Abstract
Background
Pneumococccal conjugated pneumococcal vaccine (PCV10) and pneumococcal conjugate vaccine 13 (PCV13), are used in childhood immunization programs worldwide, but direct comparisons of impacts against invasive pneumococcal disease (IPD) in equivalent populations have not been performed. We compared the vaccines (prevaccination 2007–2009 vs postvaccination 2013–2016) in Sweden, where the 21 counties use either PCV10 or PCV13 (introduced 2009–2010).
Methods
All IPD episodes (n = 16992) were recorded in Sweden during 2005–2016. Of 14186 from 2007–2016, 13468 (94.9%) were characterized with serotyping and 12235 (86.2%) with antibiotic susceptibility. Poisson models assessed changes in incidence over time.
Results
Invasive pneumococcal disease incidences decreased between 2005 and 2016 in vaccinated children (by 68.5%), and in the whole population (by 13.5%), but not among the elderly (increased by 2%), due to a substantial increase of non-vaccine types (NVTs). In 2016, NVTs constituted 72% of IPD cases in the elderly. Serotype 6A declined in PCV10 and PCV13 counties, whereas serotype 19A increased in PCV10 counties. There was no effect against serotype 3. Cross-protection was found between 6B and 6A, but not between 19F and 19A. Serotype 6C increased in PCV10 counties, but not in PCV13 counties, suggesting cross-protection with 6A, which is included in PCV13. In the elderly, the increase of NVTs excluding 6C, was more pronounced in PCV13 counties.
Conclusions
The overall impact of IPD incidences was not statistically different irrespective of vaccine used. The incidence of serotypes, where the effect of the vaccines differed, will influence the cost-effectiveness of which vaccine to use in immunization programs. The dominance of NVTs suggests a limited effect of current pediatric PCVs against IPD in the elderly.
Keywords: Pneumococcal infection, invasive pneumococcal disease, Pneumococcal conjugated vaccine, PCV10, PCV13
IPD incidence reduction was not significantly different with PCV10 and PCV13, with limited impact in the elderly. Serotypes 6C and 19A increased in PCV10 counties. NVTs dominated, and in the elderly NVTs excluding 6C was significantly higher in PCV13 counties.
Diseases caused by Streptococcus pneumoniae are a major public health problem. In 2015, it was estimated that 1.5 million people worldwide died from pneumococcal pneumonia, of which approximately 400000 were children <5 years [1, 2]. The incidence of pneumococcal diseases, especially of invasive pneumococcal disease (IPD), has declined during the 21st century in part due to immunization of children with pneumococcal conjugate vaccines (PCVs) [3–5]. Pneumococci express at least 97 different capsular serotypes, and PCVs target the most common serotypes to cause IPD among children (Supplementary Table 1) [6]. PCV7 was first introduced in 2000 in the United States, with other countries following. It was replaced by PCV10 or PCV13 around 2010 [6].
Childhood immunization programs have led to a decline of vaccine serotypes in vaccinated children, and in many countries, a reduction has also been observed in older children and adults [3, 7]. However, concomitantly there has been an increased incidence of non-vaccine serotypes (NVTs), ie, serotype-replacement [7]. Following introduction of PCV7 in the United States, an increased incidence of IPD caused by NVTs, especially of serotype 19A, that carry resistance to antibiotics was observed, and later 19A was found to expand in many other countries [3, 8–12]. Introduction of PCV10 or PCV13 has resulted in an overall reduction of IPD, but there are conflicting results regarding effects on serotypes 3 and 19A, which are included in PCV13 [13, 14]. Several studies indicate poor immunogenicity and protection against serotype 3 by PCV13, and cross-protection by PCV10 against serotype 19A is disputed [15–20]. There is a need for a comparison of the impact of PCV10 and PCV13 to prevent severe disease, but it is unlikely that a head-to-head trial will be conducted.
Sweden consists of 21 counties, each of which decides which PCV to use in the childhood immunization program. PCV7 was introduced in Sweden in January 2009, although some counties started vaccination in 2007–2008. From October 2009 onwards all counties switched from PCV7 to either PCV10 or PCV13. We compared differences in serotype-specific incidences of IPD following introduction of either PCV10 or PCV13 in Sweden.
METHODS
Study Population and Design
This is a population-based cohort study of the impact of PCVs in the childhood immunization program in Sweden (population 9.8 million). Introduction of PCV7, PCV10 or PCV13 in the childhood immunization program and the population of the 21 counties in Sweden, is presented in Supplementary Figure 1A and B. A 3-dose vaccine schedule (3, 5 and 12 months of age) was used for all PCVs, without catch-up vaccination. The vaccine coverage of 3 doses of PCV was about 97% for children aged 0–2 years in 2012 (Supplementary Table 2). Data on vaccine failures are presented in Supplementary Materials.
Since July 2004, clinicians/hospitals and laboratories have been required to report IPD cases to the Public Health Agency of Sweden, and since 2007, IPD isolates have been serotyped using gel diffusion and/or Quellung reactions [21]. Invasive pneumococcal disease was defined as isolation of pneumococci from sterile locations (blood, cerebrospinal fluid, etc.). An IPD episode was defined as pneumococci isolated >30 days apart. For 2 patients, different serotypes were isolated 19 and 30 days apart respectively. These were classified as different episodes. Five cases were excluded due to suspected double infections (isolation of 2 serotypes on the same day). Antibiotic susceptibility for penicillin G, using discs and E-test, was assessed according to EUCAST non-meningitis breakpoints (www.eucast.org) [22]. Only patients with a Swedish personal identification number were included. Data on population size for different age groups and counties was retrieved from Statistics Sweden (Supplementary Figure 1B). Nonvaccine types were defined as serotypes not included in PCV13. The study was approved by the ethical committee (Stockholms centrala etiska kommitté, EPN 2007/66-31).
Statistical Analysis
Yearly incidences of IPD in Sweden during 2005–2016 were calculated for the age groups 0–4, 5–64, and ≥65 years. For serotype-specific incidences, cases from 2007–2016 were used. Serotypes for IPD episodes with missing data were imputed using multivariate imputations by chained equations (Supplementary Material; Supplementary Table 3) [23]. Incidence rates and rate ratios were calculated with Poisson regression, and an exact method (package exactci (version1.3-1)) on the observed data was used when the imputed data contained zeros. Incidence rate ratios were estimated by comparing incidences from the period 2013–16 (post-PCV10 or -PCV13) with incidences from 2007 (pre-PCV7) and 2007–2009 (pre-PCV10 or -PCV13). The Simpson index of diversity was calculated to assess differences in serotype diversity [24]. Logistic regression analyses were used to check for significant trends regarding nonsusceptibility to Penicillin G. All statistical analyses were performed in R, version 3.4.0 (http://www.r-project-org/). Two-sided P values < .05 were considered statistically significant.
RESULTS
Patient Population
In total, 16992 IPD episodes were recorded in Sweden during 2005–2016; 15953 (93.9%) of the isolates were detected in blood, 735 (4.3%) in cerebrospinal fluid, and 304 (1.8%) from other sterile sites. Of these episodes, 635 (3.7%) occurred in children aged <5 years, 6893 (40.6%) in persons aged 5–64 years, and 9464 (55.7%) in persons aged ≥65 years of age. During 2007–2016, 13468 of 14186 (94.9%) isolates were available for serotyping (Supplementary Table 3).
