Abstract
Diagnostics are a cornerstone of the practice of infectious diseases. However, various limitations frequently lead to unmet clinical needs. In most other domains, diagnostics focus on narrowly defined questions, provide readily interpretable answers, and use true gold standards for development. In contrast, infectious diseases diagnostics must contend with scores of potential pathogens, dozens of clinical syndromes, emerging pathogens, rapid evolution of existing pathogens and their associated resistance mechanisms, and the absence of gold standards in many situations. In spite of these challenges, the importance and value of diagnostics cannot be underestimated. Therefore, the Antibacterial Resistance Leadership Group has identified diagnostics as 1 of 4 major areas of emphasis. Herein, we provide an overview of that development, highlighting several examples where innovation in study design, content, and execution is advancing the field of infectious diseases diagnostics.
Keywords: antibacterial resistance, diagnostics, devices.
The health and economic consequences of increasing antibacterial resistance have led to numerous calls for action. A meaningful impact on the resistance problem cannot come from just one domain. Rather, we require advances that span multiple domains, including limiting unnecessary antibiotic use in human and animal populations, developing new antimicrobial agents, and improving the state of infectious diseases diagnostics. Anticipating the important role that diagnostics play in combatting resistance, the Antibacterial Resistance Leadership Group (ARLG) has identified diagnostics as 1 of 4 primary emphasis areas. Specifically, the ARLG has prioritized the development and evaluation of diagnostic tests that are designed to rapidly detect or exclude bacterial infection, accurately identify bacterial pathogens, and/or inform selection of antibacterial agents (Table 1) [1–5].
Table 1.
List of Antibacterial Resistance Leadership Group Diagnostic Studies
| Study Name | Description | Status |
|---|---|---|
| BCID: Randomized Trial of Blood Culture Pathogen Identification using the FilmArray Blood Culture Identification Panel | Randomized controlled trial to evaluate an effect of rapid blood culture diagnostic on outcomes | Complete [1] |
| CEP-CON: Cepheid Control | Collection of oral and rectal swabs for development of an assay for Neisseria gonorrhoeae and Chlamydia trachomatis | Ongoing |
| CEP-CRO: Cepheid-Diagnostic for Carbapenem Resistant Organisms | Collection of endotracheal aspirate and bronchoalveolar lavage fluid matrix for development of an assay detecting carbapenem-resistant organisms | Complete |
| CEP-VAP: Cepheid-Diagnostic for Ventilator Associated Pneumonia | Collection of bronchoalveolar lavage fluid matrix for development of pathogen-detection assays | Complete |
| Diagnostics Working Group | Collaboration of industry partners, Antibacterial Resistance Leadership Group, and regulators to discuss diagnostic challenges and solutions | Ongoing |
| MASTERMIND-CT/NG: Master Protocol for Evaluating Multiple Infection Diagnostics–Chlamydia trachomatis/Neisseria gonorrhoeae | MASTERMIND study evaluating multiple companies’ diagnostics for extragenital C. trachomatis and gonorrhea infections | Protocol development; enrollment preparation [6] |
| PRIMERS I–IV: Platforms for Rapid Identification of MDR-Gram negative Bacteria and Evaluation of Resistance Studies I–IV | Development of platforms to rapidly identify antibiotic-resistant gram-negative bacteria: • PRIMERS I: Evaluation of 72 Enterobacteriaceae isolates on 4 RMD platforms • PRIMERS II: Evaluation of 196 Enterobacteriaceae isolates on 2 RMD platforms • PRIMERS III: Evaluation of 200 Acinetobacter isolates on 2 RMD platforms • PRIMERS IV: Evaluation of 197 Pseudomonas aeruginosa isolates on 3 RMD platforms |
Complete [2]Complete [2]Complete [3, 4]Complete [5] |
| RADICAL: Rapid Diagnostics in Categorizing Acute Lung Infections | Development and validation of host gene expression classifiers of bacterial, viral, or noninfectious illness in patients with acute respiratory illness | Enrollment complete; platform development and validation |
| RAPIDS-GN: Rapid Diagnostics for Gram- Negative Bacteria in Blood | Determination of the effect of rapid antimicrobial susceptibility testing on outcomes in patients with gram-negative infections | Protocol development |
| TRAP-LRTI: Use of Procalcitonin Testing to Direct Antibiotic Use in Lower Respiratory Tract Infections | Randomized, placebo-controlled trial of azithromycin vs placebo in patients with lower respiratory tract infection and a low procalcitonin | Protocol development |
Abbreviations: MDR, multidrug resistant; RMD, rapid molecular diagnostic.
