Abstract
Objectives
The expected regional variability in percent human epidermal growth factor receptor 2 (HER2)–positive breast cancers is not currently clear.
Methods
Data from the 2006 to 2011 California Cancer Registry were examined by county and health service area. The influence of demographic and pathologic features was used in a multivariable logistic regression model to compare expected with observed HER2-positive percentages by region.
Results
There was significant geographic variation by California counties (11.6%-26%). The reported HER2-positive percentage was higher when the population had higher stage, tumor size, grade, percent estrogen receptor negative, younger age, or lower socioeconomic status. Ethnic distribution of the population also influenced HER2-positive percentages. Using a multivariable logistic regression model, most regions had expected values based on their population characteristics; however, “outlier” regions were identified.
Conclusions
These results deepen our understanding of population characteristics’ influence on the distribution of HER2-positive breast cancers. Taking these factors into account can be useful when setting laboratory benchmarks and assessing test quality.
Keywords: HER2, Breast cancer, Variability, Benchmark, Quality, Positivity rate
Testing the human epidermal growth factor receptor 2 (HER2) status of every newly diagnosed primary breast cancer or newly metastatic breast cancer is recommended by the College of American Pathologists/American Society of Clinical Oncology (CAP/ASCO).1-4 HER2 testing is performed to determine whether a patient’s breast cancer is likely to benefit from targeted anti-HER2 therapies, which have dramatically improved survival when added to chemotherapy regimens in patients with this aggressive form of breast cancer.5-9
Pathologic and demographic associations with HER2 positivity have been noted. HER2-positive breast cancers are typically high grade (Nottingham grades 2-3) and are more likely to be hormone receptor negative. In comparison with the more common HER2-negative, estrogen receptor (ER)–positive breast cancers, HER2-positive breast cancers more frequently present at a higher stage and in younger patients. In addition, variability in the HER2 status of breast cancers in different ethnic groups has also been noted.10-15
The reported prevalence of HER2 positivity in breast cancer varies from as low as 10% to as high as 30% across patient samples.7,16 Given the unique demographic characteristics and more aggressive pathologic features of HER2-positive breast cancer, reported prevalence rates are likely influenced by the characteristics of the population examined. In addition, it is possible that HER2 positivity prevalence is influenced by the laboratory methods used to test for HER2. Since laboratories and regulatory organizations seek to reduce variation related to differences in laboratory methods, it would be useful for laboratories to be able to benchmark their HER2-positive rates with other locoregional laboratories while controlling for differences related to their testing population’s characteristics.
In this study, using population-based California Cancer Registry (CCR) data, we assessed the extent of regional variability, by county and health service area (HSA), in the percentage of breast cancers reported as HER2 positive. In addition, we examined the influence of patient demographics and presenting pathologic features on geographic variation. Last, we used multivariable regression analysis to develop an algorithm to compare expected HER2-positive percentages with observed percentages by health care service area.
Materials and Methods
The CCR is a population-based cancer registry that, by state mandate, collects information on all cancers diagnosed in the state, abstracting data on patient demographics, tumor characteristics, and treatment from medical records. Using these data, we selected all invasive female breast cancers diagnosed in California from 2006 to 2011. We excluded any cases diagnosed solely on autopsy or death certificate (n = 455), not microscopically confirmed (n = 1,157), American Joint Committee on Cancer (AJCC) stage 0 (n = 993), and unknown or borderline HER2 status (N = 17,151). We also excluded cases from the Alpine HSA, as case counts from this region were too small for meaningful analysis. The final study population included 121,408 cancers.
Socioeconomic and Facility Characteristics
The CCR does not collect individual-level socioeconomic data. We used a previously developed measure to assign cases to a neighborhood socioeconomic status (nSES) quintile based on the census block group of the patient’s residence at the time of diagnosis.17,18
The CCR has data on the first facility to report each cancer case. To characterize these reporting facilities, we calculated the nSES distribution of all cancer cases reported by that facility during the years of our study and also identified those facilities that were affiliated with National Cancer Institute (NCI)–designated cancer centers.
HSAs
The state of California contains 58 counties that are grouped into HSAs by the National Center for Health Statistics (NCHS). These are defined as being areas that are “relatively self-contained with respect to hospital care.”19 For our analysis, we used the NCI’s modification of the NCHS HSAs, which allocates all counties in an HSA that cross state or Surveillance, Epidemiology, and End Results (SEER) registry boundaries into one state or SEER registry, resulting in 30 NCI-modified HSAs in California.20
Statistical Analysis
We compared the proportion of HER2-positive tumors by California county and examined the distribution of patient and tumor characteristics by HER2 status.