Overall Effect of Pneumococcal Conjugate Vaccines in Sweden
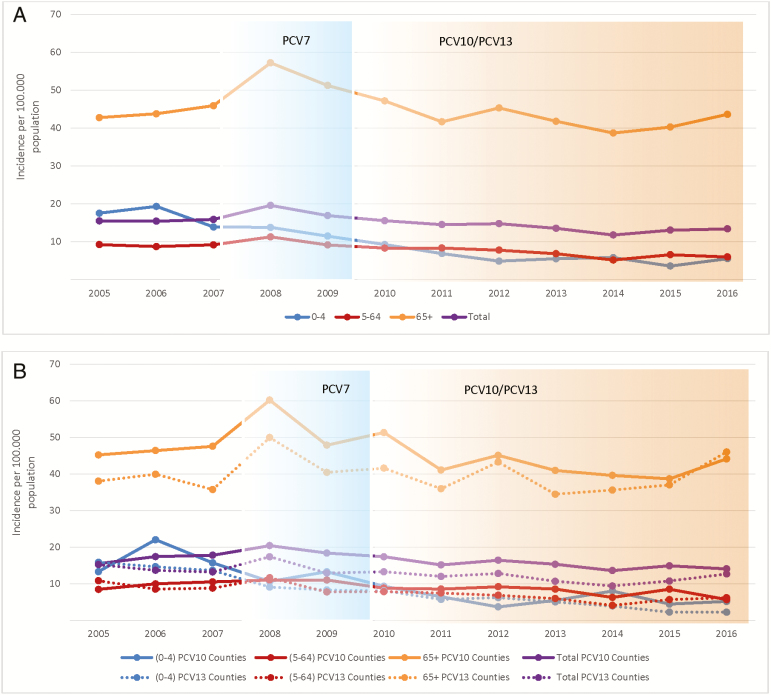
The overall IPD incidence was 15.5 per 100000 population in 2005. It then peaked at 19.6 per 100000 population in 2008, declined to 11.8 per 100000 in 2014, and increased slightly to 13.4 per 100000 population in 2016 (Figure 1A). Among children aged <5 years, there was a decrease from the peak of 19.3 per 100000 population in 2006 to 5.5 per 100000 population in 2016. Among those aged 5–64 years, there was a decrease from 9.3 per 100000 population in 2005 to 6.0 per 100000 population in 2016. Among adults aged ≥65 years, the IPD incidence was unchanged when 2005 was compared with 2016, but there was a 10% (95% confidence interval [CI], 5%–19%) reduction in incidence when 2013–2016 was compared with 2007 and 20% (95% CI, 15%–27%) reduction when 2013–2016 was compared with 2007–2009 (Table 1). PCV7 serotypes decreased and NVTs increased in both vaccinated and nonvaccinated age groups (Supplementary Figure 2). In the elderly, NVTs increased from 9.8 per 100000 population in 2007 to 31.0 per 100000 population in 2016, and 71.7% of the IPD episodes in this age group were caused by NVTs in 2016. The most prominent NVTs in 2016 were 22F, 9N, 8, 12F, 6C, 15A, 24F, 11A, 10A, 23B, and 33F, in descending order (Supplementary Figure 3). The IPD incidence of serotype 1, 5 and 7F, included in PCV10/PCV13, declined in all age groups after PCV10/PCV13 introduction. During 2013–16, only 1 case caused by these serotypes in children aged <5 years was identified (Table 1; Supplementary Figure 2). For serotypes 3, 6A, and 19A, which are only included in PCV13, there was a reduction in incidence of serotype 6A, but an overall increase in incidence of serotype 3 and 19A, although results were dependent on vaccine used.
Figure 1.
Incidence of invasive pneumococcal disease by age group in Sweden during 2005–2016. A, All counties. B, Counties using only pneumococcal conjugate vaccine 10 (PCV10) or pneumococcal conjugate vaccine 13 (PCV13). Age group 0–4 years in blue; age group 5–64 years is shown in red; age group ≥65 years is shown in green; total incidence in purple. The solid line indicates counties using PCV10. The dotted line indicates counties using PCV13. Blue background indicates when PCV7 was used in the child immunization program. Yellow background indicates when PCV10 or PCV13 was used in the child immunization program. Abbreviations: PCV7, pneumococcal vaccine 7; PCV10, pneumococcal conjugate vaccine 10; PCV13, pneumococcal conjugate vaccine 13.
Table 1.
Invasive pneumococcal disease incidence after PCV10/13 vaccination compared to before PCV7 and PCV10/13 vaccination according to serotype and age group in the whole of Sweden
| Age group (years) | Serotype | Pre-PCV7 Incidenceb (2007) | Pre-PCV10/13 Incidenceb (2007–2009) | Post-PCV10/13 Incidenceb (2013–2016) | Post-PCV10/13 vs. Pre-PCV7 Rate Ratio (95% CI)c | Post-PCV10/13 vs. Pre-PCV10/13 Rate Ratio (95% CI)c |
|---|---|---|---|---|---|---|
| 0–4 | (N = 73) | (N = 210) | (N = 120) | |||
| All serotypes | 13.9 (11.0,17.5) | 13.0 (11.4,14.9) | 5.1 (4.3,6.1) | 0.37 (0.28,0.49) | 0.39 (0.31,0.49) | |
| 4,6B,9V,14, 18C,19F,23F | 9.4 (7.0,12.5) | 7.9 (6.5,9.6) | 0.4 (0.2,0.9) | 0.05 (0.02,0.1) | 0.06 (0.03,0.11) | |
| 1,5,7F | 1.4 (0.6,2.8) | 1.6 (1.0,2.3) | 0.1 (0.02,0.4) | 0.07 (0.01,0.35) | 0.06 (0.01,0.27) | |
| 1 | 0 (0.0,0.7)a | 0.1 (0.03,0.5) | 0 (0,0.2)a | - | 0 (0,2.4)a | |
| 7F | 1.4 (0.6,2.8) | 1.4 (0.9,2.2) | 0.1 (0.02,0.4) | 0.07 (0.01,0.35) | 0.07 (0.01,0.3) | |
| 3,6A,19A | 2.1 (1.1,4.1) | 2.1 (1.4,3.1) | 1.3 (0.9,1.9) | 0.62 (0.3,1.28) | 0.62 (0.37,1.08) | |
| 3 | 0.2 (0.03,1.8) | 0.6 (0.3,1.2) | 0.7 (0.4,1.1) | 2.85 (0.36,22.8) | 1.2 (0.47,3.1) | |
| 6A | 1.2 (0.5,2.6) | 0.7 (0.4,1.2) | 0.1 (0.02,0.34) | 0.07 (0.01,0.36) | 0.13 (0.03,0.59) | |
| 19A | 0.7 (0.2,2.3) | 0.9 (0.5,1.7) | 0.6 (0.3,1.0) | 0.81 (0.22,3.0) | 0.64 (0.28,1.49) | |
| NVT | 1.0 (0.3,3.1) | 1.4 (0.8,2.5) | 3.2 (2.6,4.1) | 3.41 (1.02,11.4) | 2.27 (1.26,4.1) | |