It is clear that faster, better, and less-expensive versions of existing diagnostics represent advances. In addition, new technologies and strategies have the potential to transform infectious diseases diagnostics. However, when developing a new diagnostic, both cost and practical aspects of implementation have to be taken into consideration. For example, rapid blood culture identification of pathogens has the most meaningful impact on patient outcomes only when paired with appropriately delivered clinical decision-making guidance [1].
In addition to the standard approach of pathogen identification, diagnostics based on host response can provide useful information. For example, procalcitonin, a biomarker that correlates with bacterial infection, may be helpful to guide antibacterial use. Given the richness and complexity of host responses to infection, in some cases, single biomarkers (even very good ones) cannot be expected to capture all useful diagnostic information. As a proof of concept, the ARLG has therefore supported the development of host gene expression signatures as a tool for the differentiation between viral and bacterial infections. Although these and the other ARLG diagnostic programs are important advances, they represent only the beginning. The ARLG remains steadfastly committed to exploring, developing, and promoting the use of diagnostics in combating antibacterial resistance.
ADVANCING DIAGNOSTIC DEVELOPMENT USING A NOVEL STUDY DESIGN CALLED MASTERMIND
Obtaining regulatory approval for a new diagnostic test can be challenging due to a lack of an appropriate reference standard, limited access to good-quality, well-characterized clinical specimens, and/or costs. To address this, the Diagnostics and Devices Committee collaborated with the ARLG Statistical and Data Management [7] and Leadership and Operations [8] centers in the development of a novel study design called MASTERMIND (Master Protocol for Evaluating Multiple Infection Diagnostics), which facilitates what might have been unfeasible using conventional methods [6, 9, 10]. The MASTERMIND concept uses a single patient’s sample(s) to evaluate multiple tests, providing efficiencies of scale for simultaneous or successive investigations. For a detailed description of the MASTERMIND scheme, see “Viewpoint: MASTERMIND—Bringing Microbial Diagnostics to the Clinic” in a recent issue of Clinical Infectious Diseases [6].
The first MASTERMIND study—MASTERMIND-CT/NG—involves additional collaboration with the Special Populations Special Emphasis Panel and is designed to validate multiple companies’ nucleic acid amplification tests (NAATs) for rectal and oropharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae. Despite the US Centers for Disease Control and Prevention’s recommendation to use NAATs for this diagnosis [11], there are currently no US Food and Drug Administration (FDA)–cleared assays for extragenital sites. The involved companies already have FDA-cleared NAATs for genital C. trachomatis and N. gonorrhoeae, minimizing the technical hurdle for additional anatomic site testing. This study will return product performance data to the respective companies in support of FDA clearance for extragenital C. trachomatis and N. gonorrhoeae detection (ClinicalTrials.gov identifier: NCT02870101). In order to initiate this precedent-setting diagnostics study, the ARLG used the expertise of its infectious diseases physicians, clinical microbiologists, and statisticians; collaborated with industry; and sought input from governmental agencies.
Beyond extragenital gonorrhea and C. trachomatis, future MASTERMIND-type studies to target a number of organisms in a variety of specimen types are being discussed. Several challenges have arisen while developing this concept, including defining a reference standard when none exists, achieving consensus among participants and regulatory agencies regarding protocol design, reconciling competition and collaboration, and simultaneously evaluating multiple testing platforms from an operational perspective. Despite these challenges, the ARLG sees a prominent future for the MASTERMIND concept, particularly where diagnostics trials become cost and resource prohibitive. Our success in designing the first MASTERMIND study, MASTERMIND-CT/NG, demonstrates the feasibility of an alternative pathway for diagnostics development.
RAPID DIAGNOSIS OF BLOODSTREAM INFECTION
Novel molecular diagnostic tests that allow rapid detection of pathogens and drug resistance can in theory facilitate timely administration of “pathogen-directed” antimicrobial therapy [12–16]. However, whether these, often costly, rapid diagnostics actually improve patient-centered clinical outcomes, reduce healthcare costs, or improve antibiotic use is unknown. Thoughtful implementation strategies for rapid molecular diagnostics are essential to maximize the clinical impact of these tests. In particular, it is unclear how to most effectively communicate rapid test results to providers in order to influence clinical decision making in real time.