We then constructed a model of HER2-positive status using multivariable logistic regression. The following variables were considered for inclusion in the model: NCI-modified HSA, age at diagnosis, race/ethnicity, AJCC stage at diagnosis, tumor size, nodal involvement, histology, grade, hormone (combined estrogen and progesterone) receptor status, patient nSES quintile, marital status at diagnosis, primary payer at diagnosis, hospital nSES distribution of patients, cancer center, and year of diagnosis. Variables with a Wald type 3 P value of more than .2 (marital status and patient nSES quintile) were excluded from the final model. The variance inflation factor was used to check for multicollinearity among variables. The model was adjusted for clustering by patient and reporting hospital and weighted using inverse probability of known HER2 status (see below, propensity score weighting).
For each HSA, the expected percentage of HER2-positive tumors was predicted based on the model described above. This predicted percentage was then compared with the observed percentage, and a χ2 test was performed to test for statistical significance.
Propensity Score Weighting
The distribution of sociodemographic and tumor characteristics differed between cases with known and unknown HER2 status. We therefore used propensity scores to balance these characteristics between groups, making our study population of known HER2 status cases more representative of all breast cancer cases in the state. A propensity score was calculated based on a multivariable logistic regression model predicting known HER2 status. Variables in this model included year of diagnosis, CCR reporting region, age, race/ethnicity, AJCC stage, tumor size, nodal involvement, histology, grade, estrogen/progesterone receptor status, nSES quintile, marital status, primary payer at diagnosis, nSES distribution of cancer cases at the reporting hospital, and NCI-designated cancer center status of the reporting hospital. The inverse probability of treatment weights (IPTW), in this case representing the inverse probability of having known HER2 status, was derived from the propensity score and normalized by the mean IPTW. Use of these weights minimized differences in the distribution of the sociodemographic and tumor characteristics between the population with unknown and known HER2 status.
Results
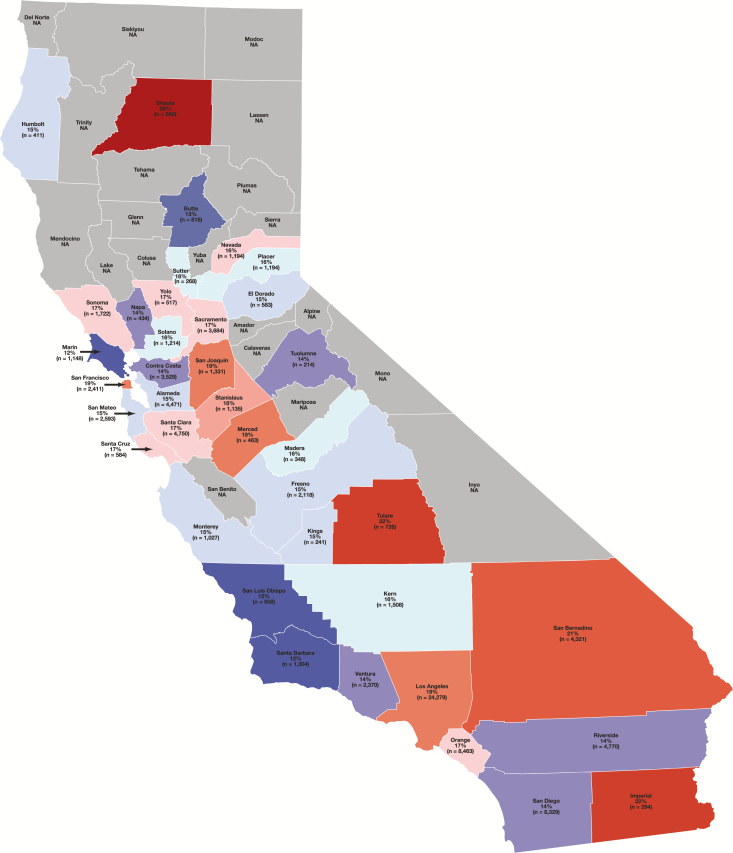
In total, 121,408 cases diagnosed between 2006 and 2011 were available in the CCR database and met inclusion criteria. The overall average percentage of HER2-positive breast cancers was 16.6%. The percentage of breast cancers that was HER2 positive varied between California counties with a range of 11.6% in Marin County to 26% in Shasta County (counties with <200 cases were excluded due to their small numbers) and a median percentage of 15.9% Table 1 and Figure 1 . In addition, there was variability in the percentage of HER2-positive breast cancers among different HSAs across the state. The lowest percentage of HER2 positivity was observed in the Santa Barbara–San Luis Obispo HSA (11.7%), while the highest percentage was in the Shasta (Redding)–Trinity HSA (23.0%) Table 2 .
Table 1 .
