A hospital-acquired and ventilator-associated bacterial pneumonia phase 3 clinical trial cost model estimates costs at $89600 per patient. The biggest cost driver is screen failure rates. Strategies to decrease screen failures are needed to improve the return on investment of these critically important studies.
Keywords: clinical trial cost, phase 3 clinical trials, hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia, habp vabp
Abstract
Background
Studies indicate that the prevalence of multidrug-resistant infections, including hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP), has been rising. There are many challenges associated with these disease conditions and the ability to develop new treatments. Additionally, HABP/VABP clinical trials are very costly to conduct given their complex protocol designs and the difficulty in recruiting and retaining patients.
Methods
With input from clinicians, representatives from industry, and the US Food and Drug Administration, we conducted a study to (1) evaluate the drivers of HABP/VABP phase 3 direct and indirect clinical trial costs; (2) to identify opportunities to lower these costs; and (3) to compare (1) and (2) to endocrine and oncology clinical trials. Benchmark data were gathered from proprietary and commercial databases and used to create a model that calculates the fully loaded (direct and indirect) cost of typical phase 3 HABP/VABP endocrine and oncology clinical trials.
Results
Results indicate that the cost per patient for a 200-site, 1000-patient phase 3 HABP/VABP study is $89600 per patient. The cost of screen failures and screen failure rates are the main cost drivers.
Conclusions
Results indicate that biopharmaceutical companies and regulatory agencies should consider strategies to improve screening and recruitment to decrease HABP/VABP clinical trial costs.
Hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) are acute infections occurring in hospitalized patients and are associated with high mortality risk [1–6]. The public health threat of multidrug-resistant (MDR) bacterial infections is increasing [2, 4, 5, 7–11].
New antibiotics are needed to treat MDR bacteria, and several entities are working to increase drug development efforts [12–16]. However, despite the critical need, biopharmaceutical organization investment in new antibiotics for HABP/VABP and other infectious diseases and new drug approvals has not kept up with the need [17–22]. Many pharmaceutical companies have chosen to invest in other therapeutic areas such as oncology or endocrinology [23].
In 2015, 2 independent surveys of pharmaceutical companies were conducted to understand the lack of R&D (research and development) investment [19, 20]. The primary barrier, stated by 80% and 84% of the 2 surveys’ respondents, was economic: High costs and low return on investment are major reasons for suspension of antibiotic clinical trial development [19, 20]. Individuals at a 2009 workshop on HABP/VABP clinical trials, cosponsored by the US Food and Drug Administration (FDA) and several professional societies, stated “extraordinarily high costs” as a major challenge and estimated that “recent studies of HABP and/or VABP cost $60000–$80000 per patient enrolled [$66513–$88684 in 2014 dollars], resulting in phase 3 trial program costs of >$75 million per study [>$82.8 million in 2014 dollars]” [24–26].
The survey respondents noted complexity and scientific risk as major barriers in all phases of development with “medical/scientific reasons” and “unreasonably long recruitment period” as frequent reasons for stopping the clinical development of new antibacterial agents [20]. The 2009 workshop proceedings describe many of the scientific and medical reasons in detail, including challenges in enrolling seriously ill patients in treatment protocols and confounding associated with prior antibiotic exposure [27]. Information from 2 multinational phase 3 HABP/VABP studies also illustrates this operational complexity, in which ≤10% of screened patients met protocol eligibility criteria, and >250 sites were required in 38 countries to enroll >1500 patients over 2.5 years [24].
To our knowledge, studies identifying key cost drivers of clinical trials have been conducted [28], but no studies have calculated the specific total cost of HABP/VABP drug development in the United States using a comprehensive cost model to identify opportunities for decreasing overall costs. To address this knowledge gap, we conducted a comprehensive study in 2015 to assess the cost of conducting a phase 3 HABP/VABP clinical trial, compare those costs to phase 3 oncology and endocrine clinical trials, and identify opportunities to lower these costs. The results of this study aim to inform future biopharmaceutical company investment in the development of much-needed antibacterial drugs.
METHODS
We developed a comprehensive cost model estimating the cost of a phase 3 HABP/VABP clinical trial with comparison to phase 3 oncology and endocrine trials. The model was developed by Tufts Center for the Study of Drug Development (CSDD) in partnership with the Clinical Trials Transformation Initiative (CTTI) HABP/VABP Studies Project [29]. A draft of the model was presented for feedback and adjustments at a February 2015 CTTI multi-stakeholder workshop including representatives from drug development companies, the FDA, practicing clinicians from academic medical centers and clinical trial research centers, and patient advocacy groups [30].
HABP/VABP model results were compared with estimates from Oracle Health Sciences ClearTrial Plan and Source Cloud Service under the same clinical trial assumptions to assess validity of the model. The cost model was created in Microsoft Excel 2013 and 2016. Total cost, total cost per patient, and analyses of cost drivers via sensitivity analyses for HABP/VABP, oncology, and endocrine phase 3 clinical trials were calculated from the model.
