Abstract
Objectives
CD200 expression has been well studied in hematopoietic malignancies; however, CD200 expression has not been well-characterized in neuroendocrine neoplasms. We examined CD200 expression in 391 neuroendocrine neoplasms from various anatomic sites.
Methods
Tissue blocks containing pulmonary small cell carcinoma, pulmonary carcinoid, large cell neuroendocrine carcinoma, pancreatic neuroendocrine tumor, gastrointestinal carcinoid, and Merkel cell carcinoma were evaluated for CD200 expression by immunohistochemistry. A set of nonneuroendocrine carcinomas was stained for comparison.
Results
CD200 was expressed in 87% of the neuroendocrine neoplasms studied, including 60 of 72 (83%) pulmonary small cell carcinomas, 15 of 22 (68%) pulmonary carcinoids, three of four (75%) pulmonary large cell neuroendocrine carcinomas, 125 of 146 (86%) Merkel cell carcinomas, 79 of 83 (95%) gastrointestinal luminal carcinoids, and 56 of 60 (93%) pancreatic neuroendocrine tumors. Thirty-two of 157 (20%) nonneuroendocrine carcinomas expressed CD200. In gastrointestinal carcinoid and pancreatic neuroendocrine neoplasms, CD200 negativity correlated with higher grade.
Conclusions
CD200 is a relatively sensitive marker of neuroendocrine neoplasms and represents a potential therapeutic target in these difficult-to-treat malignancies.
Keywords: CD200, Neuroendocrine neoplasms, Immunohistochemistry
CD200 is a membrane-bound glycoprotein normally expressed by neurons, endothelial cells, follicular dendritic cells, lymphocytes, macrophages, and granulocytes.1-3 CD200 expression may promote tumor formation and metastasis by helping malignant cells evade the immune system.4 CD200 has been well studied in hematopoietic malignancies including lymphoma, plasma cell myeloma, and acute leukemia, among others.5-12 Although less well-studied in nonhematopoietic neoplasms, CD200 expression has been reported in renal cell carcinoma, ovarian carcinoma, neuroblastoma, melanoma, cutaneous squamous carcinoma, and basal cell carcinoma, and studied but not identified in prostate carcinoma, breast carcinoma, and non–small cell lung carcinoma.13,14 Early studies reported no expression of CD200 in astrocytoma or glioblastoma, but recent studies show expression.13,15 While we have previously characterized CD200 expression in small cell lung carcinoma by both flow cytometry and immunohistochemistry, to the best of our knowledge, CD200 expression has not been characterized by immunohistochemistry in a large series of neuroendocrine neoplasms; such characterization is the primary objective of this study.16
Materials and Methods
Our study was approved by all institutional review boards in accord with the ethical standards established by the institution in which the experiments were performed, or are in accord with the Helsinki Declaration of 1975 (see Encyclopedia of Bioethics. 3rd ed. New York: Macmillan; 2003), as revised in 2008. After institutional review, archival formalin-fixed paraffin-embedded (FFPE) tissue blocks and tissue microarrays (TMA) containing neuroendocrine neoplasms (NENs) and nonneuroendocrine epithelial neoplasms (non-NENs) were identified. The NENs studied included pulmonary small cell carcinoma (SCLC), large cell neuroendocrine carcinoma (LCNEC), pulmonary carcinoid (PCT), Merkel cell carcinoma (MCC), gastrointestinal luminal carcinoid (GCT), and pancreatic neuroendocrine tumor (PanNET). Non-NENs were studied for comparison see Table 1. Uninvolved normal tissue present in tumor blocks was evaluated concurrently. Hematopoietic neoplasms were excluded from this study, given their known patterns of CD200 expression.5-12
Table 1.
