Abstract
Objectives: GATA binding factor 1 (GATA1) is a transcription factor essential for erythromegakaryocytic differentiation. Given its function in lineage specification, we sought to evaluate the immunohistochemical profile of GATA1 in normal marrow and acute leukemia and assess the use of GATA1 as a specific erythromegakaryocytic immunohistochemical marker.
Methods: Immunohistochemical studies for GATA1 expression were performed on bone marrow biopsy specimens to define its role in the evaluation of acute leukemia and other hematologic disorders.
Results: In normal marrows, intense nuclear reactivity is seen in immature erythroid precursors and megakaryocytes. Weak to moderate nuclear positivity is seen in eosinophils and mast cells. In marrows involved by acute leukemia, blasts of pure erythroleukemia and acute megakaryoblastic leukemia exhibit intense nuclear GATA1 positivity, while blasts of acute myeloid leukemia of other categories are negative. GATA1 is also absent in the blasts of acute lymphoblastic leukemia/lymphoma and in the neoplastic cells of metastatic carcinoma and plasma cell neoplasms.
Conclusions:Intense GATA1 nuclear expression is a sensitive and specific marker for cells of erythroid and megakaryocytic lineages and is an excellent marker for neoplastic cells of pure erythroleukemia and acute megakaryoblastic leukemia.
Keywords: GATA1, Acute myeloid leukemia, Acute megakaryoblastic leukemia, Pure erythroid leukemia, Immunohistochemistry
GATA binding factor 1 (GATA1) is a zinc finger transcription factor that plays a critical role in directing erythroid and megakaryocytic development.1,2 The significance of GATA1 was first demonstrated as a transcription factor that mediates the postnatal switch of γ to β hemoglobin gene expression.3,4 Subsequent studies using animal and in vitro differentiation models have shown that dysregulation of GATA1 results in failed erythropoiesis. GATA1 null mice are embryonic lethal due to an arrest at the proerythroblast stage followed by extensive apoptosis during definitive hematopoiesis, whereas reexpression of GATA1 rescues the phenotype.5‐7 A specific subset of mice harboring disruptions of gene regulatory elements adjacent to GATA1 promoters survived to adulthood with normal erythropoiesis but exhibited severe thrombocytopenia due to loss of GATA1 expression in megakaryocytes.8,9 Findings in these studies highlight the importance of GATA1 in erythro-megakaryocytic development.
In addition to erythromegakaryocytic lineages, GATA1 expression has also been reported in basophils, mast cells, eosinophils, and Sertoli cells.1 Using tissue-specific knockouts, GATA1 expression is necessary for the development of eosinophils and basophils but not mast cells.10‐12 In humans, germline mutations of GATA1, although rare, can result in varying degrees of anemia and thrombocytopenia. For instance, in X-linked dyserythropoietic anemia and thrombocytopenia, heterozygous mutation of GATA1 p.V205M abrogates the interaction with friend of GATA protein 1 (FOG1), resulting in anemia and thrombocytopenia with aberrant morphology of the platelets.13 A different mutation, p.D218G, weakens the interaction to FOG1 and results in X-linked thrombocytopenia.14 Notably, X-linked anemia with or without neutropenia and/or platelet abnormalities is associated with a germline splice site mutation (c.G332C) that results in the exclusive production of a short form of GATA1.15 In children with Down syndrome who developed transient abnormal myelopoiesis with or without progression to acute megakaryoblastic leukemia, somatic mutations that result in the exclusive production of the short form of GATA1 are nearly always present.16 These mutations underscore the importance of GATA1 in sustaining normal erythroid and megakaryocytic differentiation.