Clinical Trial Cost Element Selection and Benchmark Data Compilation
We compiled a detailed list all of cost elements prior to creating the model. Cost elements were selected based on institutional knowledge and internal databases, as well as a literature review on clinical trial financial planning models [28, 31–34]. Costs were classified as being per-patient direct costs, per-trial and per-site direct costs, and per-trial indirect costs. This classification has also been used elsewhere [28].
We gathered benchmark data to create the cost model, which calculates a fully loaded (direct and indirect) cost profile of a typical phase 3 HABP/VABP, oncology, and endocrine trial. All cost data were inflation-adjusted to reflect 2014 dollars. Data gathered from the Tufts CSDD internal databases are from a number of previous single- and multisponsored studies that collect actual clinical trial data. Commercially available data were selected based on companies that are well established, widely utilized, and offer reputable services. All groups and individuals providing data asked that the data remain confidential.
Per-patient direct costs are defined as study contact costs that vary by the number of patients (eg, cost of patient recruitment, informed consent, and screen fail). Table 1 lists all per-patient direct costs and data sources included in the model.
Table 1.
List of Per-Patient Direct Costs, Per-Trial and Per-Site Cost Elements, Per-Trial Indirect Cost Elements, and Data Sources Included in the Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trial Costing Model
| Type of Cost | Cost Element | Source(s) |
|---|---|---|
| Per-patient direct cost elements | • Patient recruitment • Patient retention • Informed consent • Protocol study procedural cost • Laboratory costs • Data entry costs • Query resolution costs |
• Medidata Solutions; median 2014 costs |
| • Clinical trial insurance cost | • Marsh Insurance • Clinical Trial Insurance Services Ltd. |
|
| • Screen-fail costs | • IMS Health [60] | |
| • Screen-fail and randomization rates | • Tufts CSDD internal databases • ClinicalTrials.gov as of January 2015 • Barriere [24], Harper and Li [38] • CTTI ABDD Pilot Study Workshop [30] • Centerphase Solutions |
|
| • Country–investigative site distribution and clinical-trial index costs by country | • IMS Health (2016) • IMS Health [35] |
|
| Per-trial and per-site direct cost elements | Personnel costs • Sponsor personnel • Clinical pharmacology • CRO/site contract management • Document manager • Clinical research associate • Physician • Statistical programmer • Study manager • Pharmaceutical technician • Product development • Site personnel • Principal investigator • Co-investigator • Research nurse/study coordinator • Technician • Other administration • Recruitment specialist • Microbiologist • Regulatory affairs • Pharmacist/pharmacy tech |
• Tufts CSDD Internal Databases • Glassdoor.com as of January 2015 |
| Per-trial site and clinical supply costs • Institutional review board fees (local to each site) • Amendment fees • Record keeping and storage • Clinical trial site recruitment costs (marketing) • Primary investigator training and travel costs/study monitor travel costs • Meeting costs for clinical travel team (venue, food, travel) • Clinical supply costs (assumed to be fixed) • Manufacturing • Comparator • Trial insurance costs |
• Tufts CSDD internal databases • Tufts University • Medidata Solutions • Centerwatch |
|
| Printing/paper/data costs • Number of translations for: • Investigator brochure • Printing • Translation • Study protocol • Printing • Translation • Informed consent • Printing • Translation • Case report form • Printing • Translation • Data costs • Server charges for EDC • IT charges for EDC • Storage costs • Data entry costs |
• Tufts CSDD internal databases • Tufts University • Medidata Solutions • Tyco Health • Ritz Carlton Hotel, Boston • Taj Hotel, Boston • IMS Health [35] |
|
| Per-trial indirect cost elements | Upper management time • Vice President • Executive (Medical) Director • Associate Director • Biostatistics Manager |
• Tufts CSDD internal databases • Glassdoor.com as of January 2015 |
| Overhead costs • Travel and meetings • Depreciation (equipment) • Depreciation (buildings) • Other infrastructure costs • Material and office supplies • IT costs |
• Tufts CSDD internal databases • Tufts University |
|
| Other costs • Administration costs • Training and professional development • Employee benefits |
• Tufts CSDD internal databases • Centerwatch • Tufts University |
Per-patient costs were adjusted by country.
Abbreviations: ABDD, Antibacterial Drug Development; CRO, contract research organization; CSDD, Center for the Study of Drug Development; CTTI, Clinical Trials Transformation Initiative; EDC, electronic data capture; IMS, Intercontinental Marketing Services; IT, Information Technology.
-
• Country-investigative site distribution. The proportion of investigative sites, clinical staff personnel, and the number of patients screened and enrolled were calculated by country. Data were provided by IMS Health in 2016. Proportions were calculated from the number of total industry-completed phase 3 clinical trials conducted worldwide for:
o Oncology and endocrine therapeutic areas in 2015.
o Nosocomial pneumonia and ventilator-associated pneumonia disease indications for 2010–2015. Data span 5 years given the limited number of clinical trials under these disease indications.
