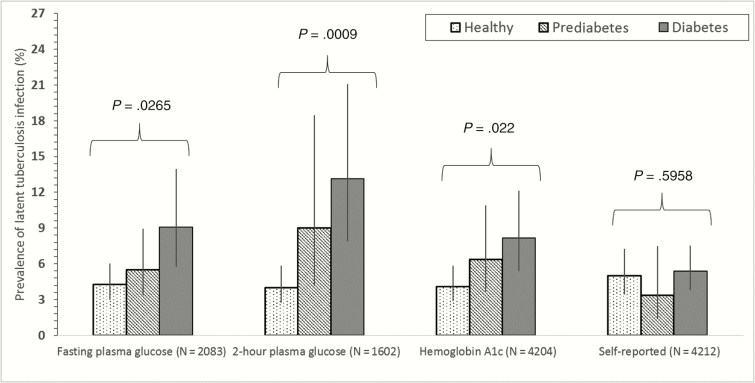
The overall weighted prevalence of tuberculosis infection was 5.0% (95% CI, 3.5–7.1; N = 404 infected; Table 2). Tuberculosis infection prevalence was elevated in individuals who did not complete high school (11.3% infected), individuals who were in household contact with a tuberculosis case (14.6% infected), or individuals who were in a household with 6 or more people (10.4% infected). Tuberculosis infection prevalence was 4.1%, 5.5%, and 7.6% in nondiabetic, prediabetic, and diabetic participants (Ptrend = .012). When we stratified by the diagnostic method used to detect diabetes (Figure 2), there was an increasing prevalence of tuberculosis infection from nondiabetic, prediabetic, and diabetic patients for FPG (Ptrend = .0265), PG (Ptrend = .0009), and HbA1c (Ptrend = .022) tests but not for self-reported diabetes (Ptrend = .5958).
Table 2.
Risk Factors for Tuberculosis Infection Among Adults Aged ≥ 20 Years in the United States, 2011–2012
| Variable | Total N a | Prevalence of Tuberculosis Infection (95% CI)a | Crude Odds Ratio (95% CI)a |
|---|---|---|---|
| N | 4215 | 5.0 (3.5–7.1) | – |
| Demographic characteristics | |||
| Age group, y | |||
| 20–29 | 406 | 3.4 (1.8–6.5) | 1 (Referent) |
| 30–39 | 1430 | 5.2 (3.3–8.2) | 1.7 (1.0–3.1) |
| 40–49 | 1470 | 5.8 (4.0–8.3) | 1.6 (1.1–2.3) |
| ≥50 | 909 | 3.8 (2.5–5.7) | 1.5 (0.9–2.4) |
| Sex | |||
| Male | 2075 | 5.4 (3.7–7.9) | 1 (Referent) |
| Female | 2140 | 4.6 (3.3–6.5) | 0.8 (0.7–1.08) |
| Birthplace | |||
| United States | 2994 | 1.8 (1.0–3.1) | 1 (Referent) |
| Foreign | 1219 | 21.2 (15.8–27.7) | 14.9 (8.0–27.7) |
| Education | |||
| Beyond high school | 2324 | 3.5 (2.6–4.8) | 1 (Referent) |
| High school | 905 | 4.6 (2.9–7.4) | 1.3 (0.9–2.0) |
| Less than high school | 984 | 11.3 (7.4–16.9) | 3.5 (2.5–4.9) |
| Household tuberculosis contact | |||
| No | 4045 | 4.7 (3.3–6.6) | 1 (Referent) |
| Yes | 145 | 14.6 (8.2–24.6) | 3.5 (1.9–6.2) |
| Cigarette smoking status | |||
| Never | 2387 | 5.0 (3.4–7.2) | 1 (Referent) |
| Former | 977 | 4.8 (3.3–7.0) | 1.0 (0.6–1.5) |
| Current | 851 | 5.4 (3.1–9.3) | 1.1 (0.7–1.8) |
| Family size | |||
| Fewer than 3 | 2095 | 3.2 (2.1–4.8) | 1 (Referent) |
| 3–5 | 1734 | 6.6 (4.2–10.4) | 2.2 (1.3–3.8) |
| 6 or more | 386 | 10.4 (6.5–16.1) | 3.6 (1.9–6.8) |
| Clinical characteristics | |||
| 2-hour plasma glucose, mg/dLb | 1602 | – | 1.0 (1.0–1.1) |
| Fasting plasma glucose, mg/dLb | 2083 | – | 1.0 (1.0–1.0) |
| Hemoglobin A1c, %b | 4202 | – | 1.2 (1.1–1.3) |
| Diabetes status | |||
| Nondiabetic | 1998 | 4.1 (2.9–5.6) | 1 (Referent) |