Few studies have examined the GATA1 transcript level in acute leukemia.17,18 These studies relied on reverse-transcriptase polymerase chain reaction (RT-PCR) of mononuclear cells fractioned with the Ficoll-Hypaque method from blood or bone marrow, making interpretation difficult. To our knowledge, to date only a single study has specifically examined the abnormal GATA1 expression pattern in primary myelofibrosis by immunohistochemistry using proprietary polyclonal rabbit antibodies.19 Therefore, the goal of this study is to assess the utility of GATA1 as an erythro-megakaryocytic lineage marker in the evaluation of acute leukemia by immunohistochemistry with a commercially available rabbit monoclonal antibody against GATA1.
Materials and Methods
Cases were obtained from the archival files in the Department of Pathology with approval from the institutional review boards of Brigham and Women’s Hospital (Boston, MA) and Boston Children’s Hospital (Boston, MA). H&E-stained slides and pathology reports were reviewed for the confirmation of diagnosis. Diagnostic criteria of acute leukemia are based on the 2008 World Health Organization’s Classification of Tumours of Haematopoietic and Lymphoid Tissues.20
Paraffin sections of 84 cases of Bouin’s or formalin-fixed bone marrow core biopsy specimens were analyzed: three cases of normal marrow, 52 cases of acute myeloid leukemia (including 14 cases of pure erythroid leukemia and five cases of acute megakaryoblastic leukemia), eight cases of B-lymphoblastic lymphoma, five cases of T-lymphoblastic lymphoma, five cases of chronic myelogenous leukemia, three cases of systemic mastocytosis, three cases of plasma cell myeloma, two cases of hypereosinophilic syndrome, and three cases of metastatic carcinomas.
Immunohistochemical studies were performed on 4-µm-thick paraffin-embedded tissue sections. Following deparaffinization, sections were treated with 3% hydrogen peroxide for 5 minutes to quench endogenous peroxidase activity. Antigen retrieval was performed using EDTA (0.001 mol/L), pH 8.0 (Invitrogen, San Francisco, CA) for 30 minutes in a steamer (model HS80; Black & Decker, Shelton, CT). The slides were allowed to remain in the hot EDTA solution for an additional 10 minutes at room temperature, then washed and placed in Tris buffer (Covance, Dedham, MA). The slides were incubated with anti-GATA1 (D52H6) rabbit monoclonal antibody (Cell Signaling Technology, Danvers, MA) at 1:200 dilution for 50 minutes at room temperature. Slides were washed and then incubated for 30 minutes with a horseradish peroxidase–labeled polymer conjugated to goat anti–rabbit immunoglobulin antibody (PowerVision; Leica Microsystems, Buffalo Grove, IL). Antibody localization was achieved using a peroxidase reaction with DAB+ (Dako, Carpinteria, CA) as the chromogen. The slides were briefly enhanced with 1% copper sulfate solution, washed, counterstained with hematoxylin, dehydrated, and mounted. The pattern and intensity of staining for each antibody were evaluated. When the staining pattern is homogenously deeply stained, resembling the pattern seen in megakaryocytes in normal marrow, it is interpreted as “strong” or “intense,” whereas the “weak” pattern refers to nuclei lightly stained in a sandy pattern as seen in the eosinophils.
Double marker studies were performed in a Leica Bond III immunostainer (Leica Microsystems). Antigen retrieval was performed using Bond epitope retrieval solution 2 (Leica Microsystems) for 30 minutes. The slides were then incubated with the respective primary antibodies for 1 hour. Finally, antibody localization was achieved using Bond Polymer Refine Detection kits (DAB and FastRed; Leica Microsystems). Rabbit polyclonal antibody specific for eosinophil peroxidase (EPX antibody; Novus Biologicals, Littleton, CO) at 1:500 dilution and a rabbit polyclonal antibody to myeloperoxidase (Dako) at 1:25,000 dilution were employed for double-marker studies with GATA1 antibody.