| 5–64 | (N = 648) | N = (2097) | (N = 1794) | |||
| All serotypes | 9.2 (8.5,9.9) | 9.9 (9.5,10.3) | 6.1 (5.9,6.4) | 0.67 (0.61,0.73) | 0.62 (0.58,0.66) | |
| 4,6B,9V,14, 18C,19F,23F | 4.7 (4.2,5.3) | 4.7 (4.4,5.1) | 0.5 (0.4,0.6) | 0.1 (0.08,0.13) | 0.1 (0.09,0.13) | |
| 1,5,7F | 1.4 (1.1,1.8) | 1.9 (1.7,2.1) | 0.5 (0.5,0.6) | 0.38 (0.29,0.51) | 0.29 (0.24,0.35) | |
| 1 | 0.2 (0.1,0.4) | 0.4 (0.3,0.5) | 0.1 (0.06,0.1) | 0.39 (0.20,0.73) | 0.26 (0.16,0.40) | |
| 7F | 1.2 (0.9,1.5) | 1.5 (1.3,1.7) | 0.4 (0.4,0.5) | 0.37 (0.27,0.51) | 0.29 (0.23,0.36) | |
| 3,6A,19A | 1.3 (1.0,1.6) | 1.3 (1.1,1.5) | 1.5 (1.3,1.6) | 1.18 (0.91,1.52) | 1.14 (0.97,1.35) | |
| 3 | 0.8 (0.5,1.0) | 0.8 (0.6,0.9) | 0.9 (0.8,1.0) | 1.22 (0.88,1.73) | 1.23 (0.99,1.54) | |
| 6A | 0.3 (0.2,0.5) | 0.3 (0.2,0.4) | 0.03 (0.02,0.07) | 0.1 (0.04,0.24) | 0.12 (0.05,0.27) | |
| 19A | 0.1 (0.06,0.3) | 0.3 (0.2,0.3) | 0.5 (0.4,0.6) | 3.52 (1.51,8.21) | 2.03 (1.42,2.91) | |
| NVT | 1.8 (1.4,2.2) | 2.0 (1.7,2.2) | 3.6 (3.4,3.9) | 2.0 (1.65,2.52) | 1.85 (1.62,2.11) | |
| ≥65 | (N = 738) | (N = 2545) | (N = 3168) | |||
| All serotypes | 45.9 (42.7,49.3) | 51.5 (49.5,53.5) | 41.1 (39.7,42.6) | 0.9 (0.83,0.97) | 0.8 (0.76,0.84) | |
| 4,6B,9V,14, 18C,19F,23F | 24.3 (21.8,27.1) | 25.4 (23.7,27.1) | 2.8 (2.5,3.3) | 0.12 (0.1,0.14) | 0.11 (0.1,0.13) | |
| 1,5,7F | 3.3 (2.4,4.6) | 3.5 (2.9,4.2) | 1.3 (1.1,1.7) | 0.4 (0.27,0.6) | 0.38 (0.29,0.51) | |
| 1 | 0.2 (0.06,0.6) | 0.3 (0.2,0.6) | 0.1 (0.04,0.2) | 0.53 (0.13,2.13) | 0.3 (0.11,0.86) | |
| 7F | 3.0 (2.1,4.2) | 3.1 (2.6,3.8) | 1.2 (1.0,1.5) | 0.41 (0.28,0.61) | 0.39 (0.29,0.53) | |
| 3,6A,19A | 8.4 (7.0,10.2) | 9.7 (8.8,10.7) | 9.8 (9.1,10.6) | 1.16 (0.95,1.42) | 1.01 (0.89,1.14) | |
| 3 | 4.6 (3.5,5.9) | 4.8 (4.1,5.5) | 6.2 (5.6,6.8) | 1.36 (1.04,1.77) | 1.3 (1.09,1.55) | |
| 6A | 2.7 (2.0,3.7) | 3.1 (2.6,3.7) | 0.5 (0.4,0.7) | 0.2 (0.12,0.31) | 0.17 (0.12,0.25) | |
| 19A | 1.2 (0.6,2.2) | 1.8 (1.4,2.4) | 3.1 (2.7,3.5) | 2.67 (1.38,5.15) | 1.68 (1.26,2.24) | |
| NVT | 9.8 (8.2,11.8) | 12.8 (11.7,14.1) | 27.1 (25.9,28.2) | 2.76 (2.28,3.34) | 2.11 (1.9,2.34) | |
| All age groups | (N = 1459) | (N = 4852) | (N = 5082) | |||
| All serotypes | 15.9 (15.1,16.7) | 17.5 (17.0,18.0) | 13.0 (12.6,13.3) | 0.82 (0.77,0.86) | 0.74 (0.71,0.77) | |
| 4, 6B, 9V, 14, 18C,19F, 23F | 8.4 (7.8,9.1) | 8.6 (8.2,9.0) | 1.0 (0.9,1.1) | 0.11 (0.1,0.13) | 0.11 (0.1,0.12) | |
| 1,5,7F | 1.7 (1.5,2.0) | 2.2 (2.0,2.3) | 0.7 (0.6,0.8) | 0.39 (0.31,0.47) | 0.31 (0.27,0.37) | |
| 1 | 0.2 (0.1,0.4) | 0.3 (0.3,0.4) | 0.09 (0.06,0.13) | 0.41 (0.23,0.72) | 0.26 (0.17,0.39) | |
| 7F | 1.5 (1.3,1.8) | 1.8 (1.6,2.0) | 0.6 (0.5,0.7) | 0.38 (0.3,0.48) | 0.32 (0.27,0.38) | |
| 3,6A,19A | 2.6 (2.2,2.9) | 2.8 (2.6,3.1) | 3.1 (2.9,3.3) | 1.21 (1.04,1.41) | 1.09 (0.99,1.2) | |
| 3 | 1.4 (1.1,1.7) | 1.5 (1.3,1.6) | 1.9 (1.8,2.1) | 1.4 (1.13,1.72) | 1.34 (1.17,1.54) | |
| 6A | 0.8 (0.6,1.0) | 0.8 (0.7,0.9) | 0.13 (0.1,0.19) | 0.17 (0.11,0.25) | 0.17 (0.12,0.24) | |
| 19A | 0.4 (0.2,0.6) | 0.6 (0.5,0.7) | 1.0 (0.9,1.1) | 2.87 (1.69,4.85) | 1.79 (1.43,2.24) | |
| NVT | 3.1 (2.7,3.6) | 3.9 (3.6,4.2) | 8.2 (7.9,8.5) | 2.61 (2.26,3.02) | 2.12 (1.96,2.3) |
aThese calculations were made by using an exact method on the observed data.
bIncidence per 100000 population in year (95% Confidence Interval)
cSignificant changes in rate ratios in bold
Comparing the Impact of Pneumococcal Conjugate Vaccine 10 or Pneumococcal Conjugate Vaccine 13 in the Childhood Immunization Program
We compared the incidence of IPD before PCV7 (year 2007), before PCV10/13 (year 2007–2009), and after PCV10/13 (2013–2016) was introduced. The overall reduction in the IPD incidence rate after vaccine introduction compared with before vaccine introduction was not significantly different between PCV10 or PCV13 counties, although there was a slightly higher incidence in 2007 among the population in counties using PCV10 (Figure 1B; Table 2). Invasive pneumococcal disease caused by PCV7 serotypes decreased significantly in all age groups using either vaccine (Supplementary Figure 4).
Table 2.