The partially ARLG-funded Randomized Trial of Blood Culture Pathogen Identification using the FilmArray Blood Culture Identification Panel study was a single-center, prospective, randomized, controlled, 3-arm trial that evaluated the clinical and economic outcomes associated with use of the FilmArray Blood Culture Identification (BCID) Panel—an FDA-approved rapid diagnostic that can identify multiple bacteria, fungi, and common antimicrobial-resistance genes (mecA, vanA/B, blaKPC) in about 1 hour following organism growth in a blood culture bottle [1]. The trial compared standard-of-care testing and reporting with 2 strategies to guide healthcare providers’ responses to the rapid test results: electronic comments with treatment guidance alone or with active oversight by an antimicrobial stewardship team.
Patients with Gram stain–positive blood cultures underwent stratified randomization to 1 of 3 groups: a control group with standard culture and antimicrobial susceptibility testing; BCID testing with treatment guidance included in the microbiology result report; or BCID testing with treatment guidance included in the microbiology result report plus real-time audit and feedback by antimicrobial stewardship interventionists. Pathogen identification was 21 hours faster in the BCID groups compared with the control group. Study groups had significant differences in antibiotic use, with both BCID arms having less broad-spectrum antibiotic use, more narrow-spectrum antibiotic use, less treatment of contaminants, and faster antibiotic escalation, compared with the control group. However, faster antibiotic deescalation occurred only in the group using BCID plus stewardship. Groups did not differ in length of stay, hospitalization costs, mortality, adverse drug events, or Clostridium difficile infection rates, although the study was not powered to detect these secondary outcomes.
The BCID trial was the first randomized, controlled trial to evaluate a rapid blood culture diagnostic in terms of clinical outcomes and implementation strategies. Results from the study suggest that rapid diagnostics implemented with automated clinical decision support systems can optimize treatment of bloodstream infections. However, clinical impact will be maximized when rapid diagnostics are used together with antimicrobial stewardship interventions. Future randomized, controlled trials of novel diagnostics and implementation strategies are planned. Other ARLG antimicrobial stewardship efforts are described elsewhere [17].
MOLECULAR DIAGNOSTIC PLATFORMS TO DETECT RESISTANCE PHENOTYPES
Conventional resistance testing relies on phenotypic antimicrobial susceptibility testing—growth of the organism in the presence of an antibiotic. However, genotypic testing is faster and therefore increasingly used. Such tests determine whether a resistance gene is present or absent. If present, one assumes the organism is resistant. The clinical utility of such genotypic, molecular tests to predict antibiotic susceptibility and resistance is straightforward when the phenotype is determined by a single gene (eg, mecA, vanA/B). However, the feasibility of this approach is less clear when the phenotype is determined by multiple genes, mutations, or combinations thereof. The PRIMERS (Platforms for Rapid Identification of MDR-Gram Negative Bacteria and Evaluation of Resistance Studies) series of studies, a collaboration with the ARLG Laboratory Center [18], focused on evaluating the performance of rapid molecular diagnostic platforms in identifying susceptibility and resistance to β-lactam antibiotics in Enterobacteriaceae (PRIMERS I and II) [2], Acinetobacter species (PRIMERS III) [3], and Pseudomonas aeruginosa (PRIMERS IV) [5]. Each platform evaluated isolates for the presence or absence of specific β-lactamase genes associated with resistance. Platform results were interpreted as “resistant” if targets were present and “susceptible” if not. Each platform was compared to the reference standard of minimum inhibitory concentrations (MICs) determined using Clinical and Laboratory Standards Institute standards.
Correct interpretation of molecular test results could not be achieved by simply reporting an isolate as susceptible or resistant. Therefore, several statistics were generated for the PRIMERS projects by the ARLG Statistical and Data Management Center [7], including the discrimination summary (DIM SUM) plot. DIM SUM can be interpreted as the probability that the platform result indicates resistance or susceptibility when the corresponding MIC is interpreted as resistant or susceptible. Also calculated were susceptibility/resistance predictive values (SPVs/RPVs), which are the probability that an MIC result will indicate susceptibility/resistance based on the platform result. SPV and RPV were shown to depend on the prevalence of susceptibility, which varies geographically and temporally. Consequently, SPVs/RPVs were plotted as a function of susceptibility prevalence for use with local antibiograms and additional information [7].
A NOVEL DIAGNOSTIC PLATFORM BASED ON HOST RESPONSE TO INFECTION
Inappropriate prescribing of antibacterial agents for viral acute respiratory illness (ARI) contributes to increased healthcare costs and unnecessary drug-related adverse effects, and it is a primary driver of antimicrobial resistance [19–22]. Most such antimicrobial use is in the outpatient setting, where the tools to identify those in need of antibacterial treatment are lacking [23–25]. An accessible, rapid, accurate, near-patient diagnostic that discriminates viral from bacterial infection has the potential to reduce inappropriate antimicrobial prescribing and stem the rising rates of antibacterial resistance.