Results
GATA1 Reactivity in Normal Bone Marrow Core Biopsy Specimens
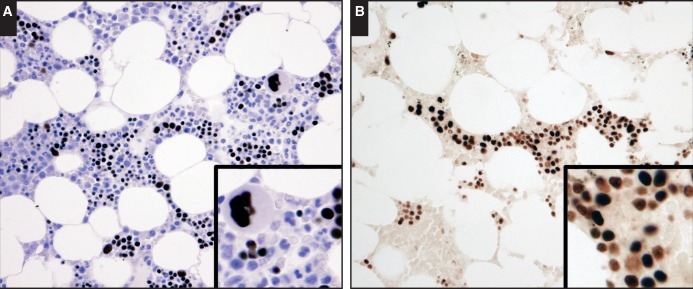
In normal bone marrow samples with maturing trilineage hematopoiesis, GATA1 reactivity was almost exclusively present in megakaryocytes and erythroid precursors Image 1A. The pattern of nuclear GATA1 reactivity was uniformly intense in megakaryocytes. In the erythroblastic islands, varying intensity of GATA1 expression was observed and could be best demonstrated in stained sections without hematoxylin counterstains Image 1B. Based on the nuclear morphology, it appeared that reactivity was strongest in the early erythroid forms and lowest in the maturing, late normoblasts. No GATA1 reactivity was detected in mature RBCs and platelets.
Image 1.
Expression of GATA binding factor 1 (GATA1) in normal bone marrow. A, Intense nuclear expression is present only in megakaryocytes and erythroid precursors (×40). B, Varying nuclear staining intensity in erythroblastic islands is best seen without hematoxylin counterstain, with strongest reactivity in the early forms and reduction in intensity with maturation (×40).
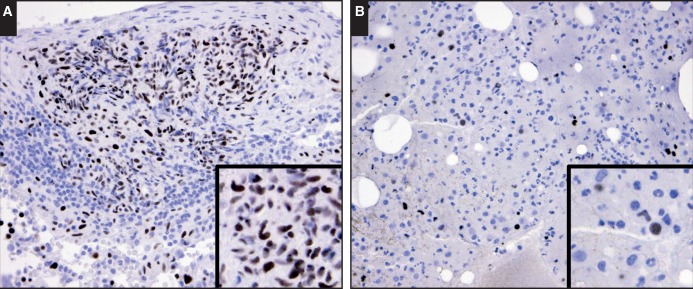
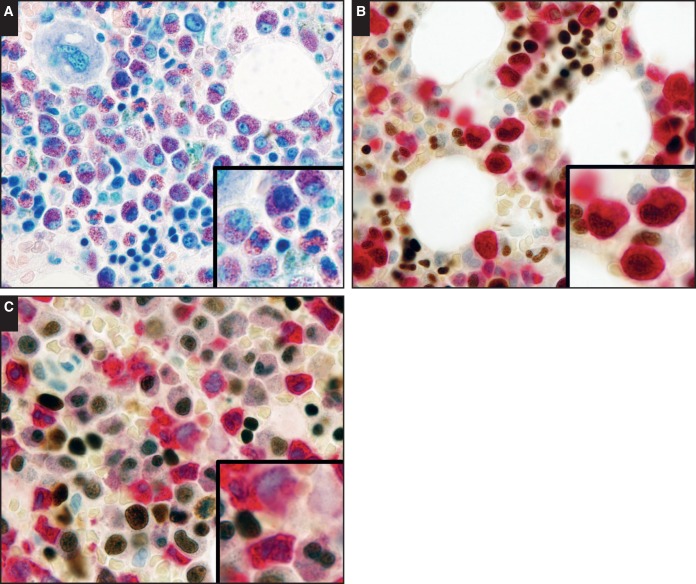
Rare myeloid cells, including mononuclear and bilobed nuclear forms, with weak to intermediate GATA1 reactivity were also seen in normal bone marrow samples. We reasoned that these cells likely represent mast cells and eosinophils, as these cell types have been reported to exhibit GATA1 expression.6,10,12 We evaluated samples enriched in both cell types. In all samples of systemic mastocytosis (n = 5), the neoplastic mast cells exhibited intermediate nuclear GATA1 reactivity Image 2A. In studying GATA1 expression in eosinophils, we took advantage of the fact that as eosinophils transition from common myeloid progenitors, the expression of EPX is upregulated while myeloperoxidase (MPO) is down-modulated.21 These two peroxidases share approximately 70% homology in amino acid sequences.22 In our hands, the EPX antibody did not appear to cross-react with MPO, while the MPO antibody cross-reacted weakly with EPX, making this protein an ideal marker for eosinophils. The dual immunostains for EPX and GATA1 in marrows with hypereosinophilic syndrome (n = 2) revealed weak to intermediate GATA1 expression in immature and mature eosinophils Image 3B. A consistent pattern was also seen in the dual immunostains for MPO and GATA1, where costaining of GATA1 