Abstract
Neural cell identity reprogramming strategies aim to treat age-related neurodegenerative disorders with newly induced neurons that regenerate neural architecture and functional circuits in vivo. The isolation and neural differentiation of pluripotent embryonic stem cells provided the first in vitro models of human neurodegenerative disease. Investigation into the molecular mechanisms underlying stem cell pluripotency revealed that somatic cells could be reprogrammed to induced pluripotent stem cells (iPSCs) and these cells could be used to model Alzheimer disease, amyotrophic lateral sclerosis, Huntington disease, and Parkinson disease. Additional neural precursor and direct transdifferentiation strategies further enabled the induction of diverse neural linages and neuron subtypes both in vitro and in vivo. In this review, we highlight neural induction strategies that utilize stem cells, iPSCs, and lineage reprogramming to model or treat age-related neurodegenerative diseases, as well as, the clinical challenges related to neural transplantation and in vivo reprogramming strategies.
Keywords: Regenerative medicine, Reprogramming, In vivo reprogramming, Transdifferentiation, Cell identity, Stem cell, Embryonic stem cell, Induced pluripotent stem cell, Neural stem cell, Induced neural stem cell, Neuron, Neurodegeneration, Alzheimer disease, Amyotrophic lateral sclerosis, Huntington disease, Parkinson disease
1. Introduction
The foremost aim of neural cell reprogramming is the treatment of age-related neurodegenerative disorders and the functional regeneration of neural circuits in vivo. This concept is particularly relevant to the central nervous system, which retains a limited capacity for self-regeneration in adulthood. The isolation of pluripotent embryonic stem cells (ESCs), in vitro neuronal differentiation, and transplantation of ESC-derived neurons to models of neurodegenerative disease marked the first milestones in the application of stem cell-related technologies to human diseases. Investigation into the molecular mechanisms underlying this pluripotency revealed that somatic cells could be reprogrammed to induced pluripotent stem cells (iPSCs) with a limited number of transcription factors. These cells enabled direct modeling of genetic and sporadic forms of Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD), and Parkinson disease (PD). Refined reprogramming strategies enabled the direct transdifferentiation of diverse neural linages and neuron subtypes both in vitro and in vivo. However, as an evolving technology, neural reprogramming still faces numerous challenges to clinical implementation. In this review, we highlight neural induction strategies that utilize stem cells, iPSCs, and transdifferentiated non-neuronal cells to model or treat age-related neurodegenerative diseases, as well as, the clinical challenges related to neuron transplantation and in vivo reprogramming strategies.
2. Stem cell-based neural induction strategies
2.1. Embryonic stem cells
2.1.1. Teratocarcinoma cells and embryonic stem cells
The isolation of mouse teratocarcinoma cells with properties highly similar to cells of the early mouse embryo provided the first in vitro experimental model of cellular pluripotency (Stevens, 1967). The in vivo transplantation of single teratocarcinoma cells isolated by enzymatic dissociation of embryonal carcinomas revealed that these cells are multipotential with the capacity to differentiate into diverse somatic lineages (Kleinsmith and Pierce, 1964). These cells provided an unprecedented opportunity to investigate the mechanisms regulating cell identity and differentiation.
Although teratocarcinoma cells are valuable as a working model of pluripotency, these cell lines often exhibit limited differentiation potential relative to stem cells derived from totipotent pre-implantation embryos. The isolation and culture of embryonic stem cells (ESCs) from proliferating mouse blastocysts established a new paradigm in stem cell research (Evans and Kaufman, 1981). Similar techniques enabled the isolation of primate (Thomson et al., 1995) and human (Thomson et al., 1998) ESC lines. The transplantation of in vitro expanded ESCs into mouse blastocysts yielded chimeric mice demonstrating that ESCs make a functional contribution to numerous differentiated tissue types throughout development (Bradley et al., 1984). Further, lineage tracing with a β-galactosidase reporter demonstrated that ESCs contribute to all parts of the central nervous system when grafted into the early mouse blastocyst (Gossler et al., 1989).
2.1.2. Somatic cell nuclear transfer and cell fusion
Prior to the isolation of ESC lines, nuclear transplantation studies using Rana pipens oocytes and nuclei from advanced blastula cells provided insight into how the nucleus endows a cell with pluripotent differentiation potential (Briggs and King, 1952). Building upon these findings, nuclei transplanted from Xenopus laevis epithelial cells into enucleated oocytes of the same species yielded viable embryos that developed into tadpoles then mature frogs (Gurdon and Laskey, 1970). The remarkable discovery that nuclei from differentiated somatic tissues retained the potential to generate functional living organisms suggested that targeted manipulation of cell differentiation mechanisms might enable genetic engineering. Reinforcing this concept, three independent mammalian nuclear transplantation studies generated a lamb (Wilmut et al., 1997), mice (Wakayama et al., 1998), and calves (Kato et al., 1998).