• Per-patient costs indexed by country. IMS Health provided an index of comparative median per-visit grant cost for select countries for 2013–2014 [35]. Per-patient clinical trial costs were adjusted by the index as per-patient costs are different across countries. Countries not included in the IMS index were assigned a mean index percentage based on geography: Western Europe, Eastern Europe, Asia Pacific, and Latin America.
Per-patient costs were aggregated into the total per-patient direct cost amount, based on the assumed total number of patients randomized in the trial.
Per-trial and per-site direct costs are defined as costs that are attributed to the trial (eg, personnel costs, trial insurance, and cost of electronic data capture). Per-trial indirect costs are not directly attributable to the trial (eg, upper management resource costs, overhead costs). The components within each category were aggregated to derive a total cost for each. Table 1 lists all per-trial and per-site direct costs and per-trial indirect costs included in the model.
Clinical Trial Assumptions and Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Cost Model Sensitivity Analyses
Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trial Assumptions
Based on feedback from the ABDD HABP/VABP Studies Team and additional stakeholders, we assumed that a phase 3 HABP/VABP trial would be conducted in 200 sites and 1000 patients globally [30]. We also assumed that the randomization rate for HABP/VABP trials is 1 patient randomized per 100 screened based on feedback from the CTTI Workshop and Barriere [24]. Table 2 summarizes all assumptions.
Table 2.
Clinical Trial Study Assumptions and Tufts Center for the Study of Drug Development Estimates of the Average Cost per Patient for Endocrine, Oncology, and Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trials
| Therapeutic Area | Total Sites (All Locations) | Total Subjects (All Locations) | Total No. of Countries | Randomization Rate | Per-Patient Direct Cost ($000) | Per-Trial Direct Cost ($000) | Per-Trial Indirect Cost ($000) | Total Cost per Patient ($000) |
|---|---|---|---|---|---|---|---|---|
| HABP/VABP | 200 sites | 1000 subjects | 52 countries | 1 patient randomized per 100 screened | $66.1 | $20.1 | $3.3 | $89.6 |
| Oncology | 279 sites | 448 subjects | 74 countries | 25 patients randomized per 100 screened | $18.1 | $61.8 | $7.5 | $87.3 |
| Endocrine | 123 sites | 582 subjects | 47 countries | 45 patients randomized per 100 screened | $9.6 | $42.3 | $5.8 | $57.7 |
Abbreviations: HABP/VABP, hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. $000 = Thousands.
Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trial Cost Model Sensitivity Analyses
Changing the following assumptions, we conducted sensitivity analyses to assess the main drivers of the cost model: number of sites, number of patients, procedural cost, screen failure rate, cost of screen fails, and the cost of patient recruitment. Items were selected based on institutional knowledge and recommendations from Knirsch et al and Donnelly et al [36, 37]. See Table 3 for sensitivity analysis details.
Table 3.
Sensitivity Analysis: Elements, Changes in Assumption, and Rationale
| Element | Change in Assumption | Rationale/References |
|---|---|---|
| No. of sites | ± 50 sites | In line with number of sites in Barriere [24] |
| No. of patients | ± 200 patients | In line with number of patients described in Barriere [24] and US Food and Drug Administration draft guidance [61] |
| Clinical trial protocol complexity (proxy measure: clinical trial procedural costs) | ± $500 per patient | Dollar amount corresponds to conducting 2 more or fewer urinalysis, hematology, and serum chemistry procedures; and 1 fewer chest radiograph and 12-lead electrocardiogram across the treatment phase |
| Eligibility criteria severity (proxy measure: the screen failure rate) | ± 10 individuals screened per 1 patient enrolled, eg, instead of 100 individuals screened to randomize 1 patient, 110 or 90 individuals are screened to randomize 1 patient | Allows to see the difference in costs resulting from a modest change in screen failure rate |
| Screening complexity (proxy measure: investigator payment per screen failure, ie, the cost of screen fails) | ± $60 per patient | Dollar amount corresponds to changes in protocol procedures, eg, conducting a clinical pulmonary infection score test vs 3 individual clinical criteria [26] |
| The cost of patient recruitment (ie, advertisement costs) | ± $50 per patient | Dollar amount corresponds to modest changes in time spent on records review, internal communications, and announcements at grand rounds |
Sensitivity analyses were conducted separately for each variable as well as by changing 2 assumptions together. Assumptions that were changed together were:
• Scenario 1: Changing the number of sites by 50 sites and changing the number of patients by 200 patients.
• Scenario 2: Changing the cost of screen fails by $60 per patient and the cost of recruitment by $50 per patient.
• Scenario 3: Changing the cost of screen fails by $60 per patient and the procedural cost by $500 per patient.