was present in eosinophils (with weak MPO reactivity) and absent in other myeloid cells with strong MPO reactivity Image 3C. In samples with chronic myelogenous leukemia (n = 5), GATA1 reactivity was largely absent. However, weak GATA1 reactivity was seen in rare myeloid cells, some with bilobed nuclei Image 2B. These staining patterns and the morphologic features were similar to that seen in eosinophils Image 3. Overall, these findings corroborate the reported expression pattern and establish our immunohistochemical technique as a sensitive and specific method for detection of GATA1 protein expression.
Image 2.
Patterns of GATA binding factor 1 (GATA1) expression in mastocytosis and chronic myelogenous leukemia (CML). A, In this bone marrow core biopsy specimen of a patient with systemic mastocytosis, the neoplastic mast cells, including spindle forms, are highlighted by intense to intermediate nuclear GATA1 staining (×40). B, In this bone marrow core biopsy specimen of a patient with CML, GATA1 reactivity is largely absent in the neoplastic myeloid cells (×40). However, rare cells, some with bilobed nuclei (see inset), are weakly positive for nuclear GATA1, likely representing eosinophilic myeloid elements. Also noted are erythroid precursors with intense nuclear GATA1 staining as a useful internal positive control.
Image 3.
Patterns of GATA binding factor 1 (GATA1) expression in hypereosinophilic syndrome. A, Giemsa stain of a bone marrow core biopsy specimen from a patient with hypereosinophilic syndrome (×40). B, A dual immunostain with GATA1 (DAB) and eosinophil peroxidase (FastRed) reveals moderate nuclear GATA1 expression in immature and mature eosinophils (×40). C, A dual immunostain with GATA1 (DAB) and myeloperoxidase (MPO; FastRed) reveals the presence of GATA1 expression in eosinophils with weak MPO reactivity and the lack of GATA1 expression in other myeloid cells with strong MPO reactivity (×40).
GATA1 Reactivity in Acute Leukemias
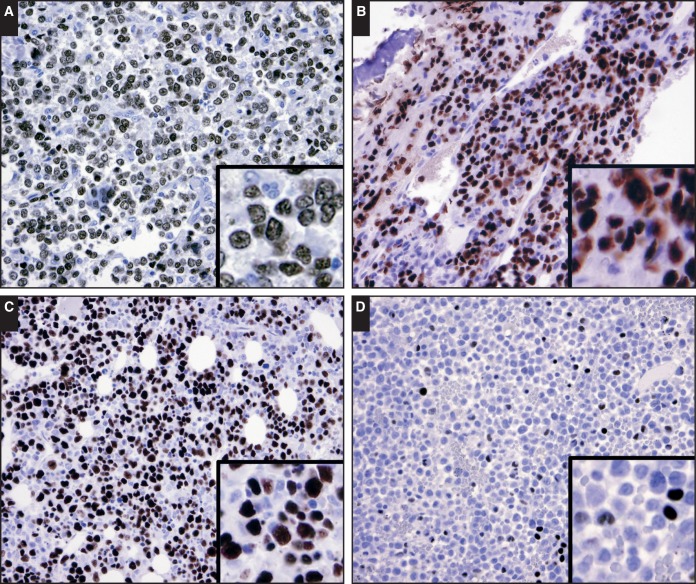
To assess whether GATA1 is a sensitive and specific marker for erythromegakaryocytic lineage in acute leukemias, we evaluated GATA1 reactivity in bone marrow biopsy specimens with pure erythroid leukemia (n = 14); acute megakaryoblastic leukemia (n = 5); acute erythroleukemia, erythroid/myeloid type (AEML; n = 6); acute monoblastic leukemia (n = 3); acute monocytic leukemia (n = 8); acute myelomonocytic leukemia (AMML; n = 4); AMML with eosinophilia (n = 5); acute promyelocytic leukemia (n = 7); B-acute lymphoblastic leukemia (n = 8); and T-acute lymphoblastic leukemia (n = 5). The results are summarized in Table 1. The blasts of pure erythroid leukemia and acute megakaryoblastic leukemia displayed uniformly intense nuclear GATA1 reactivity Image 4A and Image 4B. In AEML, the erythroid components displayed the strongest nuclear GATA1 reactivity in the early components and intermediate to weak reactivity in normoblasts—in a similar pattern seen in normal bone marrow Image 4C. In all instances of AEML, dyserythropoiesis is highlighted by the GATA1 reactivity and reassures that GATA1 is indeed expressed in the dysplastic erythroid component. However, our ability to definitively exclude ectopic GATA1 expression in the myeloblast component of AEML was obscured by the prominent erythroid compartment. In other subtypes of acute myeloid leukemia and all acute lymphoblastic leukemias, the blasts were negative for GATA1 reactivity. In cases of acute promyelocytic leukemia and AMML with eosinophilia, scattered cells displayed weak nuclear GATA1 positivity Image 4D with a similar pattern in chronic myelogenous leukemia Image 2B—likely representing eosinophils. Last, in neoplastic cells of metastatic carcinoma (n = 3) and plasma cell neoplasms (n = 3), GATA1 reactivity was absent (data not shown).
Table 1.
Summary of GATA1 Immunoreactivity in Acute Leukemia and Other Hematologic Disorders
| Diagnosis | No. of Cases | GATA1 Reactivity in Blasts, No. |
|---|---|---|
| Normal | 3 | 0 |
| Acute myeloid leukemias | ||
| Acute promyelocytic leukemia | 7 | 0 |
| Acute myelomonocytic leukemia | 4 | 0 |
| Acute myelomonocytic leukemia with eosinophilia | 5 | 0 |
| Acute monoblastic leukemia | 3 | 0 |
| Acute monocytic leukemia | 8 | 0 |
| Pure erythroleukemia | 14 | 14 |
| Acute erythroleukemia, erythroid/myeloid type | 6 | 0 |
| Acute megakaryoblastic leukemia | 5 | 5 |
| Acute lymphoblastic leukemias | ||
| B-ALL | 8 | 0 |
| T-ALL | 5 | 0 |
| Other disorders | ||
| Chronic myelogenous leukemia | 5 | Weak, rare cellsa |
| Systemic mastocytosis | 3 | Strong, intermediate |
| Hypereosinophilic syndrome | 2 | Weak, intermediateb |
| Plasma cell neoplasm | 3 | None |
| Metastatic carcinoma | 3 | None |
B-ALL, B-acute lymphoblastic leukemia; GATA1, GATA binding factor 1; T-ALL, T-acute lymphoblastic leukemia.
Likely representing eosinophilic myeloid elements.
Eosinophilic myeloid elements.
Image 4.
