Summary
Through a comparative analysis of human cases of infection with H5N6, H7N9 and H5N1, we identified similar epidemiologic characteristics and severity in cases of H5N6 and H5N1 virus infection, while severity of H7N9 cases appeared lower.
Keywords: H5N6, H5N1, H7N9, epidemiology.
Abstract
Background.
Since 2014, 17 human cases of infection with the newly emerged highly pathogenic avian influenza A(H5N6) virus have been identified in China to date. The epidemiologic characteristics of laboratory-confirmed A(H5N6) cases were compared to A(H5N1) and A(H7N9) cases in mainland China.
Methods.
Data on laboratory-confirmed H5N6, H5N1, and H7N9 cases identified in mainland China were analyzed to compare epidemiologic characteristics and clinical severity. Severity of confirmed H5N6, H5N1 and H7N9 cases was estimated based on the risk of severe outcomes in hospitalized cases.
Results.
H5N6 cases were older than H5N1 cases with a higher prevalence of underlying medical conditions but younger than H7N9 cases. Epidemiological time-to-event distributions were similar among cases infected with the 3 viruses. In comparison to a fatality risk of 70% (30/43) for hospitalized H5N1 cases and 41% (319/782) for hospitalized H7N9 cases, 12 (75%) out of the 16 hospitalized H5N6 cases were fatal, and 15 (94%) required mechanical ventilation.
Conclusion.
Similar epidemiologic characteristics and high severity were observed in cases of H5N6 and H5N1 virus infection, whereas severity of H7N9 virus infections appeared lower. Continued surveillance of human infections with avian influenza A viruses remains an essential component of pandemic influenza preparedness.
The emergence of a novel avian influenza A virus that causes severe disease in humans poses a pandemic risk that deserves close attention. Highly pathogenic avian influenza (HPAI) A(H5N1) virus emerged to cause 18 human infections in Hong Kong including 6 deaths in 1997, drawing global attention [1]. HPAI H5N1 virus has continued to circulate and evolve among poultry and wild birds beyond Asia to other regions including the Middle East, with more than 850 human infections reported from 16 countries since 2003, and a cumulative case fatality proportion of >50% [2–4]. Genetically related, but distinct subtypes of the recently emerged genetic clade 2.3.4.4 of HPAI H5 viruses, including H5N2, H5N3, H5N6, and H5N8, have been detected in wild birds and poultry in a number of countries, posing an unknown threat to public health [5]. Other non-H5 subtypes of avian influenza A viruses have caused sporadic human infections worldwide [6].
In the spring of 2013, low pathogenic avian influenza (LPAI) A(H7N9) virus emerged to cause 130 laboratory-confirmed cases of human infection in China [7]. Seasonal waves of human infections with LPAI A(H7N9) virus have occurred each subsequent winter in China, with 805 cumulative cases reported globally including sporadic cases exported from mainland China to other territories and approximately 40% case fatality proportion reported as of 30 November 2016 [8].
In May 2014, the first human case of laboratory-confirmed HPAI A(H5N6) virus infection was reported in Sichuan province, China [9]. As of 30 November 2016 there have been a cumulative total of 17 confirmed cases, 12 of which were reported in 2016. Here we assessed the epidemiologic characteristics of laboratory-confirmed human cases of HPAI A(H5N6) virus infection in mainland China, compared to confirmed cases of HPAI A(H5N1) and LPAI A(H7N9) virus infections in mainland China.
METHODS
Sources of Data
In China, all laboratory-confirmed cases of human infection with H7N9 and H5N1 viruses are reported to the Chinese Center for Disease Control and Prevention (China CDC) through a national system. Information on cases of human infection with H5N6 virus was obtained through field epidemiological investigations conducted by local provincial CDCs and literature review. We used standard forms to collect demographic, epidemiological, and basic clinical data for patients with confirmed cases of H5N6, H7N9, or H5N1 virus infections. We collected detailed epidemiological information on laboratory-confirmed H5N6, H7N9, and H5N1 cases reported as of 30 November 2016. In the current analysis, we used data on age, sex, place of residence, symptoms at illness onset, and underlying medical disorders associated with an increased risk of seasonal influenza complications; dates of illness onset, hospital admission, death or discharge; and dates of potential exposures to domestic or retail animals, visits to live poultry markets; and clinical presentation, diagnosis, and treatment. Recent exposure history to poultry including at live poultry markets and to backyard poultry during the ten days prior to illness onset were recorded through an interview with the patient or the relative of a patient [10].