• Scenario 4: Changing the cost of screen fails by $60 per patient and the screen failure rate to 99.09% and 98.89% from 99% (110:1 and 90:1 from 100:1 screened:enrolled)
Both assumptions were either increased or decreased together; that is, one assumption was not increased while the other was decreased.
Oncology and Endocrine Phase 3 Clinical Trial Assumptions
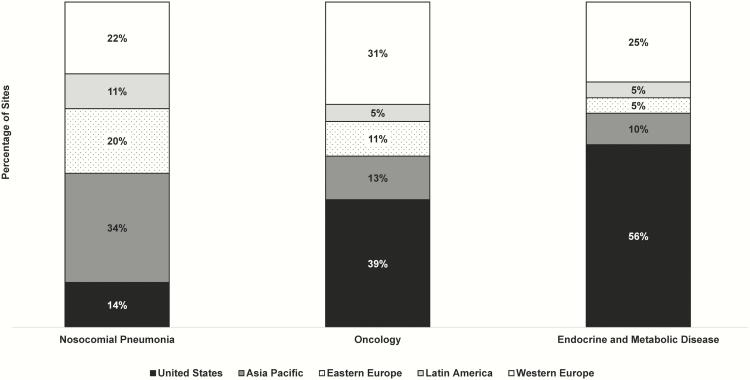
For comparison, we assumed that a typical phase 3 oncology and endocrine trial would be conducted in 279 and 123 sites globally, respectively. Assumptions are based on internal Tufts CSDD databases containing clinical trial operations data for oncology and endocrine trials from multiple biopharmaceutical companies conducted as of 2015, and Harper and Li [38] (endocrine only). Table 2 summarizes all assumptions and Figure 1 shows the percentage of sites by therapeutic area and region.
Figure 1.
Global region and site distribution assumptions in the phase 3 clinical trial costing model for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia, oncology, and endocrine.
Model without underlying data.xlsx contains the cost model without any underlying proprietary data (see Supplementary Appendix).
RESULTS
Average Cost per Patient for a Typical Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trial, Compared to Endocrine and Oncology Trials
We estimate that the overall cost of a phase 3 HABP/VABP clinical trial was $89.6 million overall, and that the overall cost per patient of a phase 3 HABP/VABP clinical trial was $89600 per patient. By comparison, we estimate that the overall cost of a phase 3 oncology clinical trial was $39.1 million overall, or $87300 per patient, and that the overall cost of a phase 3 endocrine clinical trial was $33.6 million overall, or $57700 per patient. As indicated in Table 2, per patient, phase 3 HABP/VABP clinical trials were $2200 more expensive than oncology clinical trials and $32100 more expensive than endocrine trials.
Per-patient direct costs made up the majority of phase 3 HABP/VABP trials, accounting for 74% of overall costs, whereas per-trial direct costs made up the majority of phase 3 oncology and endocrine trials (71% and 74%, respectively).
Using the same assumptions for number of global sites and number of patients, but assuming 43 countries instead of 52, Oracle’s ClearTrial solution also measured the cost of a phase 3 HABP/VABP clinical trial to be approximately $163 million, or $163000 per patient.
Cost Impact of Changing a Single Model Assumption in a Typical Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trial
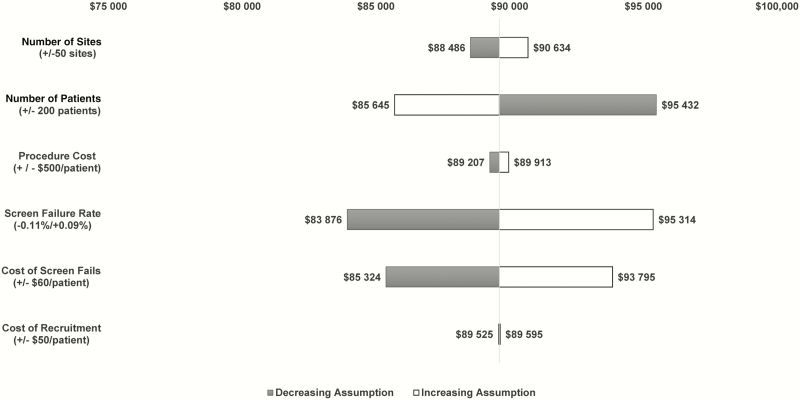
We estimated the change in overall cost and cost per patient of a typical phase 3 HABP/VABP clinical trial by modifying key assumptions in the model to determine the main cost drivers as described in the methodology. Figure 2 shows the impact of changing each assumption. Changing the screen failure rate yields the largest variation in cost. When decreasing the number of individuals screened from 100 to 90 patients to randomize 1 patient, the cost decreases by approximately $5700 per patient, or $5.7 million overall.
Figure 2.
Cost impact of changing a single model assumption in a typical phase 3 hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia trial.
Changing the cost of screen fails yields the second largest variation in cost. When the cost of screen fails is decreased by $60 per patient, the cost decreases by approximately $4200 per patient, or $4.2 million overall.