Unifying nuclear transfer and ESC isolation techniques, two novel ESC lines were isolated from non-human primate blastocysts derived from oocytes carrying the nuclei of adult rhesus macaque skin fibroblasts (Byrne et al., 2007). In an attempt to generate human pluripotent stem cells through oocyte-somatic cell genome exchange, the nucleus of an adult human skin cell was implanted into an enucleated human oocyte (Noggle et al., 2011). These oocytes arrested in late cleavage and exhibited abnormalities in gene transcription (Noggle et al., 2011). Interestingly, the addition of a somatic cell nucleus to a non-enucleated oocyte promotes cell division and development to the blastocyst stage (Noggle et al., 2011). Pluripotent cell lines derived from the inner cell mass of these blastocysts could be differentiated into cell types representative of the three germ layers (Noggle et al., 2011); however, the triploid genetic composition and ethical debate over the use of human oocytes represent significant limitations to the use of these cells as an effective therapeutic agent.
As an alternative to nuclear transfer, the chemical fusion of a pluripotent cell and differentiated somatic cell was used to generate a hybrid cell with a tetraploid genome (Miller and Ruddle, 1976; Cowan et al., 2005). This human ESC-fibroblast fusion cell retained a capacity for pluripotent differentiation (Cowan et al., 2005). Analyses of genome-wide transcription, allele-specific gene expression, and DNA methylation in these hybrid cells demonstrated that the somatic nucleus was reprogrammed to mimic the transcriptional state of a pluripotent cell (Cowan et al., 2005). These findings confirmed that nuclei from differentiated somatic cells retain the potential to adopt a pluripotent state when given a defined set of intranuclear cues.
2.1.3. Neural differentiation
Pro-neural effector molecules, similar to the cues that induce or maintain pluripotency, bias an undifferentiated cell toward a lineage-specific neural fate. Mouse ESC-derived embryoid bodies chemically directed toward a neural precursor identity in vitro were transplanted into the ventricles of embryonic rat brains and subsequently differentiated into neurons, astrocytes, and oligodendrocytes in vivo (Brüstle et al., 1997). This multipotent neural differentiation inspired the transplantation of human ESC-derived neural precursor cells in the mouse brain (Zhang et al., 2001; Reubinoff et al., 2001). Exposed to the cues of the neural microenvironment, these cells differentiated into neurons and astrocytes in multiple brain regions (Zhang et al., 2001).
The in vitro specification of neuron and glial cell subtypes from cultured ESCs promised significant advances in models of development and neurodegeneration. Cultured mouse ESC embryoid bodies were in vitro differentiated toward nestin-positive neural precursor cells using neural induction media supplemented with basic fibroblast growth factor (Okabe et al., 1996). Withdrawal of this growth factor prompted differentiation into neurons and glial cells (Okabe et al., 1996). Further, treatment with retinoic acid catalyzed the differentiation of subtype-specific GABAergic and cholinergic neurons (Bain et al., 1995; Fraichard et al., 1995). In vitro differentiated neurons formed functionally diverse excitatory and inhibitory synapses mediated by glutamate, GABAor glycine surface receptors (Finley et al., 1996; Strübing et al., 1995). Additional studies demonstrated efficient methods for the transplantation and in vivo differentiation of dopaminergic neurons (Deacon et al., 1998), serotonergic neurons (Deacon et al., 1998), spinal progenitor-derived motor neurons (Wichterle et al., 2002), and striatal progenitor-derived medium spiny neurons (Aubry et al., 2008). Moreover, ESC-derived dopaminergic neurons transplanted into the striatal nuclei of mice lesioned with 6-hydroxydopamine (6-OHDA) enhanced performance in several motor exercises relative to sham-grafted animals (Kim et al., 2002).
Aside from the ethical considerations over the use of ESCs and other pluripotent cells as therapeutic agents, the over proliferation ESC-derived neural precursors transplanted in the rat striatum (Aubry et al., 2008) and an inability to rapidly generate patient genome-specific neural cells represent detrimental barriers to clinical utility. As an in vitro model, however, human ESC-derived neurons are a powerful method for investigating the mechanisms of neurodegeneration. For instance, cultured astrocytes expressing an ALS-relevant SOD1G37R mutant protein were demonstrated to be selectively toxic to ESC-derived motor neurons (Giorgio et al., 2008; Marchetto et al., 2008). One pitfall to the use of ESC-derived neurons to model age-related neurodegenerative disorders is the lack of certain age-acquired genetic and epigenetic markers. These drawbacks pushed researchers to develop models with higher disease fidelity and patient specificity.