Percentage of Breast Cancers Reported as HER2 Positive in California by Countya
| County | Total No. of Breast Cancers | HER2 Positive, % |
|---|---|---|
| Marin | 1,148 | 11.6 |
| San Luis Obispo | 958 | 12.1 |
| Santa Barbara | 1,304 | 12.2 |
| Butte | 818 | 12.7 |
| Napa | 434 | 13.8 |
| Contra Costa | 3,529 | 13.9 |
| Tuolumne | 214 | 14.0 |
| Ventura | 2,370 | 14.3 |
| Riverside | 4,770 | 14.4 |
| San Diego | 8,329 | 14.5 |
| Kings | 241 | 14.5 |
| Monterey | 1,027 | 14.7 |
| El Dorado | 583 | 14.8 |
| Fresno | 2,118 | 14.8 |
| Humboldt | 411 | 14.8 |
| San Mateo | 2,593 | 14.8 |
| Alameda | 4,471 | 15.2 |
| Sutter | 268 | 15.7 |
| Madera | 348 | 15.8 |
| Kern | 1,508 | 16.0 |
| Placer | 1,194 | 16.1 |
| Solano | 1,214 | 16.1 |
| Orange | 8,463 | 16.5 |
| Santa Clara | 4,750 | 16.5 |
| Tolo | 517 | 16.8 |
| Sonoma | 1,722 | 16.8 |
| Sacramento | 3,684 | 17.2 |
| Nevada | 469 | 17.3 |
| Santa Cruz | 584 | 17.3 |
| Stanislaus | 1,135 | 18.4 |
| San Joaquin | 1,331 | 18.6 |
| San Francisco | 2,411 | 18.7 |
| Los Angeles | 24,279 | 18.9 |
| Merced | 463 | 19.0 |
| San Bernardino | 4,321 | 21.2 |
| Tulare | 726 | 21.6 |
| Imperial | 294 | 22.1 |
| Shasta | 565 | 26.0 |
| Overall | 95,564 | Median 15.9 |
HER2, human epidermal growth factor receptor 2.
aCounties with less than 200 cases excluded.
Figure 1 .
Map of the percentage of breast cancer cases in California counties reported as human epidermal growth factor receptor 2 (HER2) positive (counties included reported >200 cases between 2006 and 2011). The median percentage was 15.9% HER2 positive. Counties reporting percentages above this median are color-coded in red tones and counties reporting below are color-coded in blue tones to visually reflect the degree to which they deviate from the median. NA, not available.
Table 2 .
Reported HER2 Status by Demographic and Pathologic Features in California Cancer Registry Database
| Characteristic | Total No. | HER2 Positive, No. (%) |
|---|---|---|
| Overall | 121,408 | 20,142 (16.6) |
| NCI-modified health service area (California) | ||
| Santa Barbara (Santa Barbara)–San Luis Obispo | 2,779 | 325 (11.7) |
| Marin | 1,383 | 166 (12.0) |
| Inyo–Mono | 108 | 15 (13.9) |
| Butte (Chico)–Tehama | 1,364 | 196 (14.4) |
| Ventura | 2,924 | 426 (14.6) |
| San Diego (San Diego)–Imperial | 11,104 | 1,621 (14.6) |
| Fresno (Fresno)–Kings | 3,339 | 489 (14.7) |
| Alameda (Oakland)–Contra Costa | 9,796 | 1,448 (14.8) |
| Humboldt | 482 | 75 (15.6) |
| Stanislaus (Modesto)–Merced | 2,622 | 410 (15.6) |
| Sutter (Yuba City)–Yuba | 586 | 93 (15.9) |
| Solano (Vallejo)–Napa | 2,339 | 373 (16.0) |
| Santa Cruz | 769 | 124 (16.1) |
| Del Norte | 105 | 17 (16.2) |
| Santa Clara (San Jose)–Monterey | 7,280 | 1,187 (16.3) |
| Sacramento (Sacramento)–Placer | 7,650 | 1,254 (16.4) |
| Orange | 10,522 | 1,738 (16.5) |
| San Francisco (San Francisco)–San Mateo | 6,088 | 1,010 (16.6) |
| San Joaquin (Stockton)–Calaveras | 2,202 | 367 (16.7) |
| Nevada–Sierra | 575 | 98 (17.0) |
| Kern | 1,919 | 330 (17.2) |
| San Bernardino–Riverside (Riverside) | 11,280 | 1,950 (17.3) |
| Sonoma (Santa Rosa) | 2,065 | 360 (17.4) |
| Siskiyou | 178 | 32 (18.0) |
| Lassen–Plumas | 133 | 24 (18.0) |
| Los Angeles (Los Angeles) | 30,076 | 5,600 (18.6) |
| Mendocino | 189 | 38 (20.1) |
| Tulare | 955 | 193 (20.2) |
| Shasta (Redding)–Trinity | 796 | 183 (23.0) |
| Age, y | ||
| <40 | 6,077 | 1,632 (26.9) |
| 40-49 | 21,038 | 4,230 (20.1) |
| 50-64 | 46,013 | 8,292 (18.0) |
| 65+ | 48,280 | 5,988 (12.4) |
| Race/ethnicity | ||
| NH white | 78,014 | 11,297 (14.5) |
| NH black | 7,683 | 1,396 (18.2) |
| Hispanic | 20,584 | 4,148 (20.2) |
| Chinese | 3,037 | 666 (21.9) |
| Japanese | 1,337 | 188 (14.1) |
| Filipina | 4,026 | 998 (24.8) |