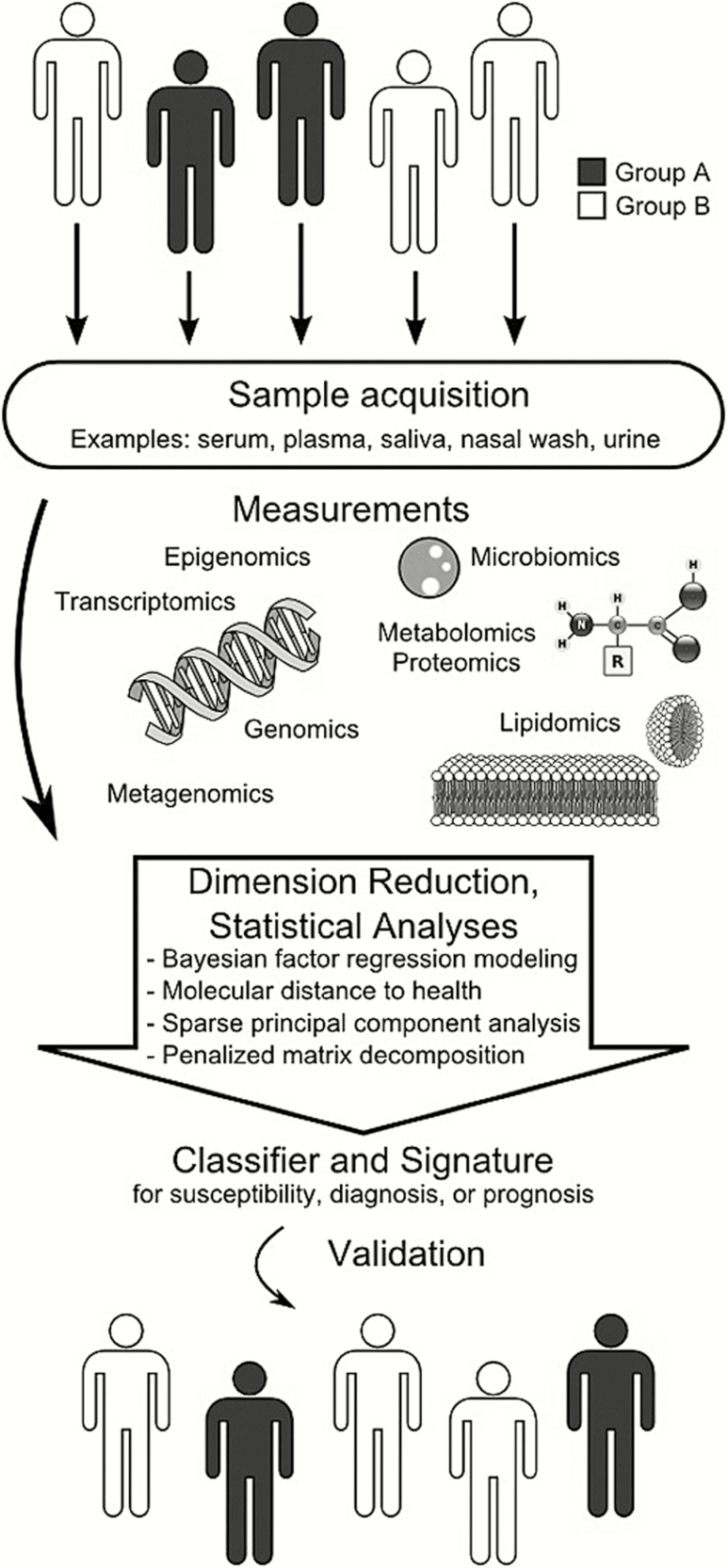
The RADICAL (Rapid Diagnostics in Categorizing Acute Lung Infections) study is predicated on the idea that external stressors induce a compensatory host response. Those responses can be detected and quantified in a variety of molecular schemes, such as the transcriptome, metabolome, and proteome settings, among others (Figure 1). Moreover, the host response to a particular stress is stereotypical. This allows machine-learning techniques, such as sparse logistic regression, to define signatures induced by viral ARI, bacterial ARI, or noninfectious illness. The ARLG has capitalized on nearly 10 years of prior work by the RADICAL team, which has defined host response signatures with the goal of translating them to clinically relevant platforms (Figure 2) [26–31].
Figure 1.