Ethical Approval
Collection and analyses of data from human cases of H5N6, H7N9, and H5N1 virus infections were part of an ongoing public health investigation of emerging outbreaks and thus were exempt from institutional review board assessment in China [10].
Statistical Analysis
We performed descriptive analyses of confirmed cases of H5N6 in comparison to H5N1 and H7N9 cases, using t-tests and χ2 tests to compare continuous variables and categorical variables respectively. We plotted the geographical location of H5N6 and H5N1 cases, in comparison to the incidence rates of H7N9 virus infections estimated at a provincial level rather than plotted individually, given the large number of reported H7N9 cases relative to H5N1 or H5N6 cases in China. We fitted parametric distributions to the time from illness onset to hospital admission, illness onset to laboratory confirmation, hospital admission to death, and hospital admission to discharge [10–12]. We compared alternative parametric distributions, including gamma, Weibull, and lognormal distributions, with non-parametric estimates and selected the best parametric distribution on the basis of the Akaike information criterion [13].
We compared the severity of H5N6, H5N1, and H7N9 cases based on the risk of severe outcomes in hospitalized cases, given that there is likely considerable under-ascertainment of mild, non-hospitalized cases [14, 15]. Specifically, mild cases have been detected through the national influenza-like illness sentinel surveillance network, which covers around 1% of outpatient consultations and only performs laboratory testing on a small fraction of influenza-like illness patients identified in the network [16]. Among hospitalized cases, we estimated the risk of admission to intensive care unit, the risk of requiring mechanical ventilation, and the risk of death, and compared these risks between H5N6, H5N1, and H7N9 cases.
Role of the Funding Source
The funding bodies had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
As of 30 November 2016, there were 17 laboratory-confirmed human cases of H5N6, compared to 45 H5N1 cases and 782 H7N9 cases reported in mainland China. H5N6 cases were significantly older and had a higher prevalence of underlying medical conditions than H5N1 cases but were significantly younger than H7N9 cases (Table 1). The sex distributions of H5N6 and H5N1 cases were similar with a largely even involvement of female and male patients, whereas H7N9 cases were more frequently reported in males. There was no difference between the prevalence of underlying medical conditions for H5N6 compared to H7N9 cases. All of the H5N6 cases reported recent exposure to poultry, with a greater (not statistically significant) frequency of recent visits to live poultry markets than for H5N1 or H7N9 cases. (Table 1).
Table 1.
Comparison of Characteristics of Laboratory-confirmed Cases of Human Infection With Avian Influenza A(H5N6), A(H5N1) and A(H7N9) Viruses Detected in Mainland China
| H5N6 (n = 17) | H5N1 (n = 45) | P-value (H5N6 vs H5N1) | H7N9 (n = 782) | P-value (H5N6 vs H7N9) | |
|---|---|---|---|---|---|
| Median age, y (IQR) | 40 (30, 47) | 26 (20, 35) | .008 | 58 (43, 68) | <.001 |
| Male | 7/17 (41%) | 23/45 (51%) | .58 | 547/782 (70%) | .016 |
| Urban residence | 10/17 (59%) | 19/43 (44%) | .39 | 476/782 (61%) | 1.00 |
| Underlying medical conditionsa | 8/13 (62%) | 5/41 (12%) | .001 | 444/711 (62%) | 1.00 |
| Poultry exposure (10 d before illness onset) | |||||
| Any exposure to poultry | 17/17 (100%) | 30/42 (71%) | .013 | 591/705 (84%) | .09 |
| Occupational exposure to live poultry | 0/9 (0%) | 4/45 (9%) | 1.00 | 63/782 (8%) | 1.00 |
| Visited live poultry market | 12/17 (71%) | 23/41 (56%) | .38 | 422/691 (61%) | .62 |
| Exposure to sick or dead poultry | 5/15 (33%) | 16/41 (39%) | .76 | 29/698 (4%) | <.001 |
| Exposure to backyard poultry | 7/16 (44%) | 21/41 (51%) | .77 | 161/632 (25%) | .14 |
Abbreviation: IQR, interquartile range.