Invasive pneumococcal disease incidence after and before vaccine introduction according to serotype and age group in counties using only PCV10 or PCV13
| Counties using PCV10 | Counties using PCV13 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group (year) | Serotype | Pre-PCV7 Incidenceb (2007) | Pre-PCV10 Incidenceb (2007–2009) | Post-PCV10 Incidenceb (2013–2016) | Post-PCV10 vs. Pre-PCV7 Rate Ratio (95% CI)c(1) | Post-PCV10 vs. Pre-PCV10 Rate Ratio (95% CI)c(2) | Pre-PCV7 Incidenceb (2007) | Pre-PCV13 Incidenceb (2007–2009) | Post-PCV13 Incidenceb (2013–2016) | Post-PCV13 vs. Pre-PCV7 Rate Ratio (95% CI)c(3) | Post-PCV13 vs. Pre-PCV13 Rate Ratio (95% CI)c(4) | Comparison of Rate Ratios (95% CI)c(4) vs. (2) | Comparison of Rate Ratios (95% CI)c(3) vs. (1) |
| 0–4 | (N = 16) | (N = 41) | (N = 26) | (N = 22) | (N = 51) | (N = 24) | |||||||
| All serotypes | 15.7 (9.6,25.6) |
13.2 (9.7,17.9) |
5.7 (3.9,8.4) |
0.36
(0.20,0.68) |
0.43
(0.27,0.71) |
13.6 (9.0,20.6) |
10.3 (7.8,13.5) |
3.3 (2.2,4.9) |
0.24
(0.14,0.43) |
0.32
(0.20,0.52) |
0.74 (0.37,1.48) |
0.67 (0.28,1.56) |
|
| PCV7e | 11.2 (6.2,20.5) |
7.5 (5.0,11.4) |
0.7 (0.2,2.0) |
0.06
(0.02,0.21) |
0.09
(0.03,0.29) |
9.3 (5.6,15.5) |
6.3 (4.4,8.9) |
0.2 (0.0,1.9) |
0.02
(0.0,0.21) |
0.03
(0.0,0.31) |
0.37 (0.03,4.87) |
0.38 (0.03,5.25) |
|
| 1, 5, 7F | 1.1 (0.1,8.5) |
2.3 (1.1,4.9) |
0.0 (0.0,0.8)a | 0.00 (0.00,4.26)a |
0.00
(0.00,0.41) a |
1.2 (0.3,4.9) |
1.0 (0.4,2.4) |
0.0 (0.0,0.5)a |
0.00
(0.0,0.77) a |
0.00
(0.00,0.68) a |
- | - | |
| 1 | 0.0 (0.0,3.7)a |
0.0 (0.0,1.2)a |
0.0 (0.0,0.8)a | - | - | 0.0 (0.0,2.3)a |
0.0 (0.0,0.8)a |
0.0 (0.0,0.5)a |
- | - | - | - | |
| 7F | 1.1 (0.1,8.5) |
2.3 (1.1,4.9) |
0.0 (0.0,0.8)a | 0.00 (0.00,4.26)a |
0.0
(0.0,0.41) a |
1.2 (0.3,4.9) |
1.0 (0.4,2.4) |
0.0 (0.0,0.5)a |
0.00
(0.0,0.77) a |
0.00
(0.00,0.68) a |
- | - | |
| 3,6A,19A | 2.5 (0.6,10.4) |
2.4 (1.1,5.2) |
2.0 (1.0,3.9) |
0.82 (0.17,3.94) |
0.83 (0.31,2.24) |
2.5 (0.9,6.8) |
2.0 (1.1,3.8) |
0.3 (0.1,1.4) |
0.13
(0.02,0.79) |
0.16
(0.03,0.81) |
0.20 (0.03,1.39) |
0.16 (0.01,2.03) |
|
| 3 | 0.0 (0.0,3.7)a |
0.7 (0.2,3.3) |
0.5 (0.1,1.9) |
Inf (0.06,Inf)a |
0.61 (0.08,4.85) |
0.0 (0.0,2.3)a |
0.2 (0.0,1.6) |
0.3 (0.1,1.4) |
Inf (0.06,Inf)a |
1.55 (0.12,19.32) |
2.52 (0.08,78) |
- | |
| 6A | 2.0 (0.5,8.3) |
1.0 (0.3,3.1) |
0.4 (0.1,1.8) |
0.22 (0.03,1.54) |
0.44 (0.07,2.66) |
0.6 (0.1,4.4) |
0.2 (0.0,1.4) |
0.0 (0.0,0.5)a |
0.00 (0.0,4.23)a |
0.00 (0.00,13.0)a |
- | - | |
| 19A | 0.0 (0.0,3.7)a |
0.7 (0.2,2.7) |
1.1 (0.5,2.7) |
Inf (0.22,Inf)a |
1.67 (0.32,8.80) |
1.9 (0.6,5.7) |
1.6 (0.8,3.2) |
0.0 (0.0,0.5)a |
0.00
(0.00,0.38) a |
0.00
(0.00,0.39) a |
- | - | |
| NVT | 0.0 (0.0,3.7)a |
0.8 (0.2,3.4) |
3.0 (1.8,5.2) |
Inf (0.77,Inf)a |
3.60 (0.82,15.84) |
0.0 (0.0,2.3)a |
1.0 (0.4,2.4) |
2.7 (1.7,4.3) |
Inf (0.98,Inf)a |
2.83 (1.00,8.04) |
0.79 (0.14,4.49) |
- | |
| 6C | 0.0 (0.0,3.7)a |
0.0 (0.0,1.2)a |
0.9 (0.3,2.4) |
Inf (0.20,Inf)a |
Inf (0.61,Inf)a |
0.0 (0.0,2.3)a |
0.2 (0.0,1.7) |
0.0 (0.0,0.5)a |
- | 0.0 (0.0,13.0)a |
- | - | |
| 5–64 | (N = 154) | (N = 476) | (N = 424) | (N = 173) | (N = 560) | (N = 469) | |||||||
| All serotypes | 10.5 (8.9,12.3) |
10.8 (9.9,11.8) |
7.2 (6.6,7.9) |
0.69
(0.57,0.83) |
0.67
(0.59,0.76) |
8.8 (7.5,10.2) |
9.4 (8.6,10.2) |
5.4 (5.0,6.0) |
0.62
(0.52,0.74) |
0.58
(0.51,0.66) |
0.87 (0.73,1.04) |
0.90 (0.70,1.16) |
|
| PCV7e | 6.3 (4.9,8.0) |
5.2 (4.5,6.0) |
0.6 (0.4,0.8) |
0.09
(0.06,0.13) |
0.11
(0.08,0.16) |
4.1 (3.2,5.3) |
4.4 (3.9,5.0) |
0.4 (0.3,0.6) |
0.10
(0.07,0.16) |
0.10
(0.07,0.14) |
0.92 (0.54,1.58) |
1.19 (0.65,2.16) |
|
| 1,5,7F | 1.2 (0.7,2.0) |
2.0 (1.6,2.5) |
0.6 (0.4,0.8) |
0.51
(0.26,0.98) |
0.30
(0.20,0.45) |
1.5 (1.0,2.2) |
1.6 (1.3,1.9) |
0.5 (0.4,0.7) |
0.35
(0.21,0.57) |
0.33
(0.23,0.48) |
1.12 (0.65,1.94) |
0.68 (0.30,1.58) |
|
| 1 | 0.2 (0.1,0.7) |
0.2 (0.1,0.4) |
0.1 (0.0,0.2) |
0.49 (0.12,2.00) |
0.44 (0.16,1.22) |
0.3 (0.1,0.7) |
0.3 (0.2,0.5) |
0.1 (0.07,0.2) |
0.42 (0.16,1.15) |
0.44
(0.20,0.94) |
1.00 (0.29,3.57) |
0.87 (0.16,4.86) |
|
| 7F | 0.9 (0.5,1.7) |
1.7 (1.4,2.2) |
0.5 (0.3,0.7) |
0.49 (0.24,1.02) |
0.27
(0.17,0.42) |
1.2 (0.8,1.9) |
1.3 (1.0,1.6) |
0.4 (0.3,0.5) |
0.32
(0.18,0.57) |
0.30
(0.20,0.46) |
1.12 (0.60,2.08) |
0.64 (0.25,1.66) |
|
| 3,6A,19A | 1.4 (0.8,2.4) |
1.5 (1.2,2.0) |
2.3 (1.9,2.7) |
1.64 (0.93,2.89) |
1.50
(1.08,2.07) |
1.2 (0.8,1.9) |
1.2 (1.0,1.6) |
0.9 (0.7,1.2) |
0.74 (0.45,1.22) |
0.75 (0.53,1.05) |
0.50
(0.31,0.82) |
0.45
(0.21,0.99) |
|
| 3 | 0.9 (0.5,1.6) |
0.9 (0.6,1.2) |
1.2 (1.0,1.6) |
1.34 (0.73,2.46) |