| Other Asian/Pacific Islander | 5,435 | 1,214 (22.3) |
| NH American Indian/other/ unknown | 1,392 | 235 (16.9) |
| Marital status | ||
| Not currently married | 50,801 | 8,160 (16.1) |
| Married | 66,475 | 11,311 (17.0) |
| Unknown | 4,132 | 671 (16.2) |
| Neighborhood quintile of SES | ||
| 1 | 14,643 | 2,932 (20.0) |
| 2 | 20,697 | 3,719 (18.0) |
| 3 | 24,687 | 4,102 (16.6) |
| 4 | 28,691 | 4,588 (16.0) |
| 5 | 32,690 | 4,801 (14.7) |
| Insurance status | ||
| Not insured/self-pay | 934 | 198 (21.2) |
| Private | 72,216 | 12,016 (16.6) |
| Public/Medicaid | 25,185 | 4,909 (19.5) |
| Medicare | 18,950 | 2,222 (11.7) |
| Military | 761 | 164 (21.6) |
| Unknown | 3,362 | 633 (18.8) |
| AJCC stage | ||
| I | 57,007 | 7,067 (12.4) |
| II | 40,496 | 7,221 (17.8) |
| III | 14,523 | 3,518 (24.2) |
| IV | 5,495 | 1,449 (26.4) |
| Unknown | 3,887 | 887 (22.8) |
| Tumor size, cm | ||
| <1 | 23,159 | 3,120 (13.5) |
| 1-1.9 | 42,187 | 5,522 (13.1) |
| 2-2.9 | 24,152 | 4,304 (17.8) |
| 3-3.9 | 11,309 | 2,357 (20.8) |
| 4-5 | 7,658 | 1,708 (22.3) |
| >5 | 9,441 | 2,233 (23.7) |
| Unknown/not recorded | 3,502 | 898 (25.6) |
| Grade | ||
| 1 | 27,244 | 1,283 (4.7) |
| 2 | 50,169 | 6,878 (13.7) |
| 3/high | 38,471 | 10,772 (28.0) |
| Unknown | 5,524 | 1,209 (21.9) |
| Histology | ||
| Ductal | 101,952 | 18,091 (17.7) |
| Lobular or with lobular component | 11,419 | 777 (6.8) |
| Other | 8,037 | 1,274 (15.9) |
| Hormone receptor status | ||
| Negative | 21,545 | 6,712 (31.2) |
| Positive | 98,853 | 13,180 (13.3) |
| Unknown/borderline | 1,010 | 250 (24.8) |
| Care at NCI cancer center | ||
| No | 114,384 | 18,874 (16.5) |
| Yes | 7,024 | 1,268 (18.1) |
| Patient SES distribution of reporting hospital | ||
| Over half of patients are high SES | 60,765 | 9,088 (15.0) |
| Over half of patients are low SES | 21,965 | 4,439 (20.2) |
| Mixed distribution | 38,678 | 6,615 (17.1) |
| Radiation therapy | ||
| No | 66,254 | 11,933 (18.0) |
| Yes | 55,154 | 8,209 (14.9) |
| Chemotherapy | ||
| No | 72,554 | 7,285 (10.0) |
| Yes | 48,854 | 12,857 (26.3) |
| Vital status | ||
| Dead | 14,990 | 2,864 (19.1) |
| Alive | 106,418 | 17,278 (16.2) |
AJCC, American Joint Committee on Cancer; HER2, human epidermal growth factor receptor 2; NCI, National Cancer Institute; NH, non-Hispanic; SES, socioeconomic status.
Available demographic and pathologic variables that might contribute to differences in the percentage of HER2-positive breast cancers in different geographic regions were examined, including age, race/ethnicity, marital status, nSES, insurance status, AJCC stage, tumor size, grade, histologic subtype, hormone receptor status, whether or not the reporting facility was a NCI-designated cancer center, patient nSES distribution of the reporting hospital, and treatment (results shown in Table 2).
A higher prevalence of HER2-positive cancers was observed in patients younger than 50 years (27.6% <50 years vs 17.9% ≥50 years). The percentage of HER2-positive cancers varied by race/ethnicity, with the highest prevalence in Filipinas (24.8%), followed by Asians/Pacific Islanders (22.3%), Chinese (21.9%), and Hispanics (20.2%). Non-Hispanic whites (14.5%) and Japanese (14.1%) had the lowest percentage of HER2-positive breast cancers. In addition, when grouped by insurance types, the highest percentage of HER2 positivity was observed in noninsured/self-pay patients (21.2%) and lowest in patients with private insurance (16.6%). Overall, the percentage of HER2-positive cases decreased with increasing socioeconomic status (SES). Reporting hospitals with more than half of patients in the highest SES category had a lower percentage of HER2-positive cancers (15.0%) compared with hospitals with the majority of patients in the lowest SES (20.2%).