| Prediabetic | 1441 | 5.5 (3.2–9.3) | 1.4 (0.9–2.1) |
| Diabetic | 776 | 7.6 (5.8–10.0) | 2.0 (1.5–2.6) |
| BMI, kg/m2 (continuous) | 4215 | – | 0.99 (0.97–1.01) |
| BMI, kg/m2 | |||
| <18.5 | 78 | 6.4 (2.3–16.8) | 1.2 (0.4–3.8) |
| 18.5–24.9 | 1198 | 5.3 (3.5–7.8) | 1 (Referent) |
| 25.0–29.9 | 1353 | 4.8 (3.4–6.8) | 0.9 (0.7–1.3) |
| ≥30 | 1537 | 5.1 (3.2–7.9) | 1.0 (0.6–1.5) |
| Hepatitis B, surface antigen | |||
| Negative | 4134 | 5.0 (3.5–7.0) | 1 (Referent) |
| Positive | 32 | 21.3 (7.5–47.2) | 5.2 (1.4–18.9) |
| Hepatitis C, antibody | |||
| Negative | 4072 | 5.0 (3.5–7.0) | 1 (Referent) |
| Positive | 81 | 8.9 (2.4–27.5) | 1.9 (0.5–7.2) |
Abbreviations: BMI, body mass index; CI, confidence interval
The tuberculin skin test was used to measure tuberculosis infection; a positive test was defined as a skin induration reaction ≥10 mm 46–76 hours after application of the test. A separate analysis was conducted using the QuantiFERON Gold In-Tube test and is included in Supplementary Materials Table E1.
aData are weighted prevalence percentages of participants in each subsample and odds ratio (95% confidence interval). The absolute number of participants from each characteristic can be seen on the left hand side.
bNot all participants took all 3 tests. FPG and PG tests were performed in morning sessions after a 9-hour fast. Participants were randomlyassigned to a morning examination session (or afternoonor evening session); therefore, only a random subset of eligibleparticipants took these 2 tests.
Figure 2.
Prevalence of tuberculosis infection by the diagnostic method used to diagnose diabetes mellitus. Vertical lines represent 95% confidence intervals. P values represent the presence of a trend after accounting for the survey design.
In a multivariate analysis controlling for age, sex, birthplace, household tuberculosis contact, cigarette smoking status, and family size, diabetes was significantly associated with tuberculosis infection (adjusted OR [AOR], 1.5; 95% CI, 1.0–2.2; Table 3). Tuberculosis infection was also associated with current smoking (AOR, 1.7; 95% CI, 1.0–2.8 compared to never smokers), household tuberculosis contact (AOR, 3.2; 95% CI, 1.5–6.9), foreign-born status (AOR, 14.9; 95% CI, 8.5–26.3 compared to US born), a family size ≥6 (AOR, 1.8; 95% CI, 1.0–3.4 compared to <3 family members), and being aged ≥50 years (AOR, 1.7; 95% CI, 1.0–2.9 compared to 20–29 years). In a separate multivariate model, adding time since arrival in the United States, the association seen among tuberculosis infection and diabetes remained unchanged (AOR, 1.5; 95% CI, 1.0–2.3).
Table 3.