2.2. Induced pluripotent stem cells
Somatic cell nuclear transfer experiments confirmed that somatic nuclei retain pluripotent potential, but the molecular pathways that catalyze a reversion to pluripotency remained undefined. In 2006, the transcriptional mechanisms underlying acquired pluripotency were unraveled using viral overexpression of stem cell-related transcription factors. Remarkably, POU5F1, SOX2, KLF4, and MYC were sufficient to induced mouse embryonic and adult fibroblasts to adopt a pluripotent fate in vitro (Takahashi and Yamanaka, 2006). Likewise, transduction of fetal and adult human fibroblasts with these factors generated iPSCs (Park et al., 2008b). Building on these findings, ectopic expression of POU5F1, SOX2, NANOG, and LIN28A in mouse and human somatic cells can similarly induce a pluripotent state (Yu et al., 2007). Additional screens demonstrated the induction of pluripotency with three factors (Wernig et al., 2008), two factors (Giorgetti et al., 2010; Huangfu et al., 2008), and POU5F1 alone under defined conditions (Kim et al., 2009).
Adult human iPSCs differentiate to glia and diverse neuronal subtypes when cultured under lineage restrictive conditions (Takahashi et al., 2007; Hu et al., 2010). A direct comparison of human ESC-derived neurons and human iPSC-derived neurons revealed that iPSCs proceed along a similar developmental time course as ESCs, but adopt neural fate with highly variable efficiencies (Hu et al., 2010). Refined differentiation protocols enabled the in vitro induction of astroglial progenitors and immature astrocytes (Krencik et al., 2011), as well as, PPP1R1B-positive medium spiny neurons (Carri et al., 2013) from human iPSCs. Importantly, motor neuron-specific differentiation of 16 iPSC lines generated from donors of varying health status demonstrated an immense potential for this technology in the clinic (Boulting et al., 2011).
2.3. Induced neural stem cells, neural progenitors, and downstream progenitor cells
2.3.1. Neural stem cells
As the earliest structures of the central nervous system emerge from the embryo, the neural plate folds into the neural tube and forms the birthplace of all neural cell types. Cascades of signaling molecules then pattern the developing brain and spinal cord by modulating transcription in neuroepithelial cells that produce neural stem cells (NSC) such as radial glia. These NSC populations generate billions of neurons throughout early neurogenesis and this process slows in adulthood with non-neurogenic cell populations predominating the mature brain and spinal cord (Götz and Huttner, 2005).
Although the rate of neurogenesis is significantly reduced in postnatal and adult neural tissues, new NSC-derived neurons are consistently produced in two regions of the brain throughout life (Altman, 1962; Altman and Das, 1965; Kaplan and Hinds, 1977). In the adult rat, NSC-derived neural progenitors in the subgranular zone of the dentate gyrus migrate to the neighboring granule cell layer and differentiate into functional granule neurons (Kaplan and Hinds, 1977). Similarly, dividing neural progenitors in the subventricular zone of the lateral ventricle generate migratory neuroblasts, which travel along the rostral migratory stream into the olfactory bulb where these cells differentiate into interneurons (Lois et al., 1996).
The isolation, in vitro culture, and directed differentiation of neural progenitor cells demonstrated that these cells retain multipotent potential in the adult brain (Gage et al., 1995). Further, the hippocampal transplantation and in vivo neuronal differentiation of cultured neural progenitors confirmed these cells retain functional multipotency in vitro (Gage et al., 1995). Neural precursors transplanted directly into the rostral migratory stream migrated to the olfactory bulb and differentiated into tyrosine hydroxylase-positive neurons demonstrating responsiveness to extracellular cues (Suhonen et al., 1996).
In addition to the subventricular and subgranular zones of the brain, proliferating cells with neural progenitor-like properties have been isolated from the adult spinal cord (Adrian and Walker, 1962; Shihabuddin et al., 1997). In vitro adult rat spinal progenitors are self-renewing, multipotent, and differentiable upon in vivo transplantation (Shihabuddin et al., 2000). The ependymal cell (Johansson et al., 1999) and pericyte (Göritz et al., 2011) origin of proliferating migratory astrocytes following spinal cord injury remains debated.
This understanding of embryonic and adult neurogenesis has informed the development of therapeutics aimed at regenerating neurons in the human central nervous system. Importantly, immunofluorescent labeling of neuronal markers and replicating DNA confirmed that limited neurogenesis occurs in the adult human hippocampus (Eriksson et al., 1998). This active field of study has primed the development of reprogramming models that utilize these mechanisms to induce NSC and neural progenitor-like cells.
2.3.2. Induced neural stem cells and induced progenitor cells
The direct induction of NSCs from mouse fibroblasts can be achieved by temporally restricting the expression of the same four transcription factors used to generate somatic cell-derived iPSCs (Kim et al., 2011b; Their et al., 2012). The direct delivery of cell-permeable POU5F1 mRNA or protein for the first five days of reprogramming combined with constitutive expression of SOX2, KLF4, and MYC generated progenitor-like cells with properties similar to endogenous NSCs (Their et al., 2012). Strict regulation of POU5F1 expression was essential since prolonged expression biased reprogramming toward a pluripotent state (Their et al., 2012). In addition to an adopted neural identity, the induced NSCs acquired tripotent differentiability to astrocytes, neurons, and oligodendrocytes (Their et al., 2012). Further, NSC-like progenitors were similarly induced with retroviral expression of SOX2, KLF4, MYC, POU3F4, and TCF3 (Han et al., 2012).