aUnderlying medical conditions include chronic respiratory disease, asthma, chronic cardiovascular disease, diabetes, chronic liver disease, chronic kidney disease, immunosuppressed status, and neuromuscular disorders, which are considered to be associated with higher risk of severe illness from seasonal influenza.
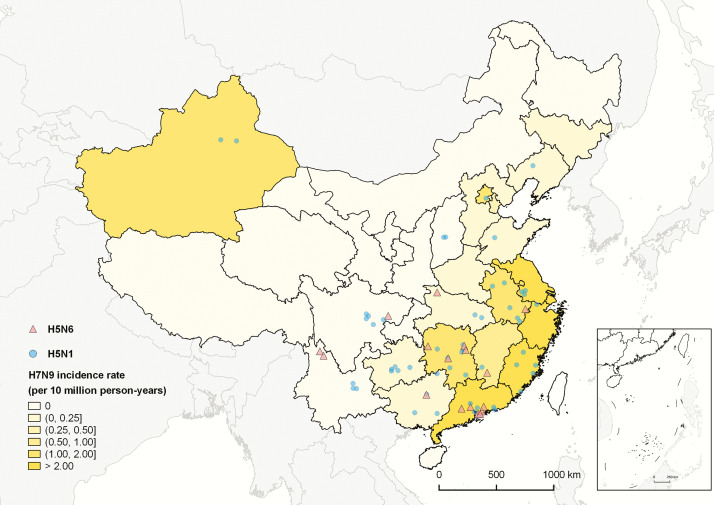
Figure 1 shows the geographic distribution of H5N6, H5N1, and H7N9 cases. The H5N6 cases have been identified in south and southwest China, whereas the H5N1 cases have occurred more sporadically across a broader part of the country. As previously noted, the incidence rate of H7N9 cases has been higher in eastern and southern China, where the density of live poultry markets is also highest [17].
Figure 1.
Geographic distributions of laboratory-confirmed cases of human infection with highly pathogenic avian influenza A(H5N6) virus (red triangles), and highly pathogenic avian influenza A(H5N1) virus (blue dots), in comparison with the incidence of laboratory-confirmed cases of human infection with low pathogenic avian influenza A(H7N9) virus in each province detected in mainland China.
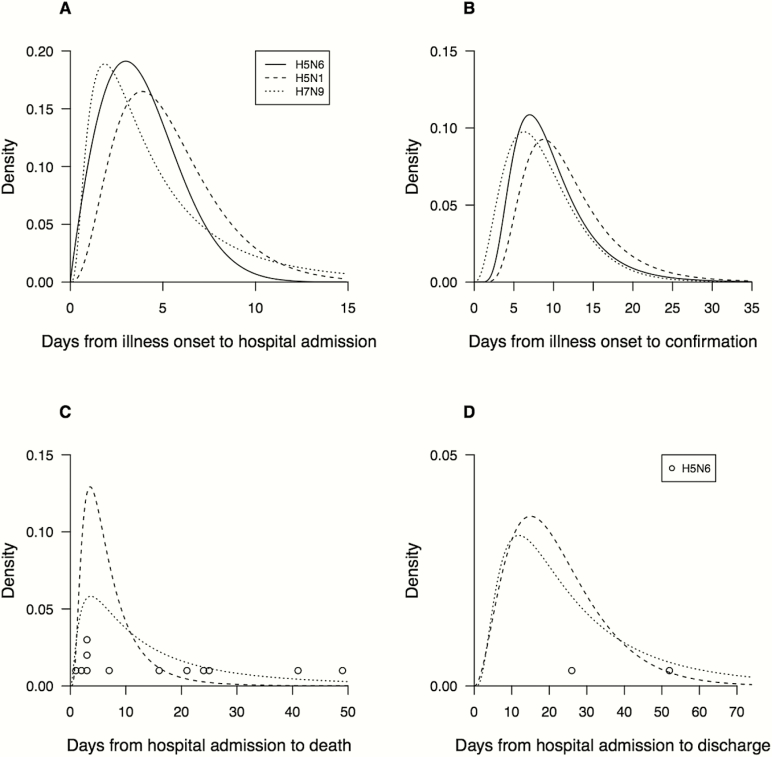
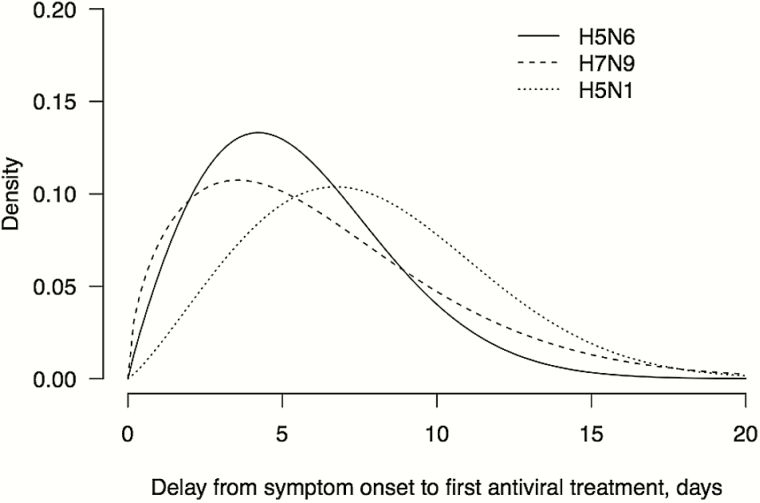
The time from symptom onset to hospital admission and the time from onset to laboratory confirmation was similar for cases infected with H5N6, H5N1, and H7N9 viruses (Figure 2A and 2B). A wide range of duration from hospital admission to death was observed in confirmed cases of infections with the 3 viruses (Figure 2C). Among cases that survived, the duration of hospitalization was mostly longer than 2 to 3 weeks, whereas scarce recovery data were available for H5N6 cases (Figure 2D). Approximately 88% of H5N6 cases and 73% of H7N9 cases received antiviral treatment, which were higher than for H5N1 cases (58%). The time from illness onset