Multivariate Logistic Regression Models of Risk Factors for Tuberculosis Infection in the United States, 2011–2012
| Variable | Adjusted Odds Ratio (95% Confidence Interval)a | P Value b |
|---|---|---|
| Age group, y | ||
| 20–29 | 1 (Referent) | |
| 30–39 | 1.2 (0.6–2.3) | .54 |
| 40–49 | 1.2 (0.8–1.0) | .31 |
| ≥50 | 1.7 (1.0–2.9) | .05 |
| Sex | ||
| Male | 1 (Referent) | |
| Female | 0.9 (0.7–1.3) | .85 |
| Birthplace | ||
| United States | 1 (Referent) | |
| Foreign | 14.9 (8.5–26.3) | <.01 |
| Household tuberculosis contact | ||
| No | 1 (Referent) | |
| Yes | 3.2 (1.5–6.9) | <.01 |
| Cigarette smoking status | ||
| Never | 1 (Referent) | |
| Former | 1.2 (0.8–1.9) | .43 |
| Current | 1.7 (1.0–2.8) | .04 |
| Family size | ||
| Fewer than 3 | 1 (Referent) | |
| 3–5 | 1.6 (0.9–2.7) | .07 |
| 6 or more | 1.8 (1.0–3.4) | .05 |
| Diabetes status | ||
| Nondiabetic | 1 (Referent) | |
| Prediabetes | 1.3 (0.8–2.1) | .28 |
| Diabetes | 1.5 (1.0–2.2) | .04 |
aData are weighted odds ratio (95% confidence interval [CI]) adjusting for the survey design. The model is adjusted for participant age, gender, household contact with a tuberculosis case, birthplace (United States or foreign born), smoking status, family size, and diabetes status. In a separate multivariate model, adding time since arrival in the United States, the association seen among tuberculosis infection, and diabetes remained unchanged (adjusted odds ratio, 1.5; 95% CI, 1.0–2.3). A sensitivity analysis was conducted using the QuantiFERON Gold In-Tube test and is included in Supplementary Materials Table E1.
b P value is an F statistic with noninteger degrees of freedom by using a second-order Rao and Scott correction, taking into account the survey design.
Diabetes Severity and Tuberculosis Infection
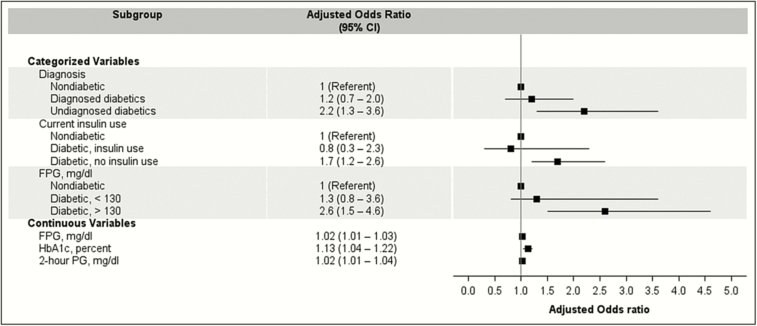
When we stratified diabetes by several distinct markers of glycemic and diabetic control (Figure 3), some characteristics modified the relationship between diabetes and tuberculosis infection. In both univariate and multivariate analyses, increasing values for HbA1c (AOR, 1.13 per 1% increase in HbA1c; 95% CI, 1.04–1.22), FPG (AOR, 1.02 per 1-mg/dL increase in FPG; 95% CI, 1.01–1.03), and PG test (AOR, 1.02 per 1-mg/dL increase in PG; 95% CI, 1.01–1.04) results predicted tuberculosis infection (Figure 3). After categorizing these laboratory tests by glycemic control targets for diabetic patients, diabetic patients with an FPG >130 mg/dL had a higher prevalence of tuberculosis infection compared to diabetics with an FPG ≤130 mg/dL and nondiabetics (9.9% vs 6.5% and 4.1%). In a multivariate model, diabetics with an FPG >130 mg/dL continued to have a significantly elevated risk of tuberculosis infection (AOR, 2.6; 95% CI, 1.5–4.6) compared to nondiabetics, while diabetics with an FPG ≤130 mg/dL (AOR, 1.3; 95% CI, 0.8–3.6) were not at increased risk. In univariate analysis, diabetic patients with HbA1c test results ≥7% were at an increased tuberculosis infection risk (9.1% prevalence in diabetics with HbA1c ≥7%, 6.7% in diabetics with HbA1c <7%, and 4.1% in nondiabetics; Ptrend = .003). However, these differences were no longer present in a multivariate model (compared to nondiabetic reference; AOR, 1.7, 95% CI, 0.9–3.0 for diabetics with HbA1c ≥7% and AOR, 1.5, 95% CI, 0.9–2.5 for diabetics with HbA1c <7%).