CD200 Expression in Neuroendocrine Neoplasms and Nonneuroendocrine Neoplasms
| Tumor Pype | No. Positive (%) | Total No. Cases |
|---|---|---|
| Neuroendocrine neoplasms | 338 (86) | 391 |
| Pulmonary small cell carcinoma | 60 (83) | 72 |
| Large cell neuroendocrine carcinoma | 4 (4) | 5 |
| Pulmonary carcinoid | 15 (65) | 23 |
| Merkel cell carcinoma | 125 (84) | 149 |
| Gastrointestinal luminal carcinoids | 78 (95) | 82 |
| Small bowel | 49 (89) | 55 |
| Appendix | 20 (95) | 21 |
| Large bowel | 4 (100) | 4 |
| Stomach | 2 (100) | 2 |
| Pancreatic neuroendocrine tumor | 56 (94) | 60 |
| Nonneuroendocrine neoplasms | 32 (20) | 157 |
| Colorectal carcinoma | 0 (0) | 12 |
| Prostatic carcinoma | 0 (0) | 8 |
| Breast carcinoma | 0 (0) | 15 |
| Pulmonary adenocarcinoma | 0 (0) | 52 |
| Pulmonary squamous cell carcinoma | 2 (10) | 20 |
| Pancreatic adenocarcinoma | 0 (0) | 5 |
| Renal cell carcinoma | 5 (72) | 7 |
| Papillary thyroid carcinoma | 10 (100) | 10 |
| Ovarian carcinoma | 6 (67) | 9 |
| Cutaneous squamous cell carcinoma | 0 (0) | 10 |
| Cutaneous basal cell carcinoma | 9 (100) | 9 |
The aforementioned neoplasms were evaluated by immunohistochemistry (IHC) for CD200 expression using Dako Link 48 autostainers and a goat antihuman CD200 polyclonal antibody (R&D Systems, Minneapolis, MN, USA) titered from 1:200 to 1:400 using the Biocare MACH4 detection system, with some samples titered to 1:500 using the Thermo Scientific Ultravision detection system. The modal intensity of staining was scored as 1+ (weak), 2+ (intermediate), or 3+ (strong). Background nerve tissue and endothelial cells typically stained at an intermediate to strong level of intensity, which was used as an internal positive control and as an intensity reference when present. The percentage of reactive cells was scored as negative (<1%), focally reactive (1%-25%), variably reactive (25%-75%), and uniformly reactive (75%-100%). Staining in more than 25% of the neoplastic cells was considered positive for CD200 expression regardless of intensity, which is the same threshold used in other immunohistochemical studies of CD200 expression7,8 All staining was reviewed blindly by two board-certified pathologists. Data analysis was performed using the standard data package included with GraphPad Prism 6.05 (GraphPad Software, La Jolla, CA).
Results
The results of the immunohistochemical staining are summarized in Table 1. CD200 was expressed in 86% of the NENs and 20% of the non-NENs (P < .0001). In this dataset, our CD200 IHC assay was 87% sensitive (0.83-0.90) and 80% specific (0.72-0.85) for the NENs studied.
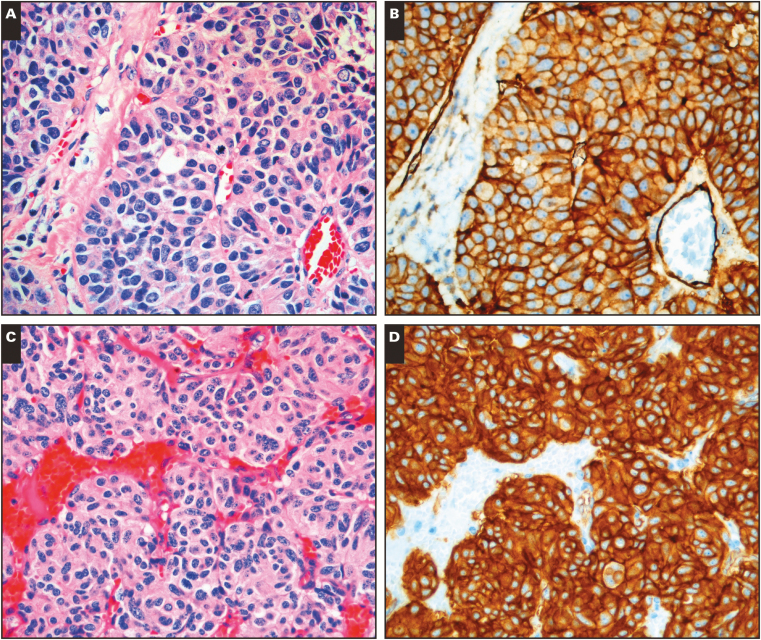
CD200 was expressed in 83% of SCLC. The typical pattern of expression was strong and diffuse membranous staining, often in a chicken-wire pattern. MCC expressed CD200 in 84% of cases with a similar staining pattern. LCNEC was CD200 positive in 75% of cases; however, we were only able to obtain four of these relatively rare neoplasms. LCNEC immunoreactivity was strong and diffuse, similar to MCC and SCLC Image 1A and Image 1B.