Overview of the development process for a host diagnostic biomarker. Beginning with a population that is dichotomized by susceptibility, diagnosis, or prognosis, biological samples are acquired. Omic measurements are run on these samples, which generates large quantities of data. Dimension reduction and statistical analyses generate a classifier or signature that distinguishes the desired characteristic from the original population. The classifier is then validated against a different population to test its generalizability. (Used with permission from Yang WE, et al. Host-based diagnostics for detection and prognosis of infectious diseases. In: Sails A, Tang YW, eds. Methods in Microbiology. Elsevier Ltd, 2015;42[13]:465–500.).
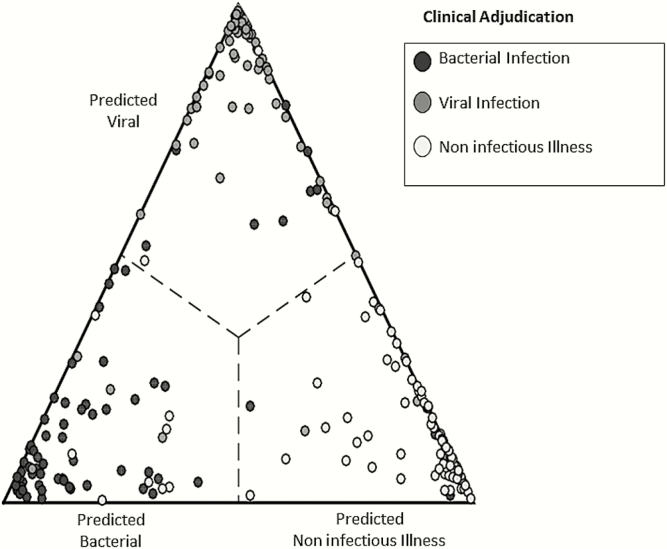
Figure 2.
Validation of the RADICAL (Rapid Diagnostics in Categorizing Acute Lung Infections) host response signature. A cohort of 273 patients encompassing bacterial acute respiratory infection (ARI) (black circles), viral ARI (dark gray circles), or noninfectious fever (white circles) was used to develop classifiers of each condition. Using leave-one-out cross-validation, 3 probabilities were determined for each patient: that of having a bacterial ARI, viral ARI, or noninfectious illness. The highest probability determined class assignment. Patients classified as having bacterial ARI appear to the left, viral ARI at the top, and noninfectious illness to the right. Closer proximity to the vertex indicates a higher probability of that condition. Overall accuracy was 87%.
In 2014, RADICAL began to validate host response signatures to viral ARI, bacterial ARI, and noninfectious illness [29]. RADICAL enrollment is intentionally broad, focusing on patients with ARI of bacterial, viral, or noninfectious etiologies in whom diagnostic testing or antibacterial therapy is being considered. Upon enrollment, peripheral whole blood is collected and banked for later gene expression analysis. The program also supports collaboration with industry to develop clinically useful tests for these host response signatures. The ideal test is envisioned as a simple sample-to-answer product, available at or near the point of care, and one that is rapid and affordable. The RADICAL project aims to validate the host response as a diagnostic strategy, as well as any novel platform that arises from its development.
Among the greatest challenges in developing a bacterial vs a viral test is the lack of a gold standard. No single diagnostic test has proven sufficiently accurate for determining if a patient’s symptoms are due to an infectious process and, if so, whether it is bacterial or viral. Therefore, the RADICAL team—in collaboration with the ARLG Steering Committee, Laboratory Center, and Diagnostics and Devices Committee—has developed a reference standard to use in the validation of a bacterial vs a viral diagnostic assay. When multiple tests are necessary for adequate classification, yet no predefined composite of tests is considered sufficiently accurate, an expert panel diagnosis is considered the best available reference standard [32, 33]. The inherent complexity of this syndrome not only requires classification of infection but also the likely etiologic agent, significance of multiple pathogens, and likelihood that an identified pathogen is causal. Such a scheme standardizes reporting of expert panel adjudications and offers levels of confidence associated with that classification. This in turn helps align the reference standard with recommended standards for reporting studies of diagnostic accuracy [34, 35].
In the next phases of the RADICAL project, enrollment will include pediatric populations and additional geographic areas. Platform development and translation will continue, including analytical validation, hopefully followed by regulatory clearance. If successful, the RADICAL project will introduce a completely new diagnostic strategy that is more accurate than current testing, with results available at the time of clinical decision making.