Patterns of GATA binding factor 1 (GATA1) expression in acute myeloid leukemias. Intense nuclear staining is present in the blasts of pure erythroid leukemia (A, ×40) and acute megakaryocytic leukemia (B, ×40). C, The erythroid component of acute erythroid/myeloid leukemia exhibits intense nuclear GATA1 staining (×40). In the more mature erythroid cells, the nuclear staining is less intense, similar to the pattern seen in normal marrow (see Image 1B). Frequent erythroid dysplasia is seen, accentuated by GATA1 (C, inset). D, In acute promyelocytic leukemia, most of the lesional cells are negative for GATA1 staining. However, rare cells with weak nuclear GATA1 staining patterns similar to the pattern seen in chronic myelogenous leukemia likely represent eosinophilic myeloid elements (×40).
Discussion
This study demonstrates that GATA1, a critical transcription factor for erythroid and megakaryocytic development, is a sensitive and specific nuclear marker for erythroid and megakaryocytic precursors. Using a rabbit monoclonal antibody against GATA1, we observed intense nuclear staining in erythroid precursors and megakaryocytes in normal marrows. We can readily distinguish the weak to intermediate staining pattern seen in eosinophils and mast cells. In acute leukemia, we found that GATA1 consistently marked the blast populations of pure erythroid leukemia and acute megakaryoblastic leukemia.
GATA1 staining alone cannot distinguish between the blast forms of erythroid and megakaryocytic lineages and needs to be combined with morphologic assessment as well as additional studies that are already routinely used. Both acute pure erythroid leukemia and acute megakaryoblastic leukemia exhibit strong nuclear GATA1 reactivity and will require additional markers, such as CD61 and CD71, for subclassification. As the conventional erythro-megakaryocytic markers comprise surface and cytoplasmic proteins, having GATA1 as a nuclear marker provides the option for double staining in combination with other surface/cytoplasmic markers in studying difficult cases. We have demonstrated the feasibility of this double-marker approach in our study for GATA1 expression in eosinophils (see Image 3), where double-marker studies for GATA1/EPX and GATA1/MPO were performed.
Interestingly, the decrescendo pattern of GATA1 reactivity in maturing erythroid precursors is reminiscent of the staining pattern for transferrin receptor (CD71).23 Based on mouse models, a low level of GATA1 transcription is detected in the common myeloid progenitors, preceding the differentiation of proeyrthroblasts.24 Differentiation into proerythroblasts is marked by a spike in GATA1 level, which is followed by a steady decrease through the course of maturation.25 These findings explain the decrescendo pattern of GATA1 staining and suggest that GATA1 reactivity may be able to detect early CD71-negative erythroblasts.
Two previous studies have specifically examined GATA1 levels in primary acute leukemia and cell lines by RT-PCR.17,18 Consistent with our study, elevated levels of GATA1 transcripts were detected in all cases of acute erythroid and megakaryoblastic leukemia. However, both studies detected increased levels of GATA1 transcripts with a wide range of variation in a moderate proportion of acute myeloid leukemia cases. It is worth noting here that RNA transcripts were isolated from mononuclear cell fractions enriched by the Ficoll-Hypaque density gradient. Possible contamination by erythroid precursors, eosinophils, mast cells, and basophils may account for the elevated level of GATA1 transcripts. Examination of well-established cell lines found that GATA1 transcripts were present in all sampled erythroid and megakaryocytic leukemia cell lines and in one acute promyelocytic leukemia line (HL-60) but not in other myelocytic, monocytic, and lymphoblastic cell lines.18 Overall, GATA1 transcript levels in cell lines and primary cells support our data.
In summary, GATA1 is a sensitive lineage marker for erythroid and megakaryocytic precursors and is particularly useful for characterization of pure erythroid leukemia and acute megakaryoblastic leukemia.
Funding
This work is in part supported by National Institutes of Health–supported Training Grant Award T32 HL007627.
Acknowledgment
Acknowledgments: We thank Mark Fleming, MD, DPhil, for helpful comments and Alyson Campbell for expert technical assistance.