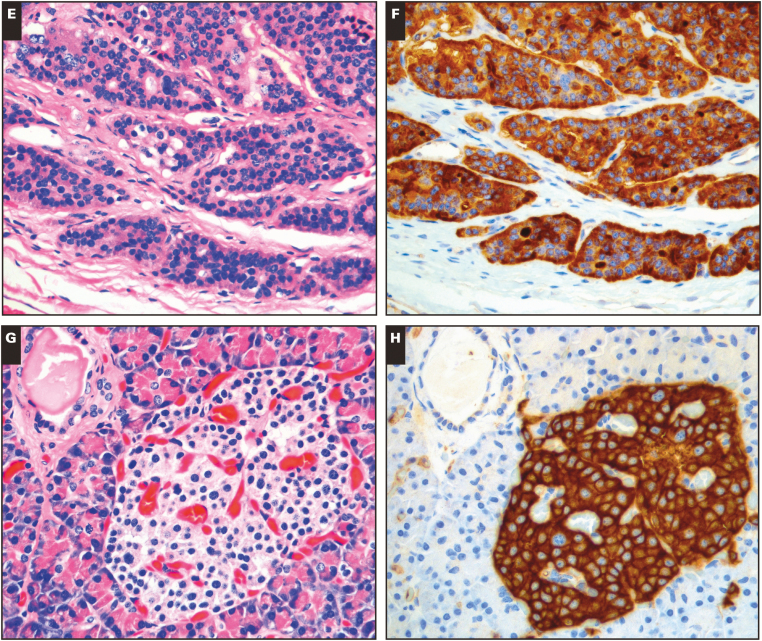
Image 1.
CD200 expression in neuroendocrine neoplasms and normal neuroendocrine tissue. A-B, Large cell neuroendocrine carcinoma (A, H&E; B, CD200). C-D, Typical pulmonary carcinoid (C, H&E; D, CD200).❚Image 1❚ (cont) E-F, Small bowel carcinoid (E, H&E; F, CD200). G-H, Normal pancreatic islet cells (G, H&E; H, CD200). (All x20.)
Ninety-five percent of GCTs, 93% of PanNETs, and 68% of PCTs were CD200 positive, and the typical expression pattern in these neoplasms was strong, diffuse membranous and cytoplasmic reactivity Image 1C and Image 1D. In cases with glandular formation, luminal deposition of stain occurred frequently Image 1E and Image 1F. Seventy of 70 (100%) low grade (G1) GCTs expressed CD200, while only eight of 11 (73%) of high-grade (G2) GCTs expressed CD200 (see Table 2 ; P = .0019). Similarly, 37 of 37 (100%) G1 PanNETs were CD200 positive, and 19 of 23 (83%) G2 PanNETs were CD200 negative; this difference was also statistically significant (P = .0182). A similar trend was found in PCT with 11 of 15 (73%) G1 PCTs expressing CD200, and four of seven (57%) G2 PCTs expressing CD200, but this was not statistically significant (P = .63).