to initiation of antiviral treatment was shorter for H5N6 cases (5 days, median) compared to H7N9 (6 days) and H5N1 cases (7 days) (Figure 3). Six out of 12 H5N6-related deaths occurred within 5 days after hospital admission, and most H5N1 and H7N9 related deaths also had a relatively short duration of hospitalization. Of the 16 hospitalized H5N6 cases, 15 (94%) required mechanical ventilation, 12 cases were fatal, and 3 patients recovered, whereas the outcome of one previously hospitalized patient is unknown. This compares to a fatality risk of 70% (30/43) for hospitalized H5N1 cases, and 41% (319/782) for hospitalized H7N9 cases that were confirmed between March 2013 and November 2016.
Figure 2.
Time to event distributions of laboratory-confirmed cases of human infection with highly pathogenic avian influenza A(H5N6) (solid lines), low pathogenic avian influenza A(H7N9) (dotted lines), and highly pathogenic avian influenza A(H5N1) (dashed lines) viruses in mainland China. The curves are constructed as smoothed versions of the histograms from the raw data. A, Days from illness onset to hospital admission. B, Days from illness onset to laboratory confirmation of infection. C, Days from hospital admission to death, with circles used to indicate the delays from admission to death for 12 cases of H5N6. D, Days from hospital admission to discharge for recovered cases, with a circle used to indicate the delay from admission to death for H5N6 cases.
Figure 3.
Days from illness onset to the initiation of antiviral treatment for laboratory-confirmed cases of human infection with highly pathogenic avian influenza A(H5N6) (solid lines), low pathogenic avian influenza A(H7N9) (dotted lines), and highly pathogenic avian influenza A(H5N1) (dashed lines) viruses in mainland China. The curves are constructed as smoothed versions of the histograms from the raw data.