1.46 (0.95,2.23) |
0.6 (0.3,1.2) |
0.7 (0.5,0.9) |
0.6 (0.4,0.8) |
0.91 (0.46,1.77) |
0.85 (0.55,1.31) |
0.58 (0.32,1.09) |
0.68 (0.27,1.68) |
|
| 6A | 0.4 (0.1,1.0) |
0.5 (0.3,0.7) |
0.1 (0.0,0.2) |
0.14
(0.03,0.62) |
0.11
(0.03,0.39) |
0.3 (0.1,0.8) |
0.3 (0.2,0.5) |
0.01 (0.0,0.1) |
0.04
(0.0,0.32) |
0.04
(0.01,0.35) |
0.40 (0.03,4.50) |
0.27 (0.02,3.46) |
|
| 19A | 0.0 (0.0,0.3)a |
0.2 (0.1,0.5) |
1.0 (0.8,1.3) |
Inf
(3.48,Inf) a |
5.14
(2.02,13.13) |
0.3 (0.1,0.8) |
0.3 (0.2,0.5) |
0.3 (0.2,0.5) |
1.33 (0.40,4.39) |
1.23 (0.62,2.42) |
0.24
(0.07,0.83) |
- | |
| NVT | 1.6 (1.0,2.7) |
2.1 (1.7,2.7) |
3.8 (3.3,4.3) |
2.35
(1.39,3.99) |
1.78
(1.37,2.30) |
1.9 (1.2,2.8) |
2.2 (1.7,2.7) |
3.6 (3.2,4.0) |
1.92
(1.25,2.94) |
1.65
(1.29,2.10) |
0.93 (0.64,1.34) |
0.82 (0.39,1.69) |
|
| 6C | 0.0 (0.0,0.3)a |
0.0 (0.0,0.1)a |
0.3 (0.2,0.4) |
Inf (0.87,Inf)a |
Inf
(2.62,Inf) a |
0.0 (0.0,0.2)a |
0.0 (0.0,0.1)a |
0.1 (0.0,0.2) |
Inf (0.23,Inf)a |
Inf (0.69,Inf)a |
- | - | |
| ≥65 | (N = 174) | (N = 583) | (N = 719) | (N = 137) | (N = 499) | (N = 726) | |||||||
| All serotypes | 47.5 (41.0,55.1) |
51.8 (47.8,56.2) |
40.8 (37.9,43.9) |
0.86 (0.73,1.01) |
0.79
(0.71,0.88) |
35.7 (30.2,42.2) |
42.0 (38.5,45.9) |
38.3 (35.6,41.2) |
1.07 (0.89,1.29) |
0.91 (0.81,1.02) |
1.16 (0.99,1.36) |
1.25 (0.98,1.60) |
|
| PCV7e | 26.0 (21.2,31.9) |
24.6 (21.8,27.7) |
2.8 (2.1,3.7) |
0.11
(0.08,0.15) |
0.11
(0.08,0.16) |
18.2 (13.6,24.3) |
21.2 (18.5,24.3) |
1.9 (1.4,2.8) |
0.11
(0.07,0.17) |
0.09
(0.06,0.14) |
0.81 (0.50,1.32) |
1.00 (0.57,1.76) |
|
| 1,5,7F | 2.4 (1.1,5.1) |
3.2 (2.3,4.6) |
1.5 (1.0,2.2) |
0.62 (0.26,1.47) |
0.46
(0.27,0.77) |
1.2 (0.3,4.5) |
2.0 (1.2,3.2) |
1.0 (0.6,1.6) |
0.83 (0.21,3.28) |
0.51
(0.27,0.99) |
1.12 (0.50,2.55) |
1.34 (0.26,6.96) |
|
| 1 | 0.0 (0.0,1.0)a |
0.3 (0.1,0.9) |
0.1 (0.0,0.5) |
Inf (0.01,Inf)a |
0.22 (0.02,2.19) |
0.0 (0.0,1.0)a |
0.1 (0.0,0.9) |
0.2 (0.1,0.6) |
Inf (0.18,Inf)a |
1.77 (0.20,16.04) |
8.23 (0.36,188) |
- | |
| 7F | 2.4 (1.1,5.1) |
2.9 (2.0,4.3) |
1.4 (0.9,2.1) |
0.60 (0.25,1.44) |
0.48
(0.28,0.83) |
1.2 (0.3,4.3) |
1.9 (1.1,3.0) |
0.8 (0.5,1.3) |
0.66 (0.17,2.60) |
0.44
(0.22,0.88) |
0.91 (0.38,2.16) |
1.11 (0.21,5.91) |
|
| 3,6A,19A | 7.9 (5.4,11.6) |
9.9 (8.2,12.0) |
10.5 (9.0,12.2) |
1.32 (0.88,1.98) |
1.06 (0.83,1.34) |
6.0 (3.6,9.9) |
8.0 (6.4,9.9) |
9.1 (7.9,10.6) |
1.53 (0.91,2.57) |
1.15 (0.88,1.49) |
1.09 (0.76,1.56) |
1.16 (0.59,2.28) |
|
| 3 | 3.4 (1.8,6.3) |
4.6 (3.4,6.1) |
5.1 (4.1,6.3) |
1.50 (0.77,2.95) |
1.11 (0.78,1.58) |
3.8 (2.1,6.9) |
3.7 (2.7,5.1) |
7.0 (5.9,8.3) |
1.84 (1.00,3.40) |
1.86
(1.31,2.66) |
1.68
(1.01,2.77) |
1.22 (0.50,3.03) |
|
| 6A | 3.6 (2.1,6.2) |
3.7 (2.7,5.1) |
0.5 (0.3,1.0) |
0.15
(0.06,0.35) |
0.14
(0.07,0.30) |
1.1 (0.4,2.9) |
2.2 (1.5,3.3) |
0.5 (0.3,1.0) |
0.48 (0.14,1.62) |
0.23
(0.10,0.52) |
1.65 (0.58,4.71) |
3.26 (0.75,14.12) |
|
| 19A | 0.9 (0.3,3.1) |
1.6 (1.0,2.7) |
4.9 (4.0,6.0) |
5.2
(1.55,17.46) |
3.00
(1.75,5.13) |
1.1 (0.3,4.0) |
2.0 (1.3,3.1) |
1.7 (1.1,2.4) |
1.57 (0.40,6.13) |
0.84 (0.47,1.52) |
0.28
(0.12,0.64) |
0.30 (0.04,1.9) |
|
| NVT | 11.2 (8.0,15.6) |
14.1 (12.0,16.5) |
26.1 (23.8,28.6) |
2.34
(1.64,3.32) |
1.85
(1.53,2.23) |
10.1 (6.4,15.6) |
10.9 (8.8,13.4) |
26.2 (24.0,28.6) |
2.59
(1.66,4.06) |
2.41
(1.91,3.0) |
1.30 (0.98,1.72) |
1.11 (0.65,1.9) |
|
| 6C | 0.0 (0.0,1.0)a |
0.2 (0.0,1.0) |
4.6 (3.6,5.7) |
Inf
(4.11,Inf) a |
22.29
(4.71,105.4) |
0.3 (0.0,3.7) |
0.5 (0.2,1.3) |
1.3 (0.9,2.0) |
3.86 (0.34,43.7) |
2.74 (0.90,8.30) |
0.12
(0.02,0.78) |
- | |
| All age groups |
(N = 344) | (N = 1100) | (N = 1169) | (N = 332) | (N = 1110) | (N = 1219) | |||||||
| All serotypes | 17.7 (16.0,19.7) |
18.8 (17.7,20.0) |
14.4 (13.6,15.3) |
0.76
(0.67,0.86) d |
0.72
(0.67,0.79) d |
13.2 (11.8,14.7) |
14.5 (13.6,15.3) |
10.8 (10.3,11.5) |
0.79
(0.70,0.89) d |
0.72
(0.67,0.78) d |
1.00 (0.89,1.12)d |
1.04 (0.88,1.23)d |
|
| PCV7e | 10.3 (8.8,12.0) |
9.0 (8.3,9.9) |
1.0 (0.8,1.3) |
0.10
(0.07,0.13) d |
0.11
(0.09,0.14) d |
6.6 (5.4,8.0) |
7.1 (6.4,7.9) |
0.7 (0.5,0.9) |
0.10
(0.07,0.14) d |
0.09
(0.07,0.12) d |
0.84 (0.58,1.21)d |
1.03 (0.67,1.57)d |
|
| 1,5,7F | 1.4 (0.9,2.2) |
2.2 (1.8,2.7) |
0.7 (0.6,1.0) |
0.52
(0.31,0.88) d |
0.33
(0.24,0.45) d |
1.5 (1.0,2.2) |
1.6 (1.3,1.9) |
0.6 (0.4,0.7) |
0.39
(0.25,0.62) d |
0.36
(0.26,0.49) d |
1.08 (0.69,1.69)d |
0.74 (0.37,1.49)d |
|
| 1 | 0.2 (0.1,0.5) |
0.2 (0.1,0.4) |
0.1 (0.0,0.2) |
0.53 (0.14,2.09) |
0.38
(0.15,0.96) |
0.2 (0.1,0.5) |
0.2 (0.1,0.4) |
0.1 (0.08,0.2) |
0.56 (0.21,1.44) |
0.54 (0.27,1.08) |