Table 2.
CD200 Expression by Grade in Pulmonary Carcinoid, Gastrointestinal Carcinoid, and PanNETa
| Tumor Type | No. Positive (%) | Total No. Cases |
|---|---|---|
| G1 typical pulmonary carcinoid | 11 (73) | 15 |
| G2 atypical pulmonary carcinoid | 4 (57) | 7 |
| G1 gastrointestinal carcinoid | 70 (100) | 70 |
| G2 gastrointestinal carcinoid | 8 (73) | 11 |
| G1 PanNET | 37 (100) | 37 |
| G2 PanNET | 19 (83) | 23 |
PanNET, pancreatic neuroendocrine tumor.
aOne G3 gastrointestinal carcinoid (negative) and one G3 PanNET (positive) were omitted.
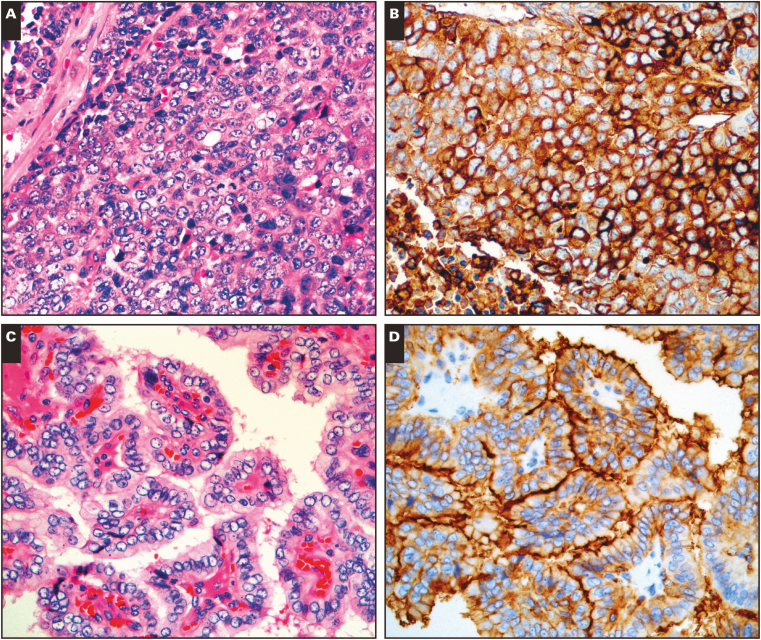
Among non-NENs tested, only 20% (32/157) expressed CD200. In the CD200 positive non-NENs, the typical staining pattern was almost always diffuse, and of weak or intermediate intensity. Only a single non-NEN showed strong CD200 staining, a serous ovarian carcinoma Image 2A and Image 2B. Overall, we demonstrated CD200 expression in six of nine (67%) ovarian carcinomas (OVCs), five of seven (71%) renal cell carcinomas (RCCs), nine of nine (100%) basal cell carcinomas (BCCs), 10 of 10 (100%) papillary thyroid carcinomas (PTCs), and two of 20 (10%) pulmonary squamous cell carcinomas (PSCs). In contrast, we found no CD200 expression in 12 colorectal carcinomas, eight prostate carcinomas, 15 breast carcinomas, 52 pulmonary adenocarcinomas, five pancreatic adenocarcinomas, and 10 cutaneous squamous cell carcinomas.
Image 2.
CD200 expression in nonneuroendocrine neoplasms. A-B, Serous ovarian carcinoma (A, H&E; B, CD200). C-D, Papillary thyroid carcinoma (C, H&E; D, CD200). (All x20.)