DISCUSSION
Human infections with HPAI H5N6 virus are the latest in a series of reported cases of human infections with avian influenza A viruses in China, following HPAI H5N1 virus infections since 2003 [10, 18], LPAI H7N9 virus infections since 2013 [10, 11, 19, 20], H10N8 virus infection in 2013 [21], and LPAI H9N2 virus infections since 1998 [22]. These continued zoonotic events pose a major threat to public health since an avian influenza A virus may be able to acquire mutations that allow for efficient and sustained human-to-human transmission and lead to the next influenza pandemic [23]. Avian influenza A viruses including HPAI H5N6 have been detected in free-range poultry and wild birds [24] and more frequently in live poultry markets [24, 25]. The newly emerged HPAI H5N6 virus belonging to the genetic clade 2.3.4.4 of H5 virus subtypes has acquired receptor binding affinity to the human-like SAα2,6Gal-linked receptor, suggesting increased pandemic potential, although HPAI H5N6 virus did not exhibit aerosol transmission and demonstrated relatively lower pathogenicity compared with HPAI H5N1 virus in the ferret model [26].
The sporadic occurrence of human H5N6 cases across southern China is somewhat consistent with the areas of highest poultry density [17], although poultry density is also high in eastern China around the Yangtze River delta where H5N6 cases have not yet been reported in the current outbreak. Human exposure to live poultry in China is also high in cities in southern China [27], where there is also a high density of live poultry markets [17]. Fourteen of the H5N6 cases reported recent exposure to live poultry markets (Table 1). However, there were distinct differences in the characteristics of H5N6 cases compared to H5N1 and H7N9 cases, with H5N6 cases having a mean age somewhat between the younger (on average) H5N1 cases and older (on average) H7N9 cases (Table 1). The majority of H5N6 cases had underlying medical conditions, which was not observed for H5N1 cases (Table 1).
In terms of the severity of hospitalized cases, 94% of H5N6 cases were admitted into an intensive care unit and required mechanical ventilation, similar to hospitalized H5N1 and H7N9 cases. To date, 12 of the 17 H5N6 cases have been fatal (71%), similar to the fatality risk of H7N9 cases identified early in 2013 [11] and the fatality risk of hospitalized H5N1 cases in mainland China over the past 13 years (70%) [10]. There was a high risk of mortality despite use of oseltamivir in most cases, although often oseltamivir treatment was not given until >5 days after illness onset.
One of the 17 H5N6 cases was detected through the influenza-like illness sentinel surveillance network [28] that covers a small fraction of ambulatory medical consultations in China, suggesting that other cases of mild illness with H5N6 virus infection have likely occurred. Therefore, the number of confirmed human H5N6 cases is likely to be an underestimate of all human H5N6 virus infections that have occurred, similar to the situation for H7N9 virus in China [14]. China CDC established systematic surveillance for pneumonia of unknown etiology in 2004 [29], and there have been improvements in laboratory surveillance for influenza, particularly since 2009. Given the increasing frequency with which human infections with avian influenza A viruses have been detected in recent years [10, 11, 18–21, 30], it is important to determine whether this pattern is purely an artefact of improvements in surveillance, or a truly increasing risk of zoonotic events in addition to improved surveillance in China.
H5N1 vaccination of poultry was introduced in China in 2006 [31], which was also the year in which the incidence of human H5N1 cases peaked in China [10]. Although H5N1 vaccination of domestic poultry protects against disease associated with H5N1 virus infection, it does not appear to protect against infection and virus shedding in the absence of obvious symptoms [32]. Recent experimental H5N1 vaccine using the HA and NA genes from the 2.3.4.4 genetic clade showed complete protection against virus shedding and disease among chickens challenged with the H5N6 virus that has been causing outbreaks worldwide [33]. This suggests that use of an H5N1 vaccine in poultry could also protect against infection with some strains of genetically related H5 subtypes, including H5N6 viruses, thereby potentially reducing the risk of zoonotic transmission to exposed persons.