Cancers with higher stage at diagnosis had higher HER2-positive percentages (14.7% for stages I-II vs 24.8% for stage ≥III). Similarly, higher grade cancers had increasingly higher percentages of HER2 positivity (4.7% for grade 1, rising to 13.7% for grade 2 and 28.0% for grade 3). Last, 31.2% of hormone receptor–negative cancers were HER2-positive compared with only 13.3% of hormone receptor–positive cancers (P < .0001).
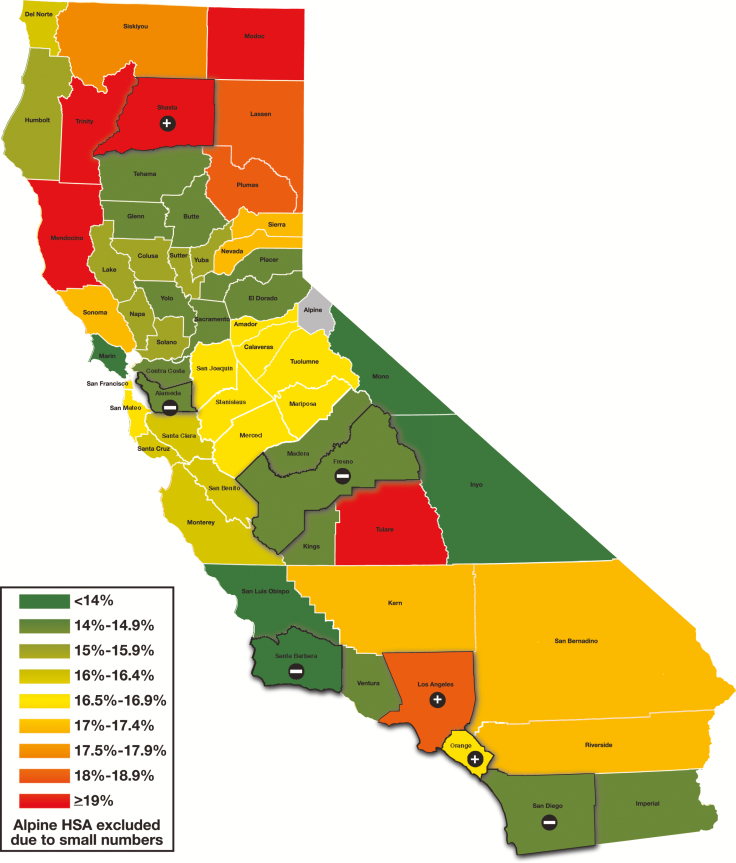
A model to predict the percentage of HER2-positive cancers was constructed using multivariable logistic regression. When the predicted percentage was compared with the observed percentage by HSA, several HSAs, including Alameda, Fresno, San Diego, and Santa Barbara, had lower than expected HER2-positive percentages, while Los Angeles, Orange, and Shasta had higher than expected percentages Table 3 and Figure 2 .
Table 3 .
Observed vs Expected HER2-Positive Rates in California by NCI-Modified Health Service Area Using Multivariate Logistic Regression Modela
| NCI-Modified Health Service Area (California) | Observed % HER2 Positive | Expected % HER2 Positive | χ 2 P Value |
|---|---|---|---|
| Santa Barbara (Santa Barbara)–San Luis Obispo | 11.69 | 14.35 | .0033 |
| Marin | 12.00 | 13.29 | .3104 |
| Inyo–Mono | 13.89 | 15.96 | .6690 |
| Butte (Chico)–Tehama | 14.37 | 14.33 | .9764 |
| Ventura | 14.57 | 15.88 | .1624 |
| San Diego (San Diego)–Imperial | 14.60 | 15.83 | .0104 |
| Fresno (Fresno)–Kings | 14.65 | 18.91 | <.0001 |
| Alameda (Oakland)–Contra Costa | 14.78 | 15.88 | .0323 |
| Humboldt | 15.56 | 14.82 | .7479 |
| Sutter (Yuba City)–Yuba | 15.87 | 16.50 | .7694 |
| Solano (Vallejo)–Napa | 15.95 | 15.83 | .9116 |
| Santa Cruz | 16.12 | 15.42 | .7055 |
| Del Norte | 16.19 | 17.40 | .8152 |
| Santa Clara (San Jose)–Monterey | 16.30 | 15.41 | .1375 |
| Sacramento (Sacramento)–Placer | 16.39 | 16.11 | .6392 |
| Orange | 16.52 | 15.37 | .0230 |
| San Francisco (San Francisco)–San Mateo | 16.59 | 15.35 | .0618 |
| San Joaquin (Stockton)–Calaveras | 16.67 | 18.12 | .2027 |
| Stanislaus (Modesto)–Merced | 16.93 | 17.73 | .4607 |
| Nevada–Sierra | 17.04 | 13.85 | .1341 |
| Kern | 17.20 | 18.03 | .4997 |
| San Bernardino–Riverside (Riverside) | 17.29 | 18.23 | .0634 |
| Sonoma (Santa Rosa) | 17.43 | 15.85 | .1721 |
| Siskiyou | 17.98 | 14.15 | .3254 |
| Lassen–Plumas | 18.05 | 14.23 | .3972 |
| Los Angeles (Los Angeles) | 18.62 | 17.72 | .0040 |
| Mendocino | 20.11 | 19.23 | .8299 |
| Tulare | 20.21 | 19.52 | .7042 |
| Shasta (Redding)–Trinity | 22.99 | 16.66 | .0015 |
HER2, human epidermal growth factor receptor 2; NCI, National Cancer Institute.