An interwoven network of transcription factors maintains NSC identity though precise genetic regulation. Increasing the activity of one or more factors in this network can unbalance this regulation and drive changes in cell identity (Tian et al., 2011). For instance, mouse embryonic fibroblasts transduced with SOX2 and FOXG1 reprogrammed to a bipotent neural precursor state, while the expression of SOX2, FOXG1, and POU3F2 generated self-renewing tripotent neural precursors (Lujan et al., 2012). The expression of this additional transcription factor primed the cellular state to adopt multipotency. Remarkably, the overexpression of SOX2 is sufficient to induce NSCs from mouse and human fibroblasts in vitro, as well as, neuroblasts from mouse striatal astrocytes in vivo (Niu et al., 2013). This ability to directly induce proliferating neural precursor cells from somatic and glial lineages beneficially enables the production of multiple neurons from each reprogrammed cell (Tian et al., 2012, 2013).
2.3.3. Induced subtype-specific neural progenitor cells
NSCs have an intrinsic potential for multi-lineage differentiation into astrocytes, oligodendrocytes, and dozens of neuronal subtypes. The directed differentiation of these precursors into a highly defined state for therapeutic use might be challenging if the underlying mechanisms of differentiation are not fully understood. Therefore, the induction of restricted neural progenitors might be better suited to regenerate specific populations of neural cells following degeneration.
Engraftable NG2-oligodendrocyte precursor cells derived from mouse and rat fibroblasts transduced with SOX10, OLIG2, and ZNF536 differentiate into mature oligodendrocytes that ensheath the axons of co-cultured neurons (Yang et al., 2013). As iPSCs have proven refractory to oligodendroglial precursor generation, this strategy of progenitor specific reprogramming enabled the production of a highly specific neural subtype and in vitro model for demyelination-related diseases. Similar work demonstrated that a combination of OLIG1, OLIG2, NKX2-2, NKX6-3, SOX10, ST18, MYRF, and MYT1 directly induce NG2-oligodendrocyte precursor cells from mouse embryonic fibroblasts (Najm et al., 2013).
With special relevance to PD, an age-related neurodegenerative disorder that selectively affects dopaminergic neurons of the substantia nigra, dopaminergic progenitor cells generated from human iPSCs (Doi et al., 2014) and mouse fibroblasts (Kim et al., 2014; Tian et al., 2015) represent an effective method for the directed differentiation of dopaminergic neurons. With further development, induced NSCs, neural progenitor cells, and subtype-specific progenitor cells hold significant therapeutic potential.
3. Neurodegenerative disorders: Ipsc models, induced neural cell transplantation, And In Vivo reprogramming
3.1. In vivo reprogramming
A central goal of regenerative medicine is the reconstruction of neural tissues damaged by age-related neurodegenerative disorders such as AD, ALS, HD, and PD. The seminal discovery that adult human fibroblasts can be induced to adopt a pluripotent stem cell-like identity in vitro by overexpression of four transcription factors (Takahashi et al., 2007) or treatment with small molecules (Hou et al., 2013) catalyzed numerous investigations into the therapeutic potential of iPSC technology. However, a high propensity for teratoma formation in vivo inherently limits the therapeutic utility of these cells (Abad et al., 2013).
To resolve this issue and demonstrate clinical feasibility, iPSC-derived neural-specific progenitors were transplanted to an injured non-human primate spinal cord with no observed tumor formation (Emborg et al., 2013). Further, lentiviral-mediated SOX2 overexpression in the mouse brain and spinal cord induces non-tumorigenic neuroblast cells poised to differentiate into functional neurons (Niu et al., 2013; Su et al., 2014). Although the transplantation of iPSC-derived and transdifferentiated cells might be clinically feasible, the direct reprogramming of endogenous cells in vivo has emerged as a potentially more versatile tool in the repair of degenerated neural networks (Table 1). Resident astrocytes, NG2 glia, and other non-neuronal cells targeted for direct reprogramming have been successfully transdifferentiated to MAP2-positive neurons in the injured cortex (Heinrich et al., 2014), glutamatergic neurons in the cortex (Grande et al., 2013; Guo et al., 2014), GABAergic neurons in the cortex (Guo et al., 2014), projection neuron-like cells in the striatum (Grande et al., 2013), and corticofugal projection neurons in the developing mouse brain (Rouaux and Arlotta, 2010).
Table 1.