Figure 3.
Adjusted logistic regression analysis of tuberculosis infection after stratification by several survey- and laboratory-based proxies of diabetes severity. Data are weighted odds ratios with 95% confidence intervals. Adjusted odds ratios for continuous variables are shown per unit increase (1-mg/dL increase in FPG or PG and for every 1% increase of HbA1C). Diabetic participants were classified as “diagnosed” if they self-reported having been previously diagnosed by a physician or health professional. Undiagnosed diabetics were classified as those who reported not previously been diagnosed with diabetes but had a HbA1C ≥6.5%, an FPG ≥126 mg/dL, or PG ≥200 mg/dL test result. Prediabetics are not shown for categorized variables. Each model is adjusted for participant age, gender, birthplace (United States or foreign born), and smoking status. Abbreviations: CI, confidence interval; HbA1c, hemoglobin A1c; FPG, fasting plasma glucose; PG, 2-hour plasma glucose.
Among survey-based proxies for diabetic control, high rates of tuberculosis infection were seen in diabetics who were undiagnosed (12.9% vs 5.4% in diagnosed cases and 4.1% in nondiabetic individuals) or were not currently taking insulin (8.8% prevalence vs 3.3% in diabetics currently taking insulin and 4.1% in nondiabetic individuals). In a multivariate model (Figure 3), undiagnosed diabetics (AOR, 2.2; 95% CI, 1.3–3.6) and diabetic patients not currently taking insulin (AOR, 1.7; 95% CI, 1.2–2.6) remained associated with tuberculosis infection, but diagnosed diabetics (AOR, 1.2; 95% CI, 0.7–2.0) and those taking insulin (AOR, 0.8; 95% CI, 0.3–2.3) were not. Other markers for disease severity, such as the time since diabetes diagnosis or diabetic pill use, did not modify the relationship between diabetes and tuberculosis infection.
Sensitivity Analysis
When we used the QFT-GIT, the prevalence of tuberculosis infection in the study population was 5.6% (95% CI, 4.6–6.7). Test agreement and kappa values were 90.6% and 0.46, respectively. The prevalence of tuberculosis infection was 4.4% (95% CI, 3.5–5.4), 5.5% (95% CI, 4.2–7.3), and 10.5% (95% CI, 7.4–10.5) in nondiabetic, prediabetic, and diabetic participants (Ptrend = .0001; Supplementary Materials Table E1). After multivariate adjustment, diabetics were 1.7 times (95% CI, 1.0–2.9) more likely to have tuberculosis infection compared to nondiabetic participants (Supplementary Materials Table E2). When we stratified by the diagnostic method used to detect diabetes (Supplementary Materials Figure E1), there was an increasing prevalence of tuberculosis infection from nondiabetic, prediabetic, and diabetic patients for PG (Ptrend = .0088), HbA1c (Ptrend = .0003), and self-report (Ptrend = .0037), as well as a suggestive trend for FPG (Ptrend = .0987).
DISCUSSION
In this population-based sample with more than 4000 participants and 700 diabetics, we found that several biomarkers, both categorical and linear, of glycemic control modified the relationship between tuberculosis infection and diabetes. Several cohort studies have demonstrated that diabetics with poor diabetic control have especially high risk for active tuberculosis [10, 12, 13]. Our results suggest these diabetics may also have increased susceptibility to tuberculosis infection.