aItalicized rows are results with significantly lower HER2 percentages than expected. Bold rows are results with significantly higher HER2 percentages than expected.
Figure 2 .
Observed vs expected human epidermal growth factor receptor 2–positive percentages in California by National Cancer Institute–modified health service area (HSA). Regions highlighted had higher or lower results than expected based on the multivariate logistic model (as indicated by a plus or minus sign). Alpine HSAs were excluded due to small numbers.
Discussion
We found that in California, there is substantial regional variation in the percentage of reported HER2-positive breast cancers. The regional variability was explained in some part by regional differences in sociodemographic factors (age, race/ethnicity, HSA, socioeconomic status, insurance status) and disease-specific factors (stage at diagnosis, hormone receptor status, tumor size, grade). Using a multivariable logistic regression model to account for these factors, most health service regions had a reported percentage of HER2-positive breast cancers that fell within the expected range. However, we also identified some regions with unexpectedly low or high HER2-positive rates that may be due to other contributing factors. Similar analyses of other population-based data sets can help laboratories set better benchmarks for expected positivity rates of cancer biomarker test results such as HER2. These population-adjusted benchmarks may allow laboratories to identify when laboratory-related factors or other factors might be contributing to unexpectedly high or low results.
While some groups have suggested that simply monitoring HER2 positivity rates by institution can be an effective way to monitor test quality, our data suggest that multiple demographic and pathologic factors will affect these rates.21 Others have reported an increased frequency of HER2-positive cancers in patients diagnosed at a younger age and having Hispanic and Asian race/ethnicity, higher SES, hormone receptor–negative breast cancer, and higher grade/stage tumors at presentation.22-27 Earlier studies of CCR data from 1999 to 2004 reported similar correlations between HER2-positive rates and these sociodemographic and clinical factors.28 A recent multicenter German study also showed that pathologic factors influenced HER2 positivity rates between centers (including grade, hormone receptor status, histologic subtype, and nodal status).29 However, no publications to our knowledge have reported on geographic variation in HER2 test results linked to demographic and pathologic factors. The current results allow comparison of different geographic regions and estimation of the contribution of population-related factors to the percentage of test results expected to be positive.
Our discovery of outlier regions in HER2 positivity raises the question of underlying reasons for the differences. In addition to further investigation of other demographic and population-specific factors (eg, potential genetic causes of HER2-positive breast cancer that may vary between racial/ethnic groups), an informative analysis should include examination of variables known to contribute to false-negative or false-positive HER2 results. These include preanalytic variables such as prolonged ischemic times, poor fixation, alternative fixation protocols, and other specimen-handling issues. Analytic variables such as test method (immunohistochemical [IHC] staining vs fluorescence in situ hybridization [FISH] vs other in situ hybridization testing techniques) and specific testing algorithm used (IHC first with reflex FISH on IHC-equivocal cases, FISH only, dual testing, etc) can also affect results.1,30 Last, postanalytic variables such as scoring method and adequacy of test validation procedures can influence test results. Some of these factors may not have been standardized in the earlier years of our study time frame (2006-2011), before publication of the first HER2 testing guidelines in 2007.3
Previous studies using the CCR reported an overall HER2 positivity rate of 22% during 1993 to 1996 and 22.7% during 1999 to 2004; however, the proportions of cases with unknown HER2 status were considerably higher in these earlier case series, and thus the HER2 positivity rates were likely artifactually inflated.14,28 In the current study using data from 2006 to 2011, this overall HER2-positive percentage was 16.6%. An analogous study in Utah reported a similar 17% overall HER2-postive percentage.31 The 2007 publication of the first ASCO/CAP HER2 testing and reporting guideline is a major factor that likely influenced this change. Prior to these guidelines, as many as 18% of HER2-positive test results were estimated to be false-positive results: this estimate is based on N9831 trial and National Surgical Adjuvant Breast and Bowel Project B-31 trial data reporting local laboratory positive results that could not be confirmed when retested by central reference laboratories.32,33 One of the issues identified as a major cause of “false-positive” cases is overinterpretation of equivocal (2+) IHC results as positive (3+) results.34 More recent studies looking at the impact of the recent 2013 guidelines update, which modified interpretation criteria for both IHC and ISH testing, suggest that the percentage of breast cancers reported as HER2 positive and HER2 equivocal will increase.1,2,35-43
Our study was limited by the data available from the CCR database during the time period studied. Detailed information about HER2 testing methods was not available. Future studies using this database will have the benefit of more detail about which type of test was performed (IHC vs ISH), which may help to elucidate its influence on HER2-positive rates. While additional validation is required to determine whether the predictive model presented here can be applied beyond California, these results support the conclusion that laboratories and regulatory agencies should consider the influence of demographic factors when comparing the distribution of HER2 test results.