References
- 1. Cantor AB, Orkin SH.. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21:3368-3376. [DOI] [PubMed] [Google Scholar]
- 2. Crispino JD, Weiss MJ.. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood. 2014;123:3080-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer DE, Orkin SH.. Update on fetal hemoglobin gene regulation in hemoglobinopathies. Curr Opin Pediatr. 2011;23:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerenyi MA, Orkin SH.. Networking erythropoiesis. J Exp Med. 2010;207:2537-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiss MJ, Orkin SH.. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci U S A. 1995;92:9623-9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pevny L, Simon MC, Robertson E, et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257-260. [DOI] [PubMed] [Google Scholar]
- 7. Simon MC, Pevny L, Wiles MV, et al. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992;1:92-98. [DOI] [PubMed] [Google Scholar]
- 8. Shivdasani RA, Fujiwara Y, McDevitt MA, et al. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orkin SH, Shivdasani RA, Fujiwara Y, et al. Transcription factor GATA-1 in megakaryocyte development. Stem Cells. 1998;16(suppl 2):79-83. [DOI] [PubMed] [Google Scholar]
- 10. Ohneda K, Moriguchi T, Ohmori S, et al. Transcription factor GATA1 is dispensable for mast cell differentiation in adult mice. Mol Cell Biol. 2014;34:1812-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zon LI, Yamaguchi Y, Yee K, et al. Expression of mRNA for the GATA-binding proteins in human eosinophils and basophils: potential role in gene transcription. Blood. 1993;81:3234-3241. [PubMed] [Google Scholar]
- 12. Nei Y, Obata-Ninomiya K, Tsutsui H, et al. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci U S A. 2013;110:18620-18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nichols KE, Crispino JD, Poncz M, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu C, Niakan KK, Matsushita M, et al. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood. 2002;100:2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hollanda LM, Lima CSP, Cunha AF, et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006;38:807-812. [DOI] [PubMed] [Google Scholar]
- 16. Gruber TA, Downing JR.. The biology of pediatric acute megakaryoblastic leukemia. Blood. 2015;126:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patmasiriwat P, Fraizer G, Kantarjian H, et al. WT1 and GATA1 expression in myelodysplastic syndrome and acute leukemia. Leukemia. 1999;13:891-900. [DOI] [PubMed] [Google Scholar]
- 18. Shimamoto T, Ohyashiki K, Ohyashiki JH, et al. The expression pattern of erythrocyte/megakaryocyte-related transcription factors GATA-1 and the stem cell leukemia gene correlates with hematopoietic differentiation and is associated with outcome of acute myeloid leukemia. Blood. 1995;86:3173-3180. [PubMed] [Google Scholar]
- 19. Vannucchi AM, Pancrazzi A, Guglielmelli P, et al. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am J Pathol. 2005;167:849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed Lyon, France: IARC; 2008. [Google Scholar]
- 21. Mori Y, Iwasaki H, Kohno K, et al. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J Exp Med. 2009;206:183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan S, Salapow MA, Breen R, et al. Eosinophil peroxidase differs from neutrophil myeloperoxidase in its ability to bind antineutrophil cytoplasmic antibodies reactive with myeloperoxidase. Int Arch Allergy Immunol. 1994;105:150-154. [DOI] [PubMed] [Google Scholar]
- 23. Marsee DK, Pinkus GS, Yu H.. CD71 (transferrin receptor): an effective marker for erythroid precursors in bone marrow biopsy specimens. Am J Clin Pathol. 2010;134:429-435. [DOI] [PubMed] [Google Scholar]
- 24. Kuhl C, Atzberger A, Iborra F, et al. GATA1-mediated megakaryocyte differentiation and growth control can be uncoupled and mapped to different domains in GATA1. Mol Cell Biol. 2005;25:8592-8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki N, Suwabe N, Ohneda O, et al. Identification and characterization of 2 types of erythroid progenitors that express GATA-1 at distinct levels. Blood. 2003;102:3575-3583. [DOI] [PubMed] [Google Scholar]