1.42 (0.45,4.47) |
1.04 (0.20,5.37) |
|
| 7F | 1.2 (0.8,2.0) |
2.0 (1.6,2.4) |
0.6 (0.5,0.8) |
0.51
(0.29,0.89) d |
0.32
(0.22,0.44) d |
1.2 (0.8,1.8) |
1.3 (1.1,1.6) |
0.4 (0.3,0.6) |
0.35
(0.21,0.59) d |
0.32
(0.22,0.45) d |
1.00 (0.61,1.64)d |
0.68 (0.31,1.49)d |
|
| 3,6A,19A | 2.7 (1.9,3.6) |
3.2 (2.7,3.7) |
4.0 (3.6,4.5) |
1.42 (1.00,2.01)d |
1.20 (0.99,1.45)d |
2.1 (1.5,2.8) |
2.3 (2.0,2.7) |
2.3 (2.0,2.6) |
1.04 (0.74,1.47)d |
0.93 (0.76,1.14)d |
0.79 (0.59,1.05)d |
0.75 (0.45,1.26)d |
|
| 3 | 1.4 (0.9,2.1) |
1.6 (1.3,1.9) |
2.0 (1.7,2.4) |
1.41 (0.88,2.28)d |
1.23 (0.95,1.61)d |
1.1 (0.7,1.7) |
1.1 (0.9,1.4) |
1.6 (1.4,1.9) |
1.42 (0.91,2.22)d |
1.39
(1.07,1.81) d |
1.15 (0.79,1.68)d |
1.04 (0.55,1.99)d |
|
| 6A | 1.1 (0.7,1.7) |
1.1 (0.9,1.5) |
0.2 (0.1,0.3) |
0.15
(0.08,0.30) d |
0.15
(0.08,0.26) d |
0.5 (0.3,0.9) |
0.6 (0.4,0.8) |
0.1 (0.0,0.2) |
0.19
(0.08,0.48) d |
0.16
(0.08,0.33) d |
1.10 (0.43,2.80)d |
1.29 (0.41,4.07)d |
|
| 19A | 0.2 (0.1,0.9) |
0.5 (0.3,0.7) |
1.8 (1.6,2.2) |
7.74
(1.83,32.8) d |
3.49
(2.25,5.42) d |
0.5 (0.2,1.0) |
0.6 (0.5,0.9) |
0.5 (0.4,0.7) |
1.06 (0.48,2.34)d |
0.83 (0.54,1.27)d |
0.24
(0.12,0.45) d |
0.14
(0.02,0.81) d |
|
| NVT | 3.4 (2.5,4.5) |
4.4 (3.8,5.0) |
8.6 (8.0,9.3) |
2.36
(1.77,3.15) d |
1.84
(1.59,2.13) d |
3.0 (2.2,4.2) |
3.4 (2.9,4.0) |
7.3 (6.8,7.8) |
2.30
(1.64,3.22) d |
2.05
(1.71,2.45) d |
1.11 (0.88,1.40)d |
0.98 (0.63,1.50)d |
|
| 6C | 0.0 (0.0,0.2)* |
0.05 (0.0,0.2) |
1.2 (1.0,1.5) |
Inf
(5.94,Inf)* |
24.08
(5.04,115) d |
0.1 (0.0,1.0) |
0.1 (0.0,0.2) |
0.3 (0.2,0.4) |
4.48 (0.38,52.8)d |
2.79 (0.98,7.98)d |
0.12
(0.02,0.67) d |
- | |
Comments: 1) In order to make the final results more clear we have decided to present age adjusted rate ratios also within vaccine group (PCV10, PCV13). 2) The estimates for serotype 1 are not age adjusted due to model fitting issues caused by the fact that there were no cases in the age group 0–4. 3) Serotype 6C has now been added to the table. 4) A new column has been added to the end of the table comparing the rate ratios of the two county groups having the year 2007 as baseline.
aThese calculations were made by using an exact method on the observed data.
bIncidence per 100000 population in year (95% Confidence Interval)
cSignificant changes in rate ratios in bold
dAge adjusted ratios
eSerotypes included are: 4, 6B, 9V, 14, 18C, 19F, 23F
For the three extra serotypes, 1, 5 and 7F, which are included in both PCV10 and PCV13, a significant decline in incidence rates in the whole population was observed in counties using either vaccine (Table 2; Supplementary Figure 4). Three years after introduction of the vaccines, no cases of these serotypes were identified in the youngest children regardless of vaccine used.
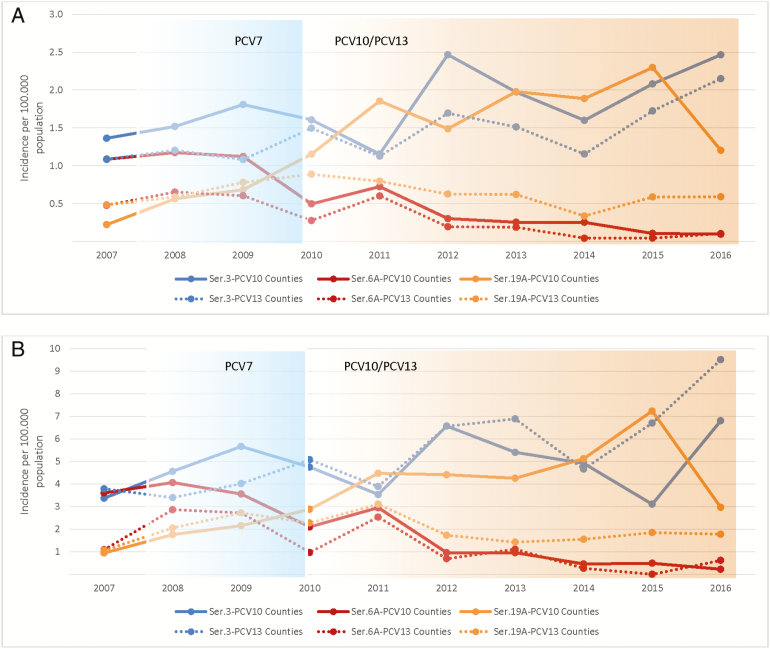
For serotypes 3, 6A, and 19A, which are included in PCV13, we found a significant decline in the incidence of serotype 6A following vaccine introduction in both PCV10 and PCV13 counties in the whole population (Table 2; Figure 2A and B). In contrast, the incidence of serotype 19A in older age groups increased significantly in PCV10 counties as compared to PCV13 counties. Although the results in children aged <5 years need to be interpreted cautiously due to few events, there was a significant reduction of serotypes 3, 6A and 19A combined in PCV13 counties, primarily due to a reduction of serotype 19A (Table 2). In PCV13 counties, there were no cases of 19A among children aged <5 years in 2013–16 compared with an incidence of 1.1 per 100000 population in PCV10 counties (Table 2; Supplementary Figure 5). The results were similar for children aged 0–2 years (Supplementary Table 4). There was no significant reduction of serotype 3 irrespective of vaccine used for any age groups.
Figure 2.
Incidence of invasive pneumococcal disease caused by serotypes 3, 6A and 19A in counties using only pneumococcal conjugate vaccine 10 (PCV10) compared with counties using pneumococcal conjugate vaccine 13 (PCV13). A, All ages. B, Those aged ≥65 years. Serotype 3 is shown in blue; serotype 6A is shown in red; and serotype19A is shown in green. The solid line indicates counties using PCV10. The dotted line indicates counties using PCV13. Blue background indicates when PCV7 was used in the child immunization program. Yellow background indicates when PCV10 or PCV13 was used in the child immunization program. Abbreviations: PCV7, pneumococcal vaccine 7; PCV10, pneumococcal conjugate vaccine 10; PCV13, pneumococcal conjugate vaccine 13; Ser., serotype.