There are some limitations of our study. First, our analysis of the epidemiology of H5N6 cases is based on case notifications, and it is possible that some human infections have occurred that were not laboratory-confirmed, for example, because of a lack of clinical suspicion or access to laboratory testing in some areas [10]. Second, only a small number of H5N6 cases have been reported to date, limiting our ability to characterize the differences with other avian influenza A virus infections or the typical illness course with H5N6 virus infection.
In conclusion, we have described the epidemiology of the first 17 laboratory-confirmed human infections with HPAI A(H5N6) virus in China. The laboratory-confirmed H5N6 cases were characterized by severe illness among the 16 hospitalized cases, with a high risk of fatal outcome. It remains to be seen whether HPAI A(H5N6) virus will continue to circulate among poultry and cause sporadic human infections in coming years, as A(H7N9) virus has done for four years. Continued global surveillance of human infections with avian influenza A viruses remains an essential component of pandemic preparedness, and further investigations are needed on the factors contributing to the observed patterns of human infections with avian influenza A viruses.
Notes
Acknowledgments. We thank staff members of the Bureau of Disease Control and Prevention and Health Emergency Response Office of the National Health and Family Planning Commission and provincial and local departments of health for providing assistance with administration and data collection; staff members at county-, prefecture-, and provincial-level Centers for Disease Control and Prevention (CDC) in the provinces where human cases of influenza A(H5N6), A(H5N1) and A(H7N9) occurred for providing assistance with field investigation, administration and data collection.
Disclaimer. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish. The views expressed are those of the authors and do not necessarily represent the official position of the China CDC, or the U.S. Centers for Disease Control and Prevention.
Financial support. This study was funded by grants from the National Science Fund for Distinguished Young Scholars (81525023), the US National Institutes of Health (Comprehensive International Program for Research on AIDS grant U19 AI51915), China CDC’s Key Laboratory of Surveillance and Early-warning on Infectious Disease, the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), a grant from the Health and Medical Research Fund of the Health, Welfare and Food Bureau of the Hong Kong SAR Government (grant no. 14131432), and the Research Grants Council of the Hong Kong Special Administrative Region, China (project no. T11-705/14N), and a commissioned grant from the Health and Medical Research Fund of the Health, Welfare and Food Bureau of the Hong Kong SAR Government, the National Natural Science Foundation of China (grant no. 81402731) and Natural Science Foundation of Shanghai (grant no. 14ZR1444500).
Potential conflicts of interest. B. J. C. has received research funding from MedImmune Inc and Sanofi Pasteur, and consults for Crucell NV. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tam JS. Influenza A (H5N1) in Hong Kong: an overview. Vaccine 2002; 20(suppl. 2:S77–81. [DOI] [PubMed] [Google Scholar]
- 2. Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science 2006; 312:384–8. [DOI] [PubMed] [Google Scholar]
- 3. Webster RG, Govorkova EA. H5N1 influenza–continuing evolution and spread. N Engl J Med 2006; 355:2174–7. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Influenza at the human-animal interface: summary and assessment, 13 June to 19 July 2016 Available at: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_07_19_2016.pdf?ua=1 Accessed 1 September 2016.
- 5. Claes F, Morzaria SP, Donis RO. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses-how is the Asian HPAI H5 lineage maintained. Curr Opin Virol 2016; 16:158–63. [DOI] [PubMed] [Google Scholar]
- 6. Freidl GS, Meijer A, de Bruin E et al. . Influenza at the animal-human interface: a review of the literature for virological evidence of human infection with swine or avian influenza viruses other than A(H5N1). Euro Surveill 2014; 19:20793. [DOI] [PubMed] [Google Scholar]
- 7. Yu H, Wu JT, Cowling BJ et al. . Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet 2014; 383:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Human infection with avian influenza A (H7N9) virus—China 17 August 2016 Available at: http://www.who.int/csr/don/17-august-2016-ah7n9-china/en/ Accessed 1 September 2016.