While evaluating the neoplastic cells in our samples, we also noted some consistent patterns of CD200 expression in nonneoplastic tissues. Nerve and vasculature were consistently CD200 positive at an intermediate level of intensity. The expression of CD200 in normal nerve and blood vessels has been reported previously, and we found it could serve as a convenient internal control.2 Normal neuroendocrine gastric enterochromaffin cells, intestinal enterochromaffin cells, and pancreatic islet cells were strongly CD200 positive Image 1G and Image 1H. A subset of renal tubules showed low-level reactivity. In sections of the ovary, fallopian tube epithelium showed weak to intermediate CD200 expression accentuated along the luminal border. To the best of our knowledge, the expression of CD200 in normal neuroendocrine cells and ovarian tubal epithelium has not been described previously.
Discussion
CD200 expression has been well studied in hematopoietic malignancies. CD200 is consistently expressed in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and CD200 expression can be used to help distinguish CLL/SLL from mantle cell lymphoma.5,9 CD200 expression has also been found in most cases of primary mediastinal diffuse large B cell lymphoma and hairy cell leukemia.5,6,10 Subsets of cases of myeloma and acute leukemia may also express CD200.7,8
In contrast, CD200 expression in NENs has not been extensively studied, aside from our previous work describing CD200 expression in SCLC by both immunohistochemistry and flow cytometry (and using a different anti-CD200 antibody in the flow cytometry).16 Our current, more extensive study suggests that CD200 is a sensitive general marker of neuroendocrine differentiation, with expression in 87% of the NENs in our dataset, with the caveat that certain NENs were underrepresented in our study, including LCNEC, grade 3 GCT, grade 3 PanNET, medulloblastoma, medullary thyroid carcinoma, pheochromocytoma, PNET, pituitary adenoma, and parathyroid adenoma. The fact that CD200 is expressed by pancreatic islet cells and other normal neuroendocrine cells in our samples provides further support for CD200 as a general marker of neuroendocrine differentiation.
In our series, 60 of 72 (83%) SCLCs expressed CD200, while 0 of 52 (0%) pulmonary adenocarcinomas, and 2 of 20 (10%) pulmonary squamous carcinomas were CD200 positive. In this set of primary pulmonary malignancies, CD200 was 83% sensitive and 97% specific as an indicator of neuroendocrine carcinoma. This suggests that CD200 may be a useful neuroendocrine marker for the diagnosis of SCLC, in a similar way to synaptophysin, chromogranin, and CD56. It has been reported that 10% of SCLCs are negative for all three of these commonly used neuroendocrine markers, and it would be interesting to see if CD200 has utility in establishing neuroendocrine differentiation in these cases.17,18 Only 15 of 22 (68%) of PCTs were CD200 positive which suggests a more limited diagnostic value in PCT.
The demonstration of at least one neuroendocrine marker is required for the diagnosis of LCNEC under the 2004 World Health Organization classification system.18 We demonstrated CD200 expression in 3 of 4 cases of LCNEC, which suggests CD200 may have utility in making this diagnosis. Additional study will be required given the low numbers of LCNEC in our cohort.
We found CD200 expression in 78 of 82 (95%) of GCTs and 56 of 60 (93%) of PanNETs with no staining in other gastrointestinal epithelial carcinomas. In this set of gastrointestinal primaries, CD200 was 94% sensitive and 100% specific as an indicator of neuroendocrine neoplasia, although the number of non-NENs tested was limited (12 cases). This suggests that CD200 could be used as an immunohistochemical marker for establishing the diagnosis of GCT and PanNET in the appropriate setting.
We found a statistically significant association between lack of CD200 expression and higher grade in small bowel GCT and PanNET, and a trend towards this in PCT (see Table 2). Further study is required to determine if lack of CD200 expression is also associated with poor outcome in these neoplasms. It should be noted that loss of neuroendocrine markers has been described previously in atypical PCT.18
In our series, 125 of 149 (84%) MCCs expressed CD200. Despite this finding, CD200 evaluation will likely not become a routine part of the diagnosis of MCC, which already has a well-defined immunoprofile of paranuclear dot-like CK20 positivity and lack of CK7 expression, typically in conjunction with positivity for Merkel cell polyomavirus.19 Given this highly specific pattern of antigen expression, the demonstration of CD200 expression does not add much to the immunohistochemical diagnosis of MCC in routine cases. Still, the expression of CD200 in MCC merits further study to determine if CD200 plays a role in the biology of the disease, if CD200 represents a potential therapeutic target, or if loss of CD200 affects prognosis.