Importantly, we found an increased incidence of NVTs that was significant in both PCV10 and PCV13 counties for all ages (Table 2, Supplementary Table 5A). The increase of NVTs was most pronounced in the elderly, with an increase of 12.0 and 15.3 per 100000 population for PCV10 and PCV 13 counties (P < .001 for both county groups), respectively, between 2007–2009 and 2013–16 (Table 2). Also, among persons aged 5–64 years, there was an increase in NVTs in both county groups. Among children aged 0–4 years, no significant increase in NVTs was observed in PCV10 (P = .09) or PCV13 (P = .051) counties; however, when all counties in Sweden were combined, NVTs increased significantly (P < .001) (Tables 1 and 2).
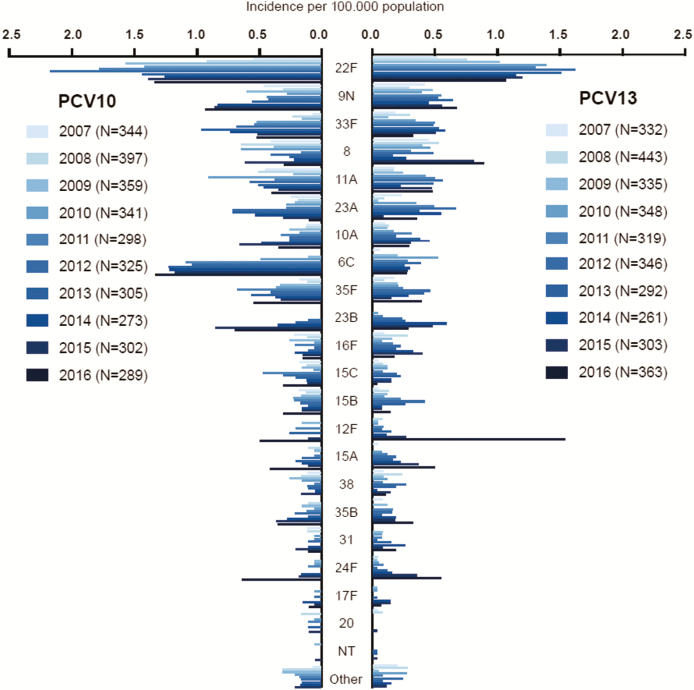
In the elderly, we observed that between 2007 and 2013–16 the incidence of 19A+NVTs increased with 18.9 per 100000 population for PCV10 counties and 16.6 per 100000 population for PCV13 counties (Supplementary Table 5B). Moreover, we assessed changes in the incidence of the most common NVTs. Serotype 6C increased more in PCV10 than in PCV13 counties (Figure 3). The incidence of serotype 6C increased from 0.05 per 100000 population in 2007–2009 to 1.2 per 100000 population in 2013–16 in PCV10 counties (Incidence rate ratio [IRR], 24.1; 95% CI, 5.0–115.0) and from 0.1 to 0.3 per 100000 population in PCV13 counties (IRR, 2.8; 95% CI, 0.98–8.0; P for interaction = .02). In 2016, we observed an increase in serotypes 12F and 15A, mainly in PCV13 counties, and of 35F and 23B in PCV10 counties. When NVTs excluding 6C were assessed in the elderly, the incidence of NVTs increased by 7.6 per 100000 in PCV10 counties and by 14.5 per 100000 population in PCV13 counties (Supplementary Table 5B).
Figure 3.
Incidence of invasive pneumococcal disease caused by serotypes not included in any conjugated vaccines in pneumococcal conjugated vaccine 10 (PCV10) and pneumococcal conjugate vaccine 13 (PCV13) counties.
Presented by calendar year. All age groups combined. Abbreviations: NT, non-typeable; PCV10, pneumococcal conjugate vaccine 10; PCV13, pneumococcal conjugate vaccine 13.
There were no differences in the Simpson Index for PCV10 or PCV13 counties when data from 2007–2009 and 2013–16 were compared (P = .97 and .07, respectively). There was not a significant difference in the Simpson Index in 2013–2016 in PCV10 counties (0.933; 95% CI, .928-.939) compared with PCV13 counties (0.941; 95% CI, .935-.946) (P = .053)], indicating limited differences in the serotype diversity.
Antibiotic Resistance
Susceptibility testing to Penicillin G was performed for 86.2% of all Swedish isolates from the period 2007–2016. Penicillin nonsusceptibility increased from 3.3% in 2007 to 5.6% in 2013–16 (P < .001) (Supplementary Figure 6). In PCV10 and PCV13 counties, nonsusceptibility went from 2.5% and 6.7% in 2007 to 3.7% and 6.7% in 2013–16, respectively. The increase among all Swedish isolates was primarily driven by expansion of nonsusceptible NVTs, which increased from 1% (n = 2/201) in 2007 to 4.1% (n = 36/886) in 2016. Serotypes 15A and 23B constituted 75% (n = 27/36) of nonsusceptible NVTs in 2016. Among PCV13 serotypes 3, 6A and 19A, there was an increase in penicillin nonsusceptible isolates from 0.4% in 2007 to 1.2% in 2016, and 14 of 15 nonsusceptible isolates in 2016 were serotype 19A. Penicillin nonsusceptible serotypes included in PCV7 declined slightly from 2.6% in 2007 to 1.1% in 2016. However, among all IPD isolates belonging to PCV7 serotypes detected in 2016, 23.7% (n = 14/59) were nonsusceptible to penicillin (mainly of serotypes 19F, 23F, and 14), as compared to 4.7% in 2007.
DISCUSSION
In this article we present 7 years of national follow-up data on serotype-specific IPD incidence after introduction of first PCV7 and then either PCV10 or PCV13 in the child immunization program in Sweden. We found that both vaccines were successful in reducing the incidence of IPD in vaccinated children, which is in agreement with studies from other countries [13, 14]. Yet, the IPD incidence in the elderly from the pre- to the postvaccination period was unchanged in Sweden (from 42.7 per 100000 population in 2005 to 43.6 per 100000 population in 2016). In contrast, the United States, England and Wales, the Netherlands and Denmark reported an overall IPD reduction of up to 25% among patients aged ≥65 years [10, 13, 14, 20]. We estimated the number of reduced cases by age group and in total for Sweden by comparing the prevaccination period 2007–2009 to the postvaccination period 2013–2016 and by calculating differences with the observed data. This resulted in a point estimate of 2082 reduced cases in the post PCV10/PCV13 period, approximately 500 cases per year. The respective estimates per age groups were 185 cases in the age group 0–4 years, 1095 cases in the age group 5–64 years, and 802 cases in the age group ≥65 years. The reduction observed for the elderly was affected by the peak in IPD incidence in 2008 (Figure 1A).
We observed a major shift in the serotype distribution, where serotypes included in PCV7 diminished, whereas NVTs increased from 3.1 per 100000 in 2007 to 9.2 per 100000 in 2016 in the whole population. The largest increase was observed among the elderly, with an increase in NVTs by 21.2 cases per 100000 population between 2007 and 2016. In 2016, 69.2% (n = 900/1300) of all serotyped isolates were NVTs, and in the elderly NVTs constituted 71.7% (n = 597/833) of all serotyped isolates. The ecological niche for pneumococci is the nasopharynx of healthy children, and in agreement with our findings, we observed that 94% of pneumococci found in nasopharyngeal childhood carriage in 2015 in Stockholm where of NVTs [25]. Previously minor NVTs expanded during 2016, some potentially carrying antibiotic resistance, such as 15A [26]. However, the serotype diversity did not change significantly (P = .97 for PCV10 and P = .07 for PCV13 counties), in contrast with what we found previously in carriage [11, 25], although PCV10 counties showed a lower Simpson Index than PCV13 counties after vaccination.
In a previous prevaccination study we found that patients infected with NVTs had more underlying diseases [27], but whether infections with NVTs show different clinical manifestations remains to be investigated.