Changing the number of patients yields the third largest variation in cost. Although the overall cost of the trial increased to $102.8 million, increasing the number of patients to 1200 decreases the cost by approximately $3915 per patient as fixed costs are spread across more patients.
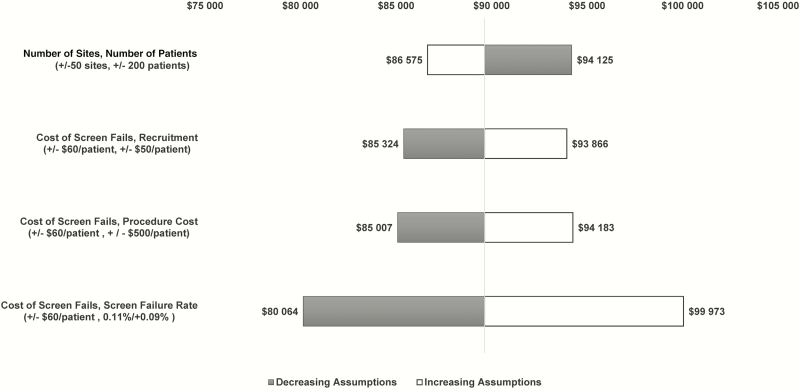
Cost Impact of Changing 2 Model Assumptions in a Typical Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia Phase 3 Clinical Trial
We estimated the change in overall cost and cost per patient of a typical phase 3 HABP/VABP clinical trial by modifying multiple key assumptions in the model to determine the main cost drivers as described in the methodology. Figure 3 shows the impact of changing each assumption. Scenario 4 (changing the cost of screen fails by $60 per patient and the screen failure rate by 10 individuals screened to randomize 1 patient) yields the largest variation in cost. When decreasing the cost of screen fails by $60 per patient and decreasing the number of individuals screened from 100 to 90 patients to randomize 1 patient, the cost decreases by approximately $9500 per patient, or $9.5 million overall. Scenarios 1–3 yield similar variations from the baseline estimate of approximately $4300 per patient.
Figure 3.
Cost impact of changing 2 model assumptions in a typical phase 3 hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia trial.
Oracle also conducted sensitivity analyses. It found that decreasing the cost of screen fails by $60 and decreasing the number of individuals screened decreases the cost by approximately $16000 per patient. Oracle’s sensitivity analysis of increasing the number of clinical trial sites by 50 and the number of patients by 200 showed an increase in overall trial costs to $195.3 million, which translated to a cost decrease of approximately $318 per patient.
DISCUSSION
We created a fully loaded (direct and indirect) cost model of a typical phase 3 HABP/VABP clinical trial. The overall cost of a phase 3 HABP/VABP clinical trial was calculated to be approximately $89.6 million, or $89600 per patient (1000 patients in 200 sites). Per patient, phase 3 HABP/VABP clinical trials were $2200 more expensive than oncology trials and $32100 more expensive than endocrine trials. Sensitivity analyses indicate that the screen failure rate and cost of screen fails are the main cost drivers for phase 3 HABP/VABP clinical trials.
Results from the model corroborate the findings from the 2009 workshop [24–26]. Our estimates are $6.8 million higher than the 2009 workshop estimate, or $920 higher than the 2009 workshop per-patient maximum. These variations could be due to study assumptions as the manuscripts do not provide detail on how the 2009 workshop estimates were calculated.
Results from our model differ from results from Oracle’s analysis by $73400 per patient. Variations could be attributed to differences in investigator payments for screen failures; Oracle’s model used the same cost of screen fails for all countries, whereas our model adjusted this cost by a country deflator. Additionally, Oracle’s model measures cost based on hours spent on each activity, calculating 409464 total hours for the baseline scenario. Our model relies on benchmark data across all disease indications, calculating 106000 total hours.
Although Oracle’s ClearTrial tool estimates are greater than our estimates, its sensitivity analyses demonstrate that although increasing the number of patients increases the overall cost of the trial, the cost per patient decreases. A possible explanation for this is that a larger percentage of patients are coming from sites in regions where costs are significantly lower (eg, Eastern Europe and Latin American).
Study results indicate that 75% of HABP/VABP clinical trial costs are attributed to per-patient direct cost variables. This could be explained by the high screen failure rate for HABP/VABP clinical trials. While the cost of screen failures for infectious disease clinical trials is lower than the cost for screen fails for oncology and endocrine clinical trials, significantly more patients fail screening; for example, to obtain 1000 patients in a HABP/VABP phase 3 trial, 100000 patients must be screened, thereby increasing the overall cost of screen fails by an order of magnitude. The literature [3, 4, 10, 17, 24–27, 37, 39–57] and sensitivity analyses corroborate this hypothesis, as demonstrated in Figure 2 and Figure 3. Decreasing both the cost of screen fails as well as the screen failure rate leads to greater decreases in clinical trial costs for HABP/VABP phase 3 clinical trials. This suggests that biopharmaceutical companies and research sites should consider ways to increase the number of potentially eligible patients. The “low-hanging fruit” of streamlining exclusion criteria, to only trial specific essential elements, has mostly been picked. Novel approaches such as using predictive models to find and consent patients earlier in the disease process should be explored [36, 58, 59]. Working to decrease screen failures will have a greater impact on lowering overall costs than trying to streamline already lean clinical trial protocols.