In vivo reprogramming methodologies. Neural in vivo reprogramming methodologies used to generate neurons or neural progenitors from diverse cell types. Cells of origin annotated with a superscript H denote human cells transplanted into rodent neural tissue for in vivo reprogramming.
| Cell of origin | Reprogramming factor(s) | Induced fate (reference) |
|---|---|---|
| Glial cell | ||
| Astrocyte | ASCL1, POU3F2, and MYT1L | Neuron (Torper et al., 2013) |
| AstrocyteH | ASCL1, POU3F2, and MYT1L | Neuron (Torper et al., 2013) |
| Cortical astrocyte | NEUROD1 | Glutamatergic neuron (Guo et al., 2014) |
| Cortical NG2 glial cell | NEUROD1 | Glutamatergic neuron (Guo et al., 2014) |
| Cortical NG2 glial cell | NEUROD1 | GABAergic neuron (Guo et al., 2014) |
| Cortical NG2 glial cell | SOX2 ± ASCL1 | Neuron (Heinrich et al., 2014) |
| Cortical glial cell | NEUROG2 ± FGF2 + EGF | Glutamatergic neuron (Grande et al., 2013) |
| Spinal astrocyte | SOX2 | Neuroblast (Su et al., 2014) |
| Spinal astrocyte | SOX2, valproic acid | Neuron (Su et al., 2014) |
| Striatal astrocyte | SOX2 | Neuroblast (Niu et al., 2013) |
| Striatal astrocyte | SOX2, valproic acid | Neuron (Niu et al., 2013) |
| Striatal glial cell | NEUROG2 ± FGF2 + EGF | GABAergic neuron (Grande et al., 2013) |
| Progenitor | ||
| Neural progenitor | FEZF2 | Cortical projection neuron (Rouaux and Arlotta, 2010) |
| Neuron | ||
| Callosal projection neuron | FEZF2 | Corticofugal projection neuron (Rouaux and Arlotta, 2013) |
| Spiny neuron | FEZF2 | Pyramidal neuron (De la Rossa et al., 2013) |
| Somatic | ||
| FibroblastH | ASCL1, POU3F2, and MYT1L | Neuron (Torper et al., 2013) |
| FibroblastH | ASCL1, POU3F2, MYT1L, OTX2, LMX1A, LMX1B, and FOXA2 | Dopaminergic neuron (Torper et al., 2013) |
Defined abbreviation: EGF + FGF2, epidermal growth factor and fibroblast growth factor 2.
These proof-of-concept in vivo studies demonstrated that functional neurons can be induced in the adult central nervous system and highlight the potential of patient-specific engineered neurons as a treatment for neurodegenerative diseases.
3.2. Alzheimer disease
3.2.1. Neuropathology of Alzheimer disease
AD is an age-related neurodegenerative disorder characterized by memory loss, cognitive impairment, behavioral changes, and an impaired ability to perform activities of daily living (Albert et al., 2011; McKhann et al., 2011). These cognitive deficits stem from disruptions of neural network activity caused by dendrite retraction, dendritic spine and synapse degeneration, and the death of cholinergic neurons in the basal forebrain and layer II of the entorhinal cortex (Whitehouse et al., 1982; Gómez-Isla et al., 1996).
The molecular etiology of AD is highly complex with multiple genetic, epigenetic, and environmental factors contributing to the overall reduction in neural network activity (Huang and Mucke, 2012). Rare early-onset genetic forms of AD have been linked to mutation or duplication of the genes encoding amyloid precursor protein (APP) (Goate et al., 1991) and the APP-processing enzymes presenilin 1 and presenilin 2 (Sherrington et al., 1995; Levy-Lahad et al., 1995). APP, an integral membrane protein with roles in synapse formation and synaptic plasticity (Priller et al., 2006), undergoes posttranslational cleavage by presenilin-containing β- and γ-secretase complexes to generate amyloid-beta (Aβ) peptides of varying lengths (De Strooper and Annaert, 2000). Intrinsically disordered Aβ peptides adopt a random coil secondary structure and are cleaved into two common alloforms, Aβ40 and Aβ42 which account for approximately 90% and 5–10% of brain-wide Aβ production, respectively (Linh and Ha-Duong, 2015). Aβ42-biased imbalances in APP processing reduce Aβ clearance from neural tissues and consequently promote the self-aggregation of Aβ monomers into neurotoxic soluble Aβ oligomers and large insoluble Aβ plaques (Walsh and Selkoe, 2007). Soluble Aβ dimers, trimers, and other low-n oligomers are potent synaptotoxins that induce synapse loss and interfere with plasticity mechanisms such as long-term potentiation (Walsh and Selkoe, 2007). In addition to rare genetic mutations, Aβ aggregation can arise from disequilibrium in Aβ synthesis and clearance. The reduced function of neprilysin or insulysin, enzymes with critical roles in Aβ degradation, might potentiate Aβ oligomerization (Iwata et al., 2000, Qiu et al., 1998). Similarly, the regulated transport of soluble Aβ monomers across the blood-brain barrier by low-density lipoprotein receptor-related protein 1, ATP binding cassette transporters, and advanced glycation end product-specific receptor is critical to maintaining non-cytotoxic levels of Aβ in the brain (Shibata et al., 2000; Deane et al., 2003). Importantly, diverse intercellular and metabolic signals such as insulin might have a direct role in the regulation of Aβ transport into and out of neural tissues (Vandal et al., 2015).