Although widely expressed in the NENs we tested, CD200 expression was only relatively specific (80%) for the abovementioned NENs. While there was little or no expression in non-NENs of colorectal, breast, pulmonary, gastric, pancreatic, or cutaneous origins, CD200 was consistently expressed in BCC and PTC, and often expressed by RCC and OVC in our data set. CD200 has been described in OVC, RCC and BCC, but the expression of CD200 in PTC (100% of cases in our series, and typically accentuated along the apical cell membrane) has not been previously reported Image 2C and Image 2D.13,14 Other authors have shown thyroid epithelial neoplasms to express neuroendocrine markers, and some have speculated thyroid epithelial neoplasms may be derived from ultimobranchial stem cells.20,21 The consistent expression of CD200 in PTC without expression in background benign thyroid follicular cells suggests that CD200 may have value as a diagnostic marker for PTC; additional study of CD200 in thyroid neoplasms would be interesting to determine if CD200 expression can confirm malignancy in problematic cases.
Five of seven (71%) RCC cases expressed CD200. In clear cell RCC, the typical pattern of expression was weak membranous staining, but stronger staining was present in a single case of papillary RCC. In contrast, the two CD200-negative renal tumors in our series were chromophobe renal cell carcinomas. Given the numerous subtypes of RCC, and that differentiation among these subtypes may be problematic, additional study of CD200 in renal tumors may be indicated to determine if any consistent associations exist.
The CD200 reactivity we observed in RCC, BCC, OVC, and PTC is not entirely surprising, as other common neuroendocrine markers, such as CD56, are also not entirely specific and stain non-NENs.18,22 Given that CD200 may be expressed in hematopoietic malignancies, NENs, and subsets of non-NENs, CD200 IHC should only be used as a part of a larger immunohistochemical panel, and interpreted in the correct morphologic and clinical context.
In conclusion, our identification of CD200 expression by NENs represents an exciting development as studies are underway to determine if anti-CD200 antibody treatment has therapeutic benefit in CD200 positive malignancies.23-26 If anti-CD200 antibody therapies are found to be viable, similar trials in NEN patients, especially those with aggressive disease such as SCLC and MCC, could be explored.
Portions of this research were supported in part by the National Institutes of Health under award numbers R01 CA162522 and K24 CA139052 to P.T.N. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Lara Lane for the retrieval, organization, and management of tissue samples used in this study.
References
- 1. McCaughan GW, Clark MJ, Barclay AN. Characterization of the human homolog of the rat mrc ox-2 membrane glycoprotein. Immunogenetics. 1987;25:329-335. [DOI] [PubMed] [Google Scholar]
- 2. Wright GJ, Jones M, Puklavec MJ et al. . The unusual distribution of the neuronal/lymphoid cell surface CD200 (ox2) glycoprotein is conserved in humans. Immunology. 2001;102:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright GJ, Cherwinski H, Foster-Cuevas M et al. . Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034-3046. [DOI] [PubMed] [Google Scholar]
- 4. Kawasaki BT, Farrar WL. Cancer stem cells, CD200 and immunoevasion. Trends Immunol. 2008;29:464-468. [DOI] [PubMed] [Google Scholar]