There are some limitations to this study. Assessment of certain variables for sensitivity analyses is limited and, moreover, the model does not account for the risk of needing to hire another contract research organization in cases where additional sites and/or countries are added due to poor enrollment.
Additionally, some cost elements in the model, eg, resource hours and clinical supply costs, are median values across all therapeutic areas and not specific to HABP/VABP, oncology, or endocrinology. Particularly, clinical supply costs are highly variable depending on the drug type, and so the median value may not be generalizable.
With a per-patient price tag of almost $90000, combined with the potential for low return on investment (ROI), HABP/VABP trials very well stand to be priced out of the market. Clinical trials of new oncology and endocrine drugs usually enroll outpatient or less acutely ill patients, which may account for the more favorable screening-to-enrollment ratios. In addition, treatment durations for oncology and endocrine drugs are longer and therefore these therapeutic areas have a larger potential for a greater ROI. In comparison, antibacterial drugs have relatively short treatment durations, decreasing the overall ROI. Therefore, it is critically important to investigate and implement ways to improve the feasibility of HABP/VABP trials.
By applying the insights gathered from this cost model, pharmaceutical and biotechnology companies—along with other clinical research stakeholders and programs—can move closer to creating a more efficient and financially viable drug development process for HABP/VABP. Specifically, biopharmaceutical companies, regulatory agencies, and research sites should consider ways to increase the number of potentially eligible patients (ie, decrease screen failures) [36, 58, 59] as an opportunity to decrease HABP/VABP clinical trial costs. This will allow the clinical trials and regulatory communities to address the need for new HABP/VABP antibiotics and provide the medical community with more clinically informative data to aid in treatment of these desperately ill patients.
Supplementary Material
Notes
Acknowledgments. The authors thank Rafael Campo at Medidata Solutions, Lance Converse at Epharma Solutions, Jeff Starkey at IMS, Michael Lange at Bioclinica, Lorie McClain at Bioclinica, Jennifer Bush at Oracle Health Sciences, Kelly Petruska at Oracle Health Sciences, Osborne Jackson of the Federal Reserve Bank of Boston, and Vance Fowler at Duke University School of Medicine, for their contributions to the study.
Disclaimer. Views expressed in this manuscript do not necessarily reflect the official policies of the US Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US government.
Financial support. This work was supported by the US Food and Drug Administration (grant number R18FD005292) and CTTI member organizations, whose annual fees support CTTI infrastructure expenses and projects.
Potential conflicts of interest. P. T. received an honorarium for Wisdom data safety monitoring board participation from University of California, San Francisco. C. A. B. is employed by EMMES as of 26 June 2017 but was not employed there during the analysis or manuscript preparation. T. L. H. has received consulting fees from Basilea Pharmaceutica. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 2. Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 2012; 33:250–6.2. [DOI] [PubMed] [Google Scholar]
- 3. Kollef MH. Review of recent clinical trials of hospital-acquired pneumonia and ventilator-associated pneumonia: a perspective from academia. Clin Infect Dis 2010; 51(suppl 1):S29–35. [DOI] [PubMed] [Google Scholar]
- 4. Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 2010; 51(suppl 1):S120–5. [DOI] [PubMed] [Google Scholar]
- 5. American Thoracic Society/Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 6. Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 2013; 19:216–28. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Antibiotic resistant threats in the United States, 2013. Atlanta, GA: CDC, 2013:114. [Google Scholar]
- 8. Weiner LM, Webb AK, Limbago B et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51(suppl 1):S81–7. [DOI] [PubMed] [Google Scholar]
- 10. Chastre J, Luyt CE. Other therapeutic modalities and practices: implications for clinical trials of hospital-acquired or ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S54–8. [DOI] [PubMed] [Google Scholar]
- 11. Patel DA, Michel A, Stephens J, Weber B, Petrik C, Charbonneau C. An economic model to compare linezolid and vancomycin for the treatment of confirmed methicillin-resistant Staphylococcus aureus nosocomial pneumonia in Germany. Infect Drug Resist 2014; 7:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magill SS, Edwards JR, Bamberg W et al. . Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Innovative Medicines Initiative. New drugs for bad bugs. Available at:http://www.imi.europa.eu/content/nd4bb. Accessed 5 September 2017.
- 14. Centers for Disease Control and Prevention. Detect and protect against antibiotic resistance Available at:https://www.cdc.gov/drugresistance/solutions-initiative/index.html. Accessed 5 September 2017.