- 9. Pan M, Gao R, Lv Q et al. . Human infection with a novel, highly pathogenic avian influenza A (H5N6) virus: virological and clinical findings. J Infect 2016; 72:52–9. [DOI] [PubMed] [Google Scholar]
- 10. Cowling BJ, Jin L, Lau EH et al. . Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 2013; 382:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng L, Wu JT, Liu X et al. . Clinical severity of human infections with avian influenza A (H7N9) virus, China, 2013/14. Euro Surveill 2014; 19:20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong JY, Zhang W, Kargbo D et al. . Assessment of the severity of Ebola virus disease in Sierra Leone in 2014–2015. Epidemiol Infect 2016; 144:1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowling BJ, Muller MP, Wong IO et al. . Alternative methods of estimating an incubation distribution: examples from severe acute respiratory syndrome. Epidemiology 2007; 18:253–9. [DOI] [PubMed] [Google Scholar]
- 14. Ip DK, Liao Q, Wu P et al. . Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: case series. BMJ 2013; 346:f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu C, Havers F, Wang L et al. . Monitoring avian influenza A(H7N9) virus through national influenza-like illness surveillance, China. Emerg Infect Dis 2013; 19:1289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu H, Cowling BJ, Feng L et al. . Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet 2013; 382:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert M, Golding N, Zhou H et al. . Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat Commun 2014; 5:4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu H, Gao Z, Feng Z et al. . Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One 2008; 3:e2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q, Zhou L, Zhou M et al. . Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med 2014; 370:520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu P, Peng Z, Fang VJ et al. . Human infection with influenza A(H7N9) virus during 3 major epidemic waves, China, 2013–2015. Emerg Infect Dis 2016; 22:964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang T, Bi Y, Tian H et al. . Human infection with influenza virus A(H10N8) from live poultry markets, China, 2014. Emerg Infect Dis 2014; 20:2076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo Y, Li J, Cheng X. Discovery of men infected by avian influenza A (H9N2) virus. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999; 13:105–8. [PubMed] [Google Scholar]
- 23. Russell CA, Fonville JM, Brown AE et al. . The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 2012; 336:1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen LJ, Lin XD, Guo WP et al. . Diversity and evolution of avian influenza viruses in live poultry markets, free-range poultry and wild wetland birds in China. J Gen Virol 2016; 97:844–54. [DOI] [PubMed] [Google Scholar]
- 25. Zhang YL, Yang SG, Li G et al. . Clinical and epidemiological characteristics of a case of avian influenza A H5N6 virus infection. J Infect 2016; 72:629–31. [DOI] [PubMed] [Google Scholar]
- 26. Sun H, Pu J, Wei Y et al. . Highly pathogenic avian influenza H5N6 viruses exhibit enhanced affinity for human type sialic acid receptor and in-contact transmission in model ferrets. J Virol 2016; 90:6235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Cowling BJ, Wu P et al. . Human exposure to live poultry and psychological and behavioral responses to influenza A(H7N9), China. Emerg Infect Dis 2014; 20:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen T, Zhang R. Symptoms seem to be mild in children infected with avian influenza A (H5N6) and other subtypes. J Infect 2015; 71:702–3. [DOI] [PubMed] [Google Scholar]
- 29. Xiang N, Havers F, Chen T et al. . Use of national pneumonia surveillance to describe influenza A(H7N9) virus epidemiology, China, 2004–2013. Emerg Infect Dis 2013; 19:1784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang Y, Li X, Zhang H et al. . Human infection with an avian influenza A (H9N2) virus in the middle region of China. J Med Virol 2015; 87:1641–8. [DOI] [PubMed] [Google Scholar]
- 31. Li XL, Liu K, Yao HW et al. . Highly pathogenic avian influenza H5N1 in Mainland China. Int J Environ Res Public Health 2015; 12:5026–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pantin-Jackwood MJ, Suarez DL. Vaccination of domestic ducks against H5N1 HPAI: a review. Virus Res 2013; 178:21–34. [DOI] [PubMed] [Google Scholar]
- 33. Zeng X, Chen P, Liu L et al. . Protective efficacy of an H5N1 inactivated vaccine against challenge with lethal H5N1, H5N2, H5N6, and H5N8 influenza viruses in chickens. Avian Dis 2016; 60(suppl. 1):253–5. [DOI] [PubMed] [Google Scholar]