- 5. Alapat D, Coviello-Malle J, Owens R et al. . Diagnostic usefulness and prognostic impact of CD200 expression in lymphoid malignancies and plasma cell myeloma. Am J Clin Pathol. 2012;137:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillai V, Pozdnyakova O, Charest K et al. . CD200 flow cytometric assessment and semiquantitative immunohistochemical staining distinguishes hairy cell leukemia from hairy cell leukemia-variant and other B-cell lymphoproliferative disorders. Am J Clin Pathol. 2013;140:536-543. [DOI] [PubMed] [Google Scholar]
- 7. Dorfman DM, Shahsafaei A. CD200 (ox-2 membrane glycoprotein) is expressed by follicular T helper cells and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2011;35:76-83. [DOI] [PubMed] [Google Scholar]
- 8. Dorfman DM, Shahsafaei A. CD200 (ox-2 membrane glycoprotein) expression in B cell-derived neoplasms. Am J Clin Pathol. 2010;134:726-733. [DOI] [PubMed] [Google Scholar]
- 9. Palumbo GA, Parrinello N, Fargione G et al. . CD200 expression may help in differential diagnosis between mantle cell lymphoma and B-cell chronic lymphocytic leukemia. Leuk Res. 2009;33:1212-1216. [DOI] [PubMed] [Google Scholar]
- 10. Brunetti L, Di Noto R, Abate G et al. . CD200/ox2, a cell surface molecule with immuno-regulatory function, is consistently expressed on hairy cell leukaemia neoplastic cells. Br J Haematol. 2009;145:665-667. [DOI] [PubMed] [Google Scholar]
- 11. Tonks A, Hills R, White P et al. . CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566-568. [DOI] [PubMed] [Google Scholar]
- 12. Moreaux J, Hose D, Reme T et al. . CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194-4197. [DOI] [PubMed] [Google Scholar]
- 13. Siva A, Xin H, Qin F et al. . Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colmont CS, Benketah A, Reed SH et al. . CD200-expressing human basal cell carcinoma cells initiate tumor growth. Proc Natl Acad Sci U S A. 2013;110:1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moertel CL, Xia J, LaRue R et al. . CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J Immunother Cancer. 2014;2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bohling SD, Davis E, Thompson K et al. . Flow cytometric analysis of CD200 expression by pulmonary small cell carcinoma. Cytometry B Clin Cytom. 2016;90:493-498. [DOI] [PubMed] [Google Scholar]
- 17. Travis WD. Update on small cell carcinioma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Path. 2012;25:S18-S30. [DOI] [PubMed] [Google Scholar]
- 18. Rekhtman N. Neuroendocrine tumors of the lung. Arch Pathol Lab Med. 2010;134:1628-1638 [DOI] [PubMed] [Google Scholar]
- 19. Wang TS, Byrne PJ, Jacobs LK et al. . Merkel cell carcinoma: update and review. Semin Cutan Med Surg. 2011;30:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kargi A, Yörükoglu, Aktaş S et al. . Neuroendocrine differentiation in non-neuroendocrine thyroid carcinoma. Thyroid. 1996;6:207-210. [DOI] [PubMed] [Google Scholar]
- 21. Satoh F, Umemura S, Yasuda M et al. . Neuroendocrine marker expression in thyroid epithelial tumors. Endocr Pathol. 2001;12:291-299. [DOI] [PubMed] [Google Scholar]
- 22. Erickson LA, Lloyd RV. Practical markers used in the diagnosis of endocrine tumors. Adv Anat Pathol. 2004;11:175-189. [DOI] [PubMed] [Google Scholar]
- 23. Moreaux J, Veyrune JL, Reme T et al. . CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun. 2008;366:117-122. [DOI] [PubMed] [Google Scholar]
- 24. Kretz-Rommel A, Qin F, Dakappagari N et al. . Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J Immunol. 2008;180:699-705. [DOI] [PubMed] [Google Scholar]
- 25. Petermann KB, Rozenberg GI, Zedek D et al. . CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest. 2007;117:3922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kretz-Rommel A, Bowdish KS. Rationale for anti-CD200 immunotherapy in B-CLL and other hematologic malignancies: new concepts in blocking immune suppression. Expert Opin Biol Ther. 2008;8:5-15. [DOI] [PubMed] [Google Scholar]