In conclusion, we have found significant regional variation in the percentage of breast cancers reported as HER2 positive and demonstrated the influence of various population characteristics on these percentages. By developing models that estimate the expected HER2-positive rates given the population demographics and pathology characteristics, more relevant benchmarks can be developed for regional laboratories to identify systematic issues as well as outliers.
Acknowledgments
We thank Norman Cyr for the figure preparation. The collection of cancer incidence data used in the current study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s SEER Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, the Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Supported by Stanford Cancer Institute and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C.
References
- 1. Wolff AC, Hammond ME, Hicks DG et al. ; American Society of Clinical Oncology; College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013. [DOI] [PubMed] [Google Scholar]
- 2. Wolff AC, Hammond ME, Hicks DG et al. ; American Society of Clinical Oncology; College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolff AC, Hammond ME, Schwartz JN et al. ; American Society of Clinical Oncology; College of American Pathologists American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118-145. [DOI] [PubMed] [Google Scholar]
- 4. Wolff AC, Hammond ME, Schwartz JN et al. ; American Society of Clinical Oncology/College of American Pathologists American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18-43. [DOI] [PubMed] [Google Scholar]
- 5. Yarden Y, Sliwkowski MX. Untangling the erbb signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [DOI] [PubMed] [Google Scholar]
- 6. Arteaga CL, Sliwkowski MX, Osborne CK et al. . Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9:16-32. [DOI] [PubMed] [Google Scholar]
- 7. Slamon DJ, Clark GM, Wong SG et al. . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [DOI] [PubMed] [Google Scholar]
- 8. Slamon D, Eiermann W, Robert N et al. ; Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baselga J, Bradbury I, Eidtmann H et al. ; NeoALTTO Study Team Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stark AT, Claud S, Kapke A et al. . Race modifies the association between breast carcinoma pathologic prognostic indicators and the positive status for HER-2/neu. Cancer. 2005;104:2189-2196. [DOI] [PubMed] [Google Scholar]
- 11. Huang HJ, Neven P, Drijkoningen M et al. . Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol. 2005;58:611-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang HJ, Neven P, Drijkoningen M et al. . Hormone receptors do not predict the HER2/neu status in all age groups of women with an operable breast cancer. Ann Oncol. 2005;16:1755-1761. [DOI] [PubMed] [Google Scholar]
- 13. Telli ML, Chang ET, Kurian AW et al. . Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carey LA, Perou CM, Livasy CA et al. . Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492-2502. [DOI] [PubMed] [Google Scholar]
- 15. Kurian AW, Fish K, Shema SJ et al. . Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gusterson BA, Gelber RD, Goldhirsch A et al. . Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049-1056. [DOI] [PubMed] [Google Scholar]
- 17. Yost K, Perkins C, Cohen R et al. . Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703-711. [DOI] [PubMed] [Google Scholar]
- 18. Yang J, Schupp CW, Harrati A et al. . Developing an Area-Based Socioeconomic Measure From American Community Survey Data. Fremont: Cancer Prevention Institute of California; 2014. [Google Scholar]
- 19. Pickle LW, Mungiole M, Jones GK et al. . Atlas of United States Mortality. Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 20. http://seer.cancer.gov/seerstat/variables/countyattribs/hsa.html.