We compared the impact of using PCV10 or PCV13 in different counties and found that the additional serotypes present in both vaccines decreased irrespective of vaccine used. However, the impact on the additional serotypes included only in PCV13 differed. The incidence of serotype 6A decreased, whereas the incidence of serotype 3 remained similar with both vaccines. In comparison, a reduction in IPD caused by serotype 3 was observed in all age groups after PCV13 introduction in England and Wales, but not in the United States [13, 14]. A limited reduction of the incidence of serotype 3 after PCV13 introduction might be due to secular trends, but poor immunogenic response induced by the vaccine, is another possible explanation [15]. In all age groups, serotype 19A increased 7-fold from 2007 to 2013–16 in Swedish counties using PCV10, whereas the incidence remained unaltered in PCV13 counties. However, in 2016 we observed a lower incidence of IPD caused by 19A in PCV10 counties, which could be due to random variation or possibly increasing herd immunity in the population [28]. Follow up studies will reveal the cause. In children aged <5 years, there was no IPD caused by serotype 19A in PCV13 counties in 2013–2016, whereas the incidence was 1.1 per 100000 population in PCV10-counties. Other countries have observed an overall reduction of IPD caused by serotype 19A after PCV13 vaccination [13, 14]. In the Netherlands a reduction of IPD caused by serotype 19A was observed after PCV10 introduction. This was not concluded to be due to cross-protection of PCV10, and 19A had already decreased in carriage in toddlers before PCV10 was introduced [20]. Vaccination with PCV7 in Sweden might have selected for serotype 19A and it has continued to increase with PCV10, but not with PCV13. The increase in serotype 19A, primarily in nonvaccinated age groups, might reflect a lack of impact of PCV10 on carriage of 19A in the nasopharynx [18].
Interestingly, we observed an increase in serotype 6C in PCV10 counties that was not observed in PCV13 counties. Possibly this indicates that serotype 6A, which is included in PCV13 but not in PCV10, offers a cross-protection against serotype 6C. However, although serotype 19A decreased in children and we found a possible cross-protection with serotype 6C using PCV13, the overall relative impact on the IPD incidence of the 2 vaccines was not significantly different (P = .99). One explanation for this could be that NVTs excluding serotype 6C were higher for PCV13 than for PCV10 counties in the elderly.
Nonsusceptibility to Penicillin G was approximately 3% in 2007–2012 but increased to approximately 6% in 2013–16, mainly due to an increase of nonsusceptible NVTs, primarily serotypes 15A and 23B. Although there was a large reduction in the incidence of PCV7 serotypes after vaccination, 24% of PCV7 serotypes in 2016 were nonsusceptible to penicillin. Hence, the prevalence of nonsusceptible isolates within PCV7 types was higher in 2016 than before vaccination, which indicates differential effects or nonvaccine related causes (eg, antibiotic pressure).
In Sweden, vaccination coverage is high, and it is mandatory to report all cases of IPD, which ensures high validity. Reporting bias of IPD is limited because both laboratories and clinicians report IPD cases. Also we found no major increase in other invasive infections (ie, of N. meningitidis or H. influenzae) (Supplementary Figure 7). However, allocation to PCV10 or PCV13 usage was nonrandom and depended on negotiation in the respective counties, and we cannot exclude underlying differences between counties using PCV10 or PCV13, although socio-demographic differences were minor (Supplementary Table 2). The largest city in Sweden, Stockholm, is though a PCV13 county. We had a limited study size to evaluate serotype-specific effects in children. Hence, our data with regard to vaccine impact in children need to be interpreted with caution. Also our data was restricted to IPD cases, and the serotype distribution might differ for pneumococcal pneumonia, which is the major manifestation of pneumococcal infections.
In conclusion, our data show a protective effect of both vaccines, with a substantial reduction of IPD in the youngest vaccinated children but a limited overall effect in the elderly due to a vast expansion of NVTs. This limits the potential impact of using current pediatric PCVs to vaccinate the elderly. By comparing PCV10 and PCV13 immunization programs in the same country, we show a cross-protection between serotypes 6B and 6A but not between serotypes 19F and 19A, and it seems that serotype 6A in PCV13 has protective effects on serotype 6C, whereas the type 3 component in PCV13 provided no protection. Yet, the overall effect on IPD incidences in the whole population was not significantly different irrespective of vaccine used. The incidence of serotypes, where the effect differed, will influence the cost-effectiveness of which vaccine to use in immunization programs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Ingrid Andersson, Christina Johansson, Gunnel Möllerberg and Tiia Lepp for excellent technical expertise, and the Clinical Microbiological laboratories in Sweden for sending isolates.
Financial support. This work was supported by grants from the Knut and Alice Wallenberg foundation, the Swedish Research Council, the Foundation for Strategic research (SSF), Stockholm County Council and the ECDC project SpIDnet.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Weekly epidemiological record. World Health Organization 2007; 82:93–104. [Google Scholar]
- 2. Mortality GBD; Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feikin DR, Kagucia EW, Loo JD, et al. ; Serotype Replacement Study Group Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013; 10:e1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitney CG, Farley MM, Hadler J, et al. ; Active Bacterial Core Surveillance of the Emerging Infections Program Network Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737–46. [DOI] [PubMed] [Google Scholar]
- 5. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. [DOI] [PubMed] [Google Scholar]
- 6. Geno KA, Gilbert GL, Song JY, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 2015; 28:871–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pilishvili T, Lexau C, Farley MM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 9. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760–8. [DOI] [PubMed] [Google Scholar]
- 10. Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066–73. [DOI] [PubMed] [Google Scholar]
- 11. Galanis I, Lindstrand A, Darenberg J, et al. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J 2016; 47:1208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J 2007; 26:123–8. [DOI] [PubMed] [Google Scholar]
- 13. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15:535–43. [DOI] [PubMed] [Google Scholar]
- 14. Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dagan R, Patterson S, Juergens C, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013; 57:952–62. [DOI] [PubMed] [Google Scholar]
- 16. Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014; 14:839–46. [DOI] [PubMed] [Google Scholar]
- 17. Domingues CM, Verani JR, Montenegro Renoiner EI, et al. ; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med 2014; 2:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammitt LL, Akech DO, Morpeth SC, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2014; 2:e397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jokinen J, Rinta-Kokko H, Siira L, et al. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children–a population-based study. PLoS One 2015; 10:e0120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knol MJ, Wagenvoort GH, Sanders EA, et al. Invasive pneumococcal disease 3 years after introduction of 10-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis 2015; 21:2040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henriqus Normark B, Christensson B, Sandgren A, et al. Clonal analysis of Streptococcus pneumoniae nonsusceptible to penicillin at day-care centers with index cases, in a region with low incidence of resistance: emergence of an invasive type 35B clone among carriers. Microb Drug Resist 2003; 9:337–44. [DOI] [PubMed] [Google Scholar]
- 22. Olsson-Liljequist B, Larsson P, Walder M, Miörner H. Antimicrobial susceptibility testing in Sweden. III. Methodology for susceptibility testing. Scand J Infect Dis Suppl 1997; 105:13–23. [PubMed] [Google Scholar]
- 23. van Buuren S,G-OK. Multivariate imputation by chained equations in R. J Stat Softw 2011; 45:1–67. [Google Scholar]
- 24. Grundmann H, Hori S, Tanner G. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J Clin Microbiol 2001; 39:4190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindstrand A, Galanis I, Darenberg J, et al. Unaltered pneumococcal carriage prevalence due to expansion of non-vaccine types of low invasive potential 8years after vaccine introduction in Stockholm, Sweden. Vaccine 2016; 34:4565–71. [DOI] [PubMed] [Google Scholar]
- 26. Kawaguchiya M, Urushibara N, Kobayashi N. Multidrug resistance in non-PCV13 serotypes of Streptococcus pneumoniae in Northern Japan, 2014. Microb Drug Resist 2017; 23:206–14. [DOI] [PubMed] [Google Scholar]
- 27. Browall S, Backhaus E, Naucler P, et al. Clinical manifestations of invasive pneumococcal disease by vaccine and non-vaccine types. Eur Respir J 2014; 44:1646–57. [DOI] [PubMed] [Google Scholar]
- 28. Wilson R, Cohen JM, Reglinski M, et al. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog 2017; 13:e1006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.