Although Aβ posttranslational processing, degradation kinetics, aggregation, and transport have been implicated in disease progression, recent mechanistic discoveries suggest that Aβ might not be the sole causative defect underlying late-onset AD. Genome-wide association studies of disease-afflicted individuals identified apolipoprotein E4 (APOE4) as a genetic risk factor (Bertram et al., 2010). As one of three APOE isoforms that differ by at most two amino acid residues (Mahley et al., 2006), APOE4 has roles in lipid redistribution for repair, maintenance, and remodeling of cells in the central nervous system (Mahley et al., 2006). Further functional analyses have shown that APOE4 forms stable complexes with Aβ peptides and plaques (Strittmatter et al., 1994), enhances Aβ deposition (Holtzman et al., 2000), and induces neurodegeneration (Buttini et al., 1999) resulting in impaired learning and memory (Raber et al., 2000). APOE4-induced neurodegeneration might result from the inhibition of neurite outgrowth (Nathan et al., 1994) due to the reduced binding affinity of APOE4 for the microtubule-stabilizing protein MAPT (Strittmatter et al., 1994). In the absence of APOE, MAPT is hyperphosphorylated resulting in microtubule destabilization and subsequent neurite degeneration (Tesseur et al., 2000; Nathan et al., 1995; Strittmatter et al., 1994; Morris et al., 2011). Hyperphosphorylated MAPT forms insoluble neurotoxic aggregates, known as neurofibrillary tangles, and is mislocalized in dendritic spines and postsynaptic densities (Hoover et al., 2010). Aβ oligomers promote this mislocalization via members of the microtubule affinity-regulating kinase family (Zempel et al., 2010) and reductions in MAPT concentration inhibit both Aβ-dependent and APOE4-dependent neuronal deficits in vitro and in vivo, respectively (Andrews-Zwilling et al., 2010; Ittner et al., 2010; Roberson et al., 2007). This interplay of Aβ, APOE4, MAPT, and other AD-related factors have provided numerous targets for pharmacological intervention.
3.2.2. Pharmacological treatments and drawbacks
The pharmacological treatment of AD currently relies on compounds that broadly inhibit acetylcholinesterase (Shah and Reichman, 2006). This inhibition increases the concentration of acetylcholine at cholinergic synapses to promote neurotransmission (Shah and Reichman, 2006). However, there is no clinical data that conclusively demonstrates these compounds reduce or reverse the functional losses imparted by the disease. Ongoing clinical trials aim to reduce synapse degeneration by targeting APP processing mechanisms, Aβ production and aggregation, MAPT production and degradation, APOE4 intra-domain interactions, and the association of soluble Aβ, MAPT, and APOE4 (Huang and Mucke, 2012). Although these treatments may improve cognitive function by reducing or even preventing disease progression, pharmacological interventions offer no mechanism to regenerate neurons for the repair of damaged neural circuits. The induction of functional subtype-specific neurons from endogenous non-neuronal cells in the basal forebrain by in vivo transdifferentiation might offer a novel mechanism for the reconstruction of degenerated neuronal circuits.
3.2.3. Alzheimer-relevant induced pluripotent stem cell models
Prior to the discovery of induced pluripotency, neurodegenerative diseases were modeled in vitro using immortal cell lines or derivatives of transformed neural tissue. The ability to induce pluripotency and direct neural differentiation of diverse disease-specific somatic cells enabled novel mechanistic studies into the progressive onset of neurodegeneration (Park et al., 2008). For instance, primary fibroblasts isolated from two patients with sporadic AD and two patients with familial APP-duplication causative AD were indirectly converted to neurons for molecular characterization (Israel et al., 2012). These neurons expressed significantly higher levels of AD-related pathological markers relative to control induced neurons (Israel et al., 2012). Treatment with β-secretase inhibitors reduced levels of phosphorylated MAPT and GSK3B yielding new insight into a direct relationship between APP proteolytic processing, GSK3B activation, and MAPT phosphorylation (Israel et al., 2012).
In addition to mechanistic investigations, in vitro models of AD enable pharmacological screening and quantification of drug response phenotypes. AD-lineage dermal fibroblasts reprogrammed to iPSCs and differentiated into functional neurons accumulated Aβ oligomers that induced oxidative stress in the endoplasmic reticulum (Kondo et al., 2013). Docosahexaenoic acid treatment of select iPSC-derived neuron lines attenuated cellular stress and reduced the generation of reactive oxygen species (Kondo et al., 2013). This suggests genetic heterogeneity and disease etiology have important roles in disease onset and pharmacological response. An alternative to pharmacological intervention, neuron replacement by transplantation of induced neurons and in vivo reprogramming are promising approaches to neural regeneration.