PROCALCITONIN-DIRECTED TREATMENT OF LOWER RESPIRATORY TRACT INFECTION
Similar to the RADICAL study, which focuses on ARI, the TRAP-LRTI (Targeted Reduction of Antibiotics using Procalcitonin in Lower Respiratory Tract Infection) study, a collaboration with the ARLG Stewardship and Infection Control Committee [17], proposes to evaluate a biomarker approach for the management of lower respiratory tract infection (LRTI). Procalcitonin was first described in the setting of sepsis, where concentrations were noted to be increased compared with noninfectious conditions [36]. Moreover, procalcitonin has been used to distinguish bacterial from viral infections based on the observation that interferon gamma production induced by viral infections inhibits procalcitonin production [37]. As a result, in several European studies, procalcitonin-guided management of ARI has been used to withhold antibiotics or shorten the duration of antibiotic therapy, without adversely affecting outcomes [38–41]. Despite this body of research, the FDA-approved indication for procalcitonin use focuses on sepsis. Specifically, procalcitonin is to be used in conjunction with other laboratory findings and clinical assessments to aid in risk assessment of critically ill patients on their first day of intensive care unit admission for progression to severe sepsis and septic shock. In collaboration with a diagnostic manufacturer and in consultation with the FDA, the ARLG has proposed TRAP-LRTI to expand the indication for use. This multicenter, double-blind, randomized, placebo-controlled trial will enroll adults presenting to the emergency department and outpatient clinics with LRTI. Patients with a procalcitonin concentration <0.1 ng/mL will be randomized to receive placebo or standard-course azithromycin. The primary outcome is to compare the efficacy of azithromycin vs placebo on study day 5 using a noninferiority approach. The hypothesis is that clinical outcomes of patients with a procalcitonin concentration <0.1ng/mL who do not receive antibiotics will be comparable, or noninferior, to those who do receive antibiotic therapy. This trial, which is in the protocol-development stage through the Vaccine and Treatment Evaluation Units (VTEUs), is intended to provide the data necessary to support an expanded indication-for-use statement.
ARLG LABORATORY CENTER AND STRAIN BIOREPOSITORIES [18]
The Laboratory Center (LC), described in detail along with their associated biorepositories elsewhere [18], is a resource developed by the ARLG to support ARLG-related projects, conduct laboratory-based research, and provide services and advice to the scientific community [38]. One of those services is the curation, maintenance, and dissemination of well-characterized bacterial strains, including clinical isolates. Distinct from typical biobanking approaches, the ARLG maintains most of these strains in a decentralized manner. Housed at multiple locations, this strain library constitutes a virtual biorepository (VB). Strains in the VB are accompanied by data such as strain type, antimicrobial susceptibility testing results, genetic characterization, and clinical information about the source of the isolate.
As the central point of contact, the LC reviews and approves strain requests, provides guidance during the selection process, and ships strains to requesting investigators. Requests are welcomed from the research community, clinical microbiologists, diagnostic companies, pharmaceutical companies, and other entities with relevant scientific interests [18].
ACCESS TO MATRIX AND SAMPLES
Diagnostics development often requires matrix (eg, blood, urine, cerebrospinal fluid, bronchoalveolar lavage fluid, rectal swab) to define parameters such as limits of detection, interference, and specimen stability, among others, through the creation of contrived (ie, spiked) samples. This can be critical to assay validation, particularly for rare analytes. In some cases, matrix is readily available (eg, urine). In other cases, it may be challenging to acquire (eg, cerebrospinal fluid, bronchoalveolar lavage fluid). The ARLG Laboratory and Leadership and Operations Centers together have developed protocols to provide such specimens to diagnostics developers using the ARLG’s network of trial sites and clinical partners. The CEP-VAP (Cepheid-Diagnostic for Ventilator Associated Pneumonia) project scavenged residual clinical bronchoalveolar lavage fluid samples that were used to develop pathogen detection assays for LRTI. The CEP-CRO (Cepheid-Diagnostic for Carbapenem Resistant Organisms) project scavenged clinical respiratory samples that were used to develop diagnostic tests to detect carbapenem-resistant gram-negative bacteria [42]. In this manner, the ARLG is able to help overcome a barrier in the diagnostic test development pathway.
CONCLUSIONS AND FUTURE DIRECTIONS
Recent technologic advances have spurred development of new tests that more rapidly and accurately detect and identify microorganisms as well as detect multiple pathogens and/or drug-resistance mechanisms simultaneously. This armamentarium of new diagnostic tests has revolutionized the portfolio of clinical microbiology laboratories, but clinical practices must also learn how best to use them. Advanced diagnostics have created a need for more implementation-science studies that explore how to operationalize and integrate these new tests into existing practice. This includes determining the ideal analyte panels for specific patient populations; the patient and clinical situation in which a test should be obtained; whether additional testing should be co-ordered or reflexively ordered; and how to report results such that they seamlessly integrate with clinical care decisions. This is especially important considering the large proliferation of novel diagnostic tests for routine clinical use.