In 3 important studies, poorly controlled diabetes (measured through HbA1c ≥7% [12], FPG >130 mg/dL [13], and diabetes complications [10]) was associated with active tuberculosis in diabetics. To our knowledge, we are the first to investigate whether glycemic control also modifies the relationship between diabetes and tuberculosis infection. We found that diabetics with poorly controlled disease (as measured by an FPG >130 mg/dL, undiagnosed diabetes, or those not using insulin) had especially high rates of tuberculosis infection. We also found the odds of tuberculosis infection significantly increased for every 1-mg/dL increase in FPG or PG and for every 1% increase in HbA1c. This corresponds well with results from a recent large study from Taiwan that showed an increasing hazard for active tuberculosis for every 10-mg/dL increase in FPG (AOR, 1.1 [95% CI, 1.0–1.1]) [13]. We suggest part of the risk for active tuberculosis among diabetics seen in many studies [11] may relate to a higher rate for tuberculosis infection, especially among those with poorly controlled glucose levels. However, our study was not longitudinal and we did not measure active tuberculosis. Therefore, we cannot state the contributory role of primary progressive disease in this process. Future cohort studies estimating the risk of active tuberculosis among diabetic patients should measure and control for tuberculosis infection at baseline.
In a recent metaanalysis in which AORs from 13 observational studies were pooled, a slightly higher, but significant, rate of tuberculosis infection among diabetics was found [15]. However, only 2 included studies showed a statistically higher rate of tuberculosis infection among diabetics. There may be several reasons for the differing results from our study. First, since NHANES performs comprehensive laboratory testing, we identified diabetics using physician diagnosis, HbA1C, PG, and FPG tests. Past studies identified diabetics through medical records or self-report [15]. Only 1 study [30] used a combination of self-report and laboratory testing to diagnose diabetics. These diagnostic definitions may substantially underestimate diabetes [6, 16, 31]. In a recent metaanalysis of more than 300000 diabetics, the sensitivity of HbA1C to detect undiagnosed diabetes was reported to be 52.8% and 32.7% compared to FPG and PG [32]. We found that undiagnosed diabetics had especially high rates of tuberculosis infection, and this may influence previous study results if these patients are misclassified. Second, many of these studies had sample sizes with few overall participants or few diabetics, leading to low statistical power when tuberculosis infection was analyzed. Last, all prior studies were conducted in high-risk populations (tuberculosis contacts, refugees, hemodialysis patients, or patients with human immunodeficiency virus) [15] that are unlikely to be representative of the general population.
The mechanisms that underlie the association between tuberculosis infection and diabetes seen in this study are unclear and cannot be definitively ascertained from the epidemiological study design used here. Previously, researchers have postulated that this interaction may be influenced by impairment of innate or adaptive immunity through diabetes-induced dysglycemia [30, 33]. In addition, human leukocyte antigen-DQ β gene alleles (especially at codon 57) may enhance the risk for tuberculous infection and disease progression and confer concurrent risk of diabetes mellitus [34, 35]. Regardless of the cause of this increased risk, policy on screening of tuberculosis infection in diabetic patients should be discussed. Currently, screening of diabetic patients for tuberculosis infection is not recommended by global health organizations [8]. Our study results must be confirmed in further studies, and cost-effectiveness analyses are still needed. However, screening tuberculosis infection in diabetic patients with poor glycemic control may provide an opportunity to effectively target a high-risk population for tuberculosis transmission and primary progressive disease [10, 12, 13] while also limiting expended resources to manageable levels for tuberculosis control programs from low-income settings. Additional research is needed to ascertain the proportion of tuberculosis disease that is attributable to increased tuberculosis infection. In a recent study [36], researchers found that compared to tuberculosis contacts, self-reported diabetics had lower prevalence of interferon-gamma positivity but a higher rate of tuberculosis disease, suggesting that although diabetes may be a risk factor for both tuberculosis infection and disease, the increased risk of disease progression may be especially potent.