3.2.4. Alzheimer-relevant regeneration strategies
The direct transdifferentiation of mouse embryonic and postnatal fibroblasts into functional neurons was first demonstrated in vitro using viral overexpression of the transcription factors ASCL1, POU3F2, and MYT1L (Vierbuchen et al., 2010). With the inclusion of a fourth transcription factor, NEUROD1, this same technique was successfully applied to human fibroblasts (Pang et al., 2011). Further demonstrating how targeted modulation of the transcriptome can transform cell identity, human adult fibroblasts were directly reprogrammed to neurons with the microRNA miR-124 and transcription factors POU3F2 and MYT1L (Ambasudhan et al., 2011), as well as, miR-124, miR-9/9*, NEUROD2, ASCL1, and MYT1L (Yoo et al., 2011).
The application of these foundational in vitro findings to the regeneration of cholinergic neurons in the basal forebrain requires the high-efficiency induction of homogeneous fate-specific neurons. In a step toward therapeutic utility, the transcription factors NEUROG2 and SOX11 were combined with the small molecules forskolin and dorsomorphin to rapidly reprogram adult human fibroblasts to functional, homogeneous cholinergic neurons with nearly 90% efficiency (Liu et al., 2013). This pro-neurogenic activity of NEUROG2 further enabled retroviral-induced transdifferentiation of mouse postnatal cortical astroglia to TUBB3-positive neurons (Berninger et al., 2007) and functional, synapse-forming glutamatergic neurons (Heinrich et al., 2010).
Transitioning these in vitro reprogramming achievements in vivo, human astrocytes and fibroblasts transfected with doxycycline-regulated ASCL1, POU3F2, and MYT1L lentiviruses were implanted into the striatum of adult rats for in vivo transdifferentiation (Torper et al., 2013). Transplanted human astrocytes survived in the striatal microenvironment and, following doxycycline treatment, reprogrammed to neural-cell-adhesion-molecule-positive neurons (Torper et al., 2013). A similar cell transplantation study demonstrated that self-renewing, multipotent NSCs derived from mouse fibroblasts could also survive, differentiate, and mature into RBFOX3-positive neurons in the mouse cortical microenvironment (Ring et al., 2012).
These proof of concept in vitro reprogramming and cell transplantation studies demonstrated the feasibility of directly generating functional neurons from non-neuronal cells with high efficiency and subtype specificity. The application of these findings to in vivo systems enabled researchers to directly transform resident non-neuronal glia of the adult mouse striatum to RBFOX3-positive neurons using retroviral-mediated expression of ASCL1, POU3F2, and MYT1L (Torper et al., 2013). The induction of GABAergic neurons in the striatum and glutamatergic neurons in the neocortex by retroviral overexpression of NEUROG2 following focal injury highlights the intrinsic regional differences in glia of the mouse brain (Grande et al., 2013). Additional treatment with fibroblast growth factor 2 and epidermal growth factor enhanced NEUROG2-mediated reprogramming and robustly induced GABA-positive striatal neurons, while reprogramming in the neocortex was significantly more restricted (Grande et al., 2013).
The direct conversion of resident cortical astrocytes to functional neurons in a transgenic mouse model of AD illuminated the clinical potential of cell reprogramming technologies (Guo et al., 2014). NEUROD1-encoding retrovirus transformed reactive astrocytes and NG2 glia of the mouse somatosensory cortex into neurons with robust synaptic activity within 16 days (Guo et al., 2014). Importantly, the efficiency of this neuronal conversion process increased in aged mice (Guo et al., 2014). NEUROD1 rapidly transformed glia to bipolar doublecortin-positive cells within three days, which matured into neurons exhibiting extensive neurite outgrowth with functional synapses within three weeks (Guo et al., 2014). Interestingly, reactive astrocytes adopted an exclusively excitatory glutamatergic identity, while NG2 glia gave rise to a heterogeneous population of glutamatergic and GABAergic neurons (Guo et al., 2014). The long-term survival of these neurons was validated two months following induction (Guo et al., 2014). These findings support the premise that engineered neurons might enable the functional regeneration of neuronal networks following the onset of AD-related neurodegeneration.
3.3. Amyotrophic lateral sclerosis
3.3.1. Neuropathology of amyotrophic lateral sclerosis
ALS is an adult-onset, multifactorial disease characterized by the degeneration of upper motor neurons in the motor cortex and lower motor neurons localized to the brain stem and ventral horn of the spinal cord (Hardlman et al., 2011). As the axons of these motor neurons retract and eliminate neuromuscular synapses, neuroinflammation triggers reactive astrogliosis and the proliferation of microglia (Philips and Robberecht, 2011). This progressive degeneration manifests as muscle atrophy, fasciculations, hyperreflexia, spasticity, and a reduced ability to initiate voluntary movement (Hardlman et al., 2011).