Going forward, cost-effectiveness or clinical utility studies, akin to the study by Banerjee et al [1], will help determine the optimal use of new technologies. The important outcome variables that future diagnostic studies should assess include impact on antimicrobial usage, time to effective or optimal therapy, patient length of stay, cost, mortality, and emergence and spread of resistance. Multicenter studies will likely be required to have enough patients or clinical specimens to make statistically meaningful observations. The value of these tests in outpatient settings will likewise need to be defined. However, the costs to perform such studies can be substantial and must therefore be weighed against the available resources, low reimbursement for diagnostic testing, and anticipated clinical impact. The ARLG, along with its academic and industry partners, have made progress in answering these challenges.
Notes
Financial support. The development of this article was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH; UM1AI104681). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplement sponsorship. This article appears as part of the supplement “Antibacterial Resistance Leadership Group (ARLG): Productivity and Innovation,” sponsored by the Antibacterial Resistance Leadership Group.
Potential conflicts of interest. E. L. T.: consultant: Immunexpress, CytoVale, Liquidia; research support: Novartis, Defense Advanced Research Projects Agency, NIH/NIAID, Defense Threat Reduction Agency, Bill and Melinda Gates Foundation, Veterans Health Administration; equity relationship: Host Response, Inc.; filed patent: methods of identifying infectious diseases and assays for identifying infectious disease; patent pending: host gene expression signatures of Staphylococcusaureus and Escherichia coli infections. R. A. B.: research funding: NIH and VA Merit Review Board; research grants: Merck, Wockhardt, Allergan, and Roche for preclinical work on beta-lactamase inhibitors. S. R. E.: grants: NIAID/NIH, Fogarty; personal fees: American Statistical Association, Society for Clinical Trials, Drug Information Association, FDA, NIH, City of Hope, Huntington’s Study Group, Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials, Preclinical Pain Research Consortium for Investigating Safety and Efficacy, Muscle Study Group, DeGruyter (Statistical Communications in Infectious Diseases), Takeda, Pfizer, Roche, Novartis, Merck, Achaogen, Auspex, Alcon, Chelsea, Mannkind, QRx Pharma, Genentech, Affymax, FzioMed, Amgen, GlaxoSmithKline, Sunovion, Boehringer-Ingelheim, Cubist, AstraZeneca, Teva, Repros, Dexcom, Zeiss, University of Rhode Island, New Jersey Medical School/Rutgers, University of Vermont, Osaka University, and the National Cerebral and Cardiovascular Center of Japan. R. P.: consultant: Curetis, Diaxonhit; payments: Mayo Clinic (employer), American Society for Microbiology (travel reimbursement, editor’s stipend), Up-To-Date (royalties), Diaxonhit and Curetis (consulting, money paid to employer), United States Medical Licensing Examination (honorarium), Infectious Diseases Board Review Course (honorarium); research grants: nanoMR, Curetis, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, the Medicines Company, Allergan; patents: Bordetella pertussis polymerase chain reaction, anti-biofilm substance, device/method for sonication, anti-biofilm substance. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans SR, Hujer AM, Jiang H, et al. ; Antibacterial Resistance Leadership Group Rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: PRIMERS I and II. Clin Infect Dis 2016; 62:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans S, Hujer A, Jiang H, et al. Informing antibiotic treatment decisions: evaluating rapid molecular diagnostics (RMDs) to identify susceptibility and resistance to carbapenems against Acinetobacter spp. PRIMERS III. J Clin Microbiol 2016; 55:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans SR, Pennello G, Pantoja-Galicia N, et al. ; Antibacterial Resistance Leadership Group Benefit-risk evaluation for diagnostics: a framework (BED-FRAME). Clin Infect Dis 2016; 63:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans S, Tran T, Hujer A, et al. Choosing ceftazidime/avibactam and ceftolozane/tazobactam as empiric therapies against Pseudomonas aeruginosa (Pa) using rapid molecular diagnostics (RMDs): PRIMERS IV. IDWeek. New Orleans, LA, 2016. [Google Scholar]
- 6. Patel R, Tsalik EL, Petzold E, et al. Viewpoint: MASTERMIND—bringing microbial diagnostics to the clinic. Clin Infect Dis 2017; 64:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huvane J, Komarow L, Hill C, et al. Fundamental and catalytic innovation: the Statistical and Data Management Center of the Antibacterial Resistance Leadership Group. Clin Infect Dis 2017; 64(Suppl 1):S18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cross HR, Harris A, Arias RM, et al. Transforming concepts into clinical trials and creating a multisite network: the Leadership and Operations Center of the Antibacterial Resistance Leadership Group. Clin Infect Dis 2017; 64(Suppl 1): S8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 2009; 86:97–100. [DOI] [PubMed] [Google Scholar]