There are limitations to our analyses. First, the prevalence of tuberculosis infection increases with age [25, 37, 38], and we cannot distinguish whether participants acquired tuberculosis infection before or after developing diabetes. Although reverse causality may have occurred in our study, we believe this is unlikely for 2 reasons: (1) tuberculosis infection is unlikely to cause an increased risk of diabetes development; (2) we found a dose-response relationship between tuberculosis infection and differing severities of diabetes. Second, like all observational studies, unmeasured and residual confounding is always a concern. Third, a repeat measurement after a single positive HbA1c, FPG, or PG result is recommended to confirm diagnosis [16]. Since NHANES participants only have 1 study visit, this was not possible and diabetes overdiagnosis is possible [19]. However, if present, this bias would bring the association between tuberculosis infection and diabetes toward the null and therefore our estimates may be conservative. Lastly, tuberculosis infection rates were low, limiting our statistical power to assess some markers of diabetes severity.
In conclusion, in a large population-based study, we found that diabetic patients had elevated rates of tuberculosis infection, and diabetics with poor glycemic control modified this relationship.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. Conception and/or design: L. M.; data analyses: L. M.; interpretation of study results: L. M., L. Z., M. E. C., B. D. H., Q. L., C. C., C. C. W.; writing of first draft: L. M.; subsequent drafting of manuscript for important intellectual content: L. M., L. Z., M. E. C., B. D. H., Q. L., C. C., C. C. W.; review and edit of manuscript: L. M., L. Z., M. E. C., B. D. H., Q. L., C. C., C. C. W.; and approval of final version of the manuscript: L. M., L. Z., M. E. C., B. D. H., Q. L., C. C., C. C. W.
Acknowledgments. We thank the research groups, Epidemiology in Action (EIA), and the Jiangsu Province Tuberculosis Control Division at China’s Centers for Disease Control and Prevention (CDC) for their useful and instructive comments on presentations of this project. Members of EIA include Anita Nsubuga, Srijita Chakraburty, Robert Kakaire, Jane Mutanga, Simon Mutembo, and Andreas Handel. Members of the Jiangsu Province Tuberculosis Control Division at China’s CDC include Yan Shao, Honghuan Song, Guoli Li, Yan Li, and Wei Lu. Last, L. M. thanks Matías Straub Martinez, Manuela Straub Martinez, Nicolás Straub Martinez, Penelope Thomas-Martinez, and Paloma Thomas-Martinez for their constant support.
Financial support. L. M. and C. C. W. were supported in part by an investigator initiated grant (AI 093856) and a diversity supplement grant (3R01AI093856-05W1) from the National Institutes of Allergy and Infectious Diseases. M. E. C. was supported by the Schlumberger Foundation Faculty for the Future Fellowship.
Disclaimer. The funders had no involvement in the design, collection, analysis, or interpretation of the data; in writing the report; or in the decision to submit.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Raviglione M, Director GT. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva, World Health Organization; 2013. [Google Scholar]
- 2. Raviglione M, Marais B, Floyd K et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012; 379:1902–13. [DOI] [PubMed] [Google Scholar]
- 3. Odone A, Houben RM, White RG, Lönnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014; 2:754–64. [DOI] [PubMed] [Google Scholar]
- 4. Pan HQ, Bele S, Feng Y et al. Analysis of the economic burden of diagnosis and treatment of tuberculosis patients in rural China. Int J Tuberc Lung Dis 2013; 17:1575–80. [DOI] [PubMed] [Google Scholar]
- 5. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract 2014; 103:150–60. [DOI] [PubMed] [Google Scholar]
- 7. Harries A, Satyanarayana S, Kumar A et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review. Public Health Action 2013; 3(Supp 1):S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes. 2011. [PubMed] [Google Scholar]
- 9. Harries AD, Kumar AM, Satyanarayana S et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg 2016; 110:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker MA, Lin H-H, Chang H-Y, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis 2012; 54:818–25. [DOI] [PubMed] [Google Scholar]