- 21. Choritz H, Büsche G, Kreipe H; Study Group HER2 Monitor Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch. 2011;459:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cronin KA, Harlan LC, Dodd KW et al. . Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fehrenbacher L, Habel L, Capra A et al. . Incidence and demographic and tumor characteristics of HER2-positive invasive breast cancer in a large, unselected population, 2000-2006. Cancer Res. 2009;69(suppl):3058. [Google Scholar]
- 24. Konecny G, Pauletti G, Pegram M et al. . Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142-153. [DOI] [PubMed] [Google Scholar]
- 25. Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000-2010. BMC Cancer. 2013;13:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Howlader N, Altekruse SF, Li CI et al. . US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beltjens F, Bertaut A, Pigeonnat S et al. . HER2-positivity rates in breast cancer: no variation over time when clinicopathological features and testing are stable [published online October 27, 2015]. Eur J Cancer Care (Engl). [DOI] [PubMed] [Google Scholar]
- 28. Parise CA, Bauer KR, Brown MM et al. . Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15:593-602. [DOI] [PubMed] [Google Scholar]
- 29. Rüschoff J, Lebeau A, Kreipe H et al. ; Nicht-interventionelle Untersuchung (NIU) HER2 Study Group Assessing HER2 testing quality in breast cancer: variables that influence HER2 positivity rate from a large, multicenter, observational study in Germany. Mod Pathol. 2017;30:217-226. [DOI] [PubMed] [Google Scholar]
- 30. Penault-Llorca F, Bilous M, Dowsett M et al. . Emerging technologies for assessing HER2 amplification. Am J Clin Pathol. 2009;132:539-548. [DOI] [PubMed] [Google Scholar]
- 31. Stenehjem DD, Yoo M, Unni SK et al. . Assessment of HER2 testing patterns, HER2+ disease, and the utilization of HER2-directed therapy in early breast cancer. Breast Cancer (Dove Med Press). 2014;6:169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roche PC, Suman VJ, Jenkins RB et al. . Concordance between local and central laboratory HER2 testing in the Breast Intergroup trial n9831. J Natl Cancer Inst. 2002;94:855-857. [DOI] [PubMed] [Google Scholar]
- 33. Paik S, Bryant J, Tan-Chiu E et al. . Real-world performance of HER2 testing—National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852-854. [DOI] [PubMed] [Google Scholar]
- 34. Grimm EE, Schmidt RA, Swanson PE et al. . Achieving 95% cross-methodological concordance in HER2 testing: causes and implications of discordant cases. Am J Clin Pathol. 2010;134:284-292. [DOI] [PubMed] [Google Scholar]
- 35. Lim TH, Lim AS, Thike AA et al. . Implications of the updated 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations on human epidermal growth factor receptor 2 gene testing using immunohistochemistry and fluorescence in situ hybridization for breast cancer. Arch Pathol Lab Med. 2016;140:140-147. [DOI] [PubMed] [Google Scholar]
- 36. Muller KE, Marotti JD, Memoli VA et al. . Impact of the 2013 ASCO/CAP HER2 guideline updates at an academic medical center that performs primary HER2 FISH testing: increase in equivocal results and utility of reflex immunohistochemistry. Am J Clin Pathol. 2015;144:247-252. [DOI] [PubMed] [Google Scholar]
- 37. Xu Y, Bai QM, Yang F et al. . Impact of 2013 American Society of Clinical Oncology/College of American Pathologist guidelines on borderline immunostaining results for HER2: a retrospective study on HER2 FISH results in 1,780 cases of invasive breast cancers [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2016;45:545-549. [DOI] [PubMed] [Google Scholar]
- 38. Singh K, Tantravahi U, Lomme MM et al. . Updated 2013 College of American Pathologists/American Society of Clinical Oncology (CAP/ASCO) guideline recommendations for human epidermal growth factor receptor 2 (HER2) fluorescent in situ hybridization (FISH) testing increase HER2 positive and HER2 equivocal breast cancer cases; retrospective study of HER2 FISH results of 836 invasive breast cancers. Breast Cancer Res Treat. 2016;157:405-411. [DOI] [PubMed] [Google Scholar]
- 39. Press MF, Villalobos I, Santiago A et al. . Assessing the new American Society of Clinical Oncology/College of American Pathologists guidelines for HER2 testing by fluorescence in situ hybridization: experience of an academic consultation practice [published online April 15, 2016]. Arch Pathol Lab Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan YS, Casas CE, Peng J et al. . HER2 FISH classification of equivocal HER2 IHC breast cancers with use of the 2013 ASCO/CAP practice guideline. Breast Cancer Res Treat. 2016;155:457-462. [DOI] [PubMed] [Google Scholar]
- 41. Polónia A, Leitão D, Schmitt F. Application of the 2013 ASCO/CAP guideline and the SISH technique for HER2 testing of breast cancer selects more patients for anti-HER2 treatment. Virchows Arch. 2016;468:417-423. [DOI] [PubMed] [Google Scholar]
- 42. Stoss OC, Scheel A, Nagelmeier I et al. . Impact of updated HER2 testing guidelines in breast cancer: re-evaluation of HERA trial fluorescence in situ hybridization data. Mod Pathol. 2015;28:1528-1534. [DOI] [PubMed] [Google Scholar]
- 43. Long TH, Lawce H, Durum C et al. . The new equivocal: changes to HER2 FISH results when applying the 2013 ASCO/CAP guidelines. Am J Clin Pathol. 2015;144:253-262. [DOI] [PubMed] [Google Scholar]