- 10. Mandrekar SJ, Dahlberg SE, Simon R. Improving clinical trial efficiency: thinking outside the box. Am Soc Clin Oncol Educ Book 2015: e141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Workowski KA, Bolan GA; Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 12. Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program’s impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 2010; 51:1074–80. [DOI] [PubMed] [Google Scholar]
- 13. Forrest GN, Roghmann MC, Toombs LS, et al. Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother 2008; 52:3558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57:1237–45. [DOI] [PubMed] [Google Scholar]
- 15. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 2013; 137:1247–54. [DOI] [PubMed] [Google Scholar]
- 16. Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J Clin Microbiol 2013; 51:4008–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson DJ, Jenkins TC, Evans SR, et al. The role of stewardship in addressing antibacterial resistance: the Stewardship and Infection Control Committee of the Antibacterial Resistance Leadership Group. Clin Infect Dis 2017; 64(Suppl 1): S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manca C, Hill C, Hujer AM, et al. Leading antibacterial laboratory research by integrating conventional and innovative approaches: the Laboratory Center of the Antibacterial Resistance Leadership Group. Clin Infect Dis 2017; 64(Suppl 1): S13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gould IM. Antibiotic resistance: the perfect storm. Int J Antimicrob Agents 2009; 34:S2–5. [DOI] [PubMed] [Google Scholar]
- 20. Kim JH, Gallis HA. Observations on spiraling empiricism: its causes, allure, and perils, with particular reference to antibiotic therapy. Am J Med 1989; 87:201–6. [DOI] [PubMed] [Google Scholar]
- 21. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 2014; 12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The White House. Executive Order—Combating antibiotic-resistant bacteria. Office of the Press Secretary, 2014. [Google Scholar]
- 23. Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365:579–87. [DOI] [PubMed] [Google Scholar]
- 24. Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011; 128:1053–61. [DOI] [PubMed] [Google Scholar]
- 25. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 26. Huang Y, Zaas AK, Rao A, et al. Temporal dynamics of host molecular responses differentiate symptomatic and asymptomatic influenza a infection. PLoS Genet 2011; 7:e1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McClain MT, Nicholson BP, Park LP, et al. A genomic signature of influenza infection shows potential for presymptomatic detection, guiding early therapy, and monitoring clinical responses. Open Forum Infect Dis 2016; 3:ofw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woods CW, McClain MT, Chen M, et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS One 2013; 8:e52198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaas AK, Burke T, Chen M, et al. A host-based RT-PCR gene expression signature to identify acute respiratory viral infection. Sci Transl Med 2013; 5:203ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaas AK, Chen M, Varkey J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe 2009; 6:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsalik EL, Henao R, Nichols M, et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med 2016; 8:322ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reitsma JB, Rutjes AW, Khan KS, Coomarasamy A, Bossuyt PM. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 2009; 62:797–806. [DOI] [PubMed] [Google Scholar]
- 33. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bossuyt PM, Reitsma JB, Bruns DE, et al. ; Standards for Reporting of Diagnostic Accuracy The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003; 138:W1–12. [DOI] [PubMed] [Google Scholar]
- 35. Whiting P, Rutjes A, Dinnes J, Reitsma J, Bossuyt PM, Kleijnen J. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 2004; 8:1–234. [DOI] [PubMed] [Google Scholar]
- 36. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Linscheid P, Seboek D, Nylen ES, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 2003; 144:5578–84. [DOI] [PubMed] [Google Scholar]
- 38. Albrich WC, Dusemund F, Bucher B, et al. ; ProREAL Study Team Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an international, multicenter poststudy survey (ProREAL). Arch Intern Med 2012; 172:715–22. [DOI] [PubMed] [Google Scholar]
- 39. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med 2008; 168:2000–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 40. Schuetz P, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
- 41. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012; CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doi Y, Bonomo RA, Hooper DC, et al. Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the Antibacterial Resistance Leadership Group. Clin Infect Dis 2017; 64(Suppl 1):S30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]