- 15. Infectious Diseases Society of America. Antibiotic development: the 10 x ’20 initiative: bringing new antibiotics to patients who need them. Available at:http://www.idsociety.org/templates/twocolumnnavigation10X20.aspx?pageid=32212258833. Accessed 5 September 2017.
- 16. Woodcock J. Three encouraging steps towards new antibiotics. Available at:http://blogs.fda.gov/fdavoice/index.php/tag/gain-act/. Accessed 5 September 2017.
- 17. Power E. Impact of antibiotic restrictions: the pharmaceutical perspective. Clin Microbiol Infect 2006; 12(suppl 5):25–34. [DOI] [PubMed] [Google Scholar]
- 18. Braine T. Race against time to develop new antibiotics. Bull World Health Organ 2011;. 89:88–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Review on Antimicrobial Resistance. Survey of actors involved in the development of antimicrobials: high level results. Available at: https://amr-review.org/sites/default/files/Surveyofactors.pdf. Accessed 5 September 2017.
- 20. Bettiol E, Wetherington JD, Schmitt N, Harbarth S; COMBACTE Consortium Challenges and solutions for clinical development of new antibacterial agents: results of a survey among pharmaceutical industry professionals. Antimicrob Agents Chemother 2015; 59:3695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boucher HW, Talbot GH, Benjamin DK Jr et al. . Infectious Diseases Society of America 10 x ’20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kermani F. Boosting new antibiotic drug development in Europe. Available at: http://www.pharmtech.com/boosting-new-antibiotic-drug-development-europe. Accessed 5 September 2017.
- 23. Center for Drug Evaluation and Research, Food and Drug Administration. Segmentation of active FDA-regulated clinical trials for drugs by therapeutic category. In: Mathieu MP, ed. Parexel Biopharmaceutical R&D statistical sourcebook, 2016/2017. Waltham, MA: Parexel, 2016:90. [Google Scholar]
- 24. Barriere SL. Challenges in the design and conduct of clinical trials for hospital-acquired pneumonia and ventilator-associated pneumonia: an industry perspective. Clin Infect Dis 2010; 51(suppl 1):S4–9. [DOI] [PubMed] [Google Scholar]
- 25. Talbot GH. Considerations in undertaking a clinical development program for hospital-acquired bacterial pneumonia and/or ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51(suppl 1):S144–9. [DOI] [PubMed] [Google Scholar]
- 26. Spellberg B, Talbot G; Infectious Diseases Society of America, American College of Chest Physicians, American Thoracic Society, Society of Critical Care Medicine Recommended design features of future clinical trials of antibacterial agents for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51(suppl 1):S150–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bartlett JG, Barie PS, Niederman MS, Wunderink RG. Workshop on clinical trials of antibacterial agents for hospital-acquired pneumonia and ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S1–3. [DOI] [PubMed] [Google Scholar]
- 28. Sertkaya A, Wong HH, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials 2016; 13:117–26. [DOI] [PubMed] [Google Scholar]
- 29. Clinical Trials Transformation Initiative. ABDD HABP/VABP studies project. Available at:https://www.ctti-clinicaltrials.org/projects/habpvabp-studies. Accessed 5 September 2017.
- 30. Clinical Trials Transformation Initiative. ABDD HABP/VABP Pilot Study Workshop. Available at:https://www.ctti-clinicaltrials.org/briefing-room/meetings/abdd-habpvabp-pilot-study-workshop; https://www.ctti-clinicaltrials.org/files/final_attendee_list_habpvabp_pilotstudy_24feb2015.pdf. Accessed 5 September 2017.