- 11. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leung CC, Lam TH, Chan WM et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol 2008; 167:1486–94. [DOI] [PubMed] [Google Scholar]
- 13. Lee PH, Fu H, Lai TC, Chiang CY, Chan CC, Lin HH. Glycemic control and the risk of tuberculosis: a cohort study. PLoS Med 2016; 13:e1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009; 9:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee M-R, Huang Y-P, Kuo Y-T et al. Diabetes mellitus and latent tuberculosis infection: a systemic review and meta-analysis. Clin Infect Dis 2017; 64:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2016; 39(Supp 1):S13–22. [DOI] [PubMed] [Google Scholar]
- 17. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15:539–53. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. 2011. [PubMed] [Google Scholar]
- 19. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314:1021–9. [DOI] [PubMed] [Google Scholar]
- 20. Xu Y, Wang L, He J et al. ; 2010 China Noncommunicable Disease Surveillance Group Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310:948–59. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association. 5. Glycemic targets. Diabetes Care 2016; 39:S39–46. [DOI] [PubMed] [Google Scholar]
- 22. Walsh MC, Camerlin AJ, Miles R et al. The sensitivity of interferon-gamma release assays is not compromised in tuberculosis patients with diabetes. Int J Tuberc Lung Dis 2011; 15:179–84, i–iii. [PMC free article] [PubMed] [Google Scholar]
- 23. Faurholt-Jepsen D, Aabye MG, Jensen AV et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand J Infect Dis 2014; 46:384–91. [DOI] [PubMed] [Google Scholar]
- 24. Choi JC, Jarlsberg LG, Grinsdale JA et al. Reduced sensitivity of the QuantiFERON(®) test in diabetic patients with smear-negative tuberculosis. Int J Tuberc Lung Dis 2015; 19:582–8. [DOI] [PubMed] [Google Scholar]
- 25. Mancuso JD, Diffenderfer JM, Ghassemieh BJ, Horne DJ, Kao TC. The prevalence of latent tuberculosis infection in the United States. Am J Respir Crit Care Med 2016; 194:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghassemieh BJ, Attia EF, Koelle DM, Mancuso JD, Narita M, Horne DJ. Latent tuberculosis infection test agreement in the national health and nutrition examination survey. Am J Respir Crit Care Med 2016; 194:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25. [PubMed] [Google Scholar]
- 28. Rao JN, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 1984; 12:46–60. [Google Scholar]
- 29. Rao JN, Scott AJ. The analysis of categorical data from complex sample surveys: chi-squared tests for goodness of fit and independence in two-way tables. J Am Stat Assoc 1981; 76:221–30. [Google Scholar]
- 30. Hensel RL, Kempker RR, Tapia J, Oladele A, Blumberg HM, Magee MJ. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int J Tuberc Lung Dis 2016; 20:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cowie CC, Rust KF, Byrd-Holt DD et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010; 33:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NCD Risk Factor Collaboration. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331 288 participants. Lancet Diabetes Endocrinol 2015; 3:624–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stevenson CR, Critchley JA, Forouhi NG et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn 2007; 3:228–45. [DOI] [PubMed] [Google Scholar]
- 34. Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014; 44:617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Delgado JC, Baena A, Thim S, Goldfeld AE. Aspartic acid homozygosity at codon 57 of HLA-DQ beta is associated with susceptibility to pulmonary tuberculosis in Cambodia. J Immunol 2006; 176:1090–7. [DOI] [PubMed] [Google Scholar]
- 36. Koesoemadinata RC, McAllister SM, Soetedjo NNM et al. Latent TB infection and pulmonary TB disease among patients with diabetes mellitus in Bandung, Indonesia. Trans R Soc Trop Med Hyg 2017; 111:81–9. [DOI] [PubMed] [Google Scholar]
- 37. Martinez L, Arman A, Haveman N et al. Changes in tuberculin skin test positivity over 20 years in periurban shantytowns in Lima, Peru. Am J Trop Med Hyg 2013; 89:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-seropositive patients with tuberculosis in a high-burden African setting. Am J Respir Crit Care Med 2016; 194:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.