- 31. DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 2016; 47:20–33. [DOI] [PubMed] [Google Scholar]
- 32. Spilker B. Cost of clinical trials, projects, and pharmaceutical development. guide to clinical trials. New York: Raven Press, Ltd, 1991. [Google Scholar]
- 33. Morgan S, Grootendorst P, Lexchin J, Cunningham C, Greyson D. The cost of drug development: a systematic review. Health Policy 2011; 100:4–17. [DOI] [PubMed] [Google Scholar]
- 34. Emanuel EJ, Schnipper LE, Kamin DY, Levinson J, Lichter AS. The costs of conducting clinical research. J Clin Oncol 2003; 21:4145–50. [DOI] [PubMed] [Google Scholar]
- 35. IMS Health: indexed clinical trial costs: cost per visit in clinical trials, 2013-2013. In: Mathieu MP, ed. Parexel biopharmaceutical R&D statistical sourcebook 2014/2015. Waltham, MA: Parexel, 2014:3. [Google Scholar]
- 36. Knirsch C, Alemayehu D, Botgros R et al. . Improving conduct and feasibility of clinical trials to evaluate antibacterial drugs to treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: recommendations of the Clinical Trials Transformation Initiative antibacterial drug development project team. Clin Infect Dis 2016; 63(suppl 2):S29–36. [DOI] [PubMed] [Google Scholar]
- 37. Donnelly H, Alemayehu D, Botgros R et al. . Streamlining safety data collection in hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia trials: recommendations of the Clinical Trials Transformation Initiative antibacterial drug development project team. Clin Infect Dis 2016; 63(suppl 2):S39–45. [DOI] [PubMed] [Google Scholar]
- 38. Harper B, Lee G. Emerging clinical trial recruitment benchmarking metrics: the recruitment funnel analysis. In: Mathieu MP, ed. Parexel pharmaceutical R&D statistical sourcebook 2015/2016. Waltham, MA: Parexel, 2015. [Google Scholar]
- 39. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51(suppl 1):S81–7. [DOI] [PubMed] [Google Scholar]
- 40. Niederman MS. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis 2010; 51(suppl 1):S12–7. [DOI] [PubMed] [Google Scholar]
- 41. Gilbert DN. Scenario 1: a patient with hospital-acquired bacterial pneumonia–introduction to clinical trial design issues. Clin Infect Dis 2010; 51(suppl 1):S10–1. [DOI] [PubMed] [Google Scholar]
- 42. Niederman MS. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis 2010; 51(suppl 1):S12–7. [DOI] [PubMed] [Google Scholar]
- 43. Powers JH. Recommendations for improving the design, conduct, and analysis of clinical trials in hospital-acquired pneumonia and ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sorbello A, Komo S, Valappil T, Nambiar S. Registration trials of antibacterial drugs for the treatment of nosocomial pneumonia. Clin Infect Dis 2010; 51(suppl 1):S36–41. [DOI] [PubMed] [Google Scholar]
- 45. File TM., Jr Recommendations for treatment of hospital-acquired and ventilator-associated pneumonia: review of recent international guidelines. Clin Infect Dis 2010; 51(suppl 1):S42–7. [DOI] [PubMed] [Google Scholar]
- 46. Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S48–53. [DOI] [PubMed] [Google Scholar]
- 47. Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis 2010; 51(suppl 1):S59–66. [DOI] [PubMed] [Google Scholar]
- 48. Napolitano LM. Use of severity scoring and stratification factors in clinical trials of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S67–80. [DOI] [PubMed] [Google Scholar]
- 49. Chastre J, Trouillet JL, Combes A, Luyt CE. Diagnostic techniques and procedures for establishing the microbial etiology of ventilator-associated pneumonia for clinical trials: the pros for quantitative cultures. Clin Infect Dis 2010; 51(suppl 1):S88–92. [DOI] [PubMed] [Google Scholar]
- 50. Niederman MS. The argument against using quantitative cultures in clinical trials and for the management of ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S93–9. [DOI] [PubMed] [Google Scholar]
- 51. Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap!Clin Infect Dis 2010; 51(suppl 1):S103–10. [DOI] [PubMed] [Google Scholar]
- 52. Kleppinger CF, Ball LK. Building quality in clinical trials with use of a quality systems approach. Clin Infect Dis 2010; 51(suppl 1):S111–6. [DOI] [PubMed] [Google Scholar]
- 53. Laessig KA. End points in hospital-acquired pneumonia and/or ventilator-associated pneumonia clinical trials: Food and Drug Administration perspective. Clin Infect Dis 2010; 51(suppl 1):S117–9. [DOI] [PubMed] [Google Scholar]
- 54. Wunderink RG. Surrogate markers and microbiologic end points. Clin Infect Dis 2010; 51(suppl 1):S126–30. [DOI] [PubMed] [Google Scholar]
- 55. Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis 2010; 51(suppl 1):S131–5. [DOI] [PubMed] [Google Scholar]
- 56. Bradley JS. Considerations unique to pediatrics for clinical trial design in hospital-acquired pneumonia and ventilator-associated pneumonia. Clin Infect Dis 2010; 51(suppl 1):S136–43. [DOI] [PubMed] [Google Scholar]
- 57. Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 2010; 51(suppl 1):S120–5. [DOI] [PubMed] [Google Scholar]
- 58. Corneli A, Perry B, Collyar D, Powers JH, Donnelly HK, Anda CD.. Formative research findings on the design of an early enrollment clinical trial on hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP). In: Annual Meeting of the Society for Clinical Trials/International Clinical Trials Methodology Conference, Liverpool, UK, 2017. [Google Scholar]
- 59. Bergin SP, Coles A, Farley J, Santiago J, Calvert SB. Predicting pneumonia: a prospective observational study of the risk factors for hospital-acquired and ventilator-associated bacterial pneumonia (A2642). In: American Thoracic Society International Conference Washington, DC, 2017. [Google Scholar]
- 60. IMS Health: measures of clinical trial costs, 2011–2012. In: Mathieu MP, ed. Parexel biopharmaceutical R&D statistical sourcebook 2013/2014. Waltham, MA: Parexel, 2013. [Google Scholar]
- 61. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance for industry: hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment (draft guidance, revision 2). Silver Spring, MD: FDA, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.