Familial ALS is a genetically heritable condition that accounts for approximately 5% of known ALS cases, while non-hereditary, sporadic forms of the disease are responsible for nearly 95% of ALS cases (Byrne et al., 2011). Although a well-defined etiology for sporadic ALS has yet to be elucidated (Ferraluolo et al., 2011), cytological evidence suggests that cytotoxic oligomers and protein-rich inclusions in the soma and axon of motor neurons disrupt axonal transport (Bilsland et al., 2010), impair mitochondrial function (Mattiazzi et al., 2002), and trigger glutamate-mediated excitotoxicity (Vucic et al., 2008).
Familial ALS has been linked to more than 15 heritable genetic mutations (Anderson and Al-Chalabl, 2011) in genes such as SOD1 (Rosen et al., 1993), TARDBP (Sreedharan et al., 2008), and FUS (Deng et al., 2010). Genetic mutations that disrupt the tertiary structure of SOD1, a free radical scavenger that regulates the level of superoxide radicals generated in the mitochondria, result in misfolded protein aggregates that can inhibit both the proteasomal pathway and autophagy (Robberecht and Philips, 2013). TDP-43 (TARDBP) mutations similarly result in protein-rich inclusions that trigger an unfolded protein response, microglial activation, and cell death (Neumann et al., 2006; Robberecht and Philips, 2013). This complex, heterogeneous etiology combined with age-related physiological stressors has hampered the development of pharmacological interventions and ALS-specific therapeutics.
3.3.2. Pharmacological treatments and drawbacks
At present, Riluzole is the only pharmacological treatment approved to treat ALS symptoms. This compound relies on the non-specific inhibition of glutamatergic synaptic transmission via sodium channel blockade (Nagoshi et al., 2015). Although numerous small molecules and other therapeutics remain in clinical trials, none are anticipated to both significantly enhance motor function and promote the regeneration of lost neuromuscular synapses. Therefore, much focus has shifted to cell-based strategies that aim to induce additional muscle-targeting motor neurons in the spinal cord to recover motor function and promote neuronal survival.
3.3.3. ALS-relevant induced pluripotent stem cell models
Functional motor neurons were successfully induced from numerous diseased iPSC lines (Boulting et al., 2011) and skin fibroblast-derived iPSCs originating from a patient with familial ALS (Dimos et al., 2008). Specific mutations in TARDBP have been causatively linked to ALS onset (Neumann et al., 2006; Robberecht and Philips, 2013); therefore, induced motor neurons generated from TARDBP-mutant iPSCs provided a unique opportunity to investigate TARDBP-related proteinopathies in vitro (Bilican et al., 2012). This model demonstrated that elevated levels of soluble TDP-43 reduced neuronal survival and enhanced susceptibility to PI3K signaling inhibition (Bilican et al., 2012). These models have provided valuable genetic and mechanistic evidence for disease progression and informed motor neuron-specific induction techniques targeting in vivo regeneration.
3.4. ALS-relevant regeneration strategies
The direct induction of motor neurons from embryonic and adult mouse fibroblasts was first achieved in vitro by viral overexpression of seven transcription factors (Son et al., 2011). Building on the discovery that ASCL1, POU3F2, and MYT1L could induce functional neurons from mouse fibroblasts (Vierbuchen et al., 2010), these factors combined with LHX3, ISL1, MNX1, and NEUROG2 were co-expressed in vitro to generate morphologically mature motor neurons with 5–10% efficiency (Son et al., 2011). The inclusion of an eighth transcription factor, NEUROD1, induced vesicular choline acetyltransferase-positive functional motor neurons from human fibroblasts (Son et al., 2011).
Transcriptional profiling of these induced motor neurons revealed a transcriptomic signature that co-segregated with endogenous motor neurons and significantly differed from the cell-of-origin fibroblast (Son et al., 2011). Critically, these induced motor neurons formed functional neuromuscular junctions when co-cultured with chick myotubes and repetitively fired trains of action potentials (Son et al., 2011). To evaluate whether in vitro reprogrammed neurons would have functional attributes similar to endogenous motor neurons, induced neurons were transplanted into the developing chick spinal cord (Son et al., 2011). Remarkably, these cells migrated to sites of integration, successfully engrafted, and extended axons into neighboring tissue (Son et al., 2011).
Wild-type motor neurons co-cultured with glia isolated from the SOD1G93A mouse model of ALS exhibit a sharp reduction in survival as compared to co-culture with wild-type astroglia (Son et al., 2011). Similar reductions in survival and function were observed for induced motor neurons, indicating susceptibility to the same ALS-related degenerative stimuli (Son et al., 2011). This both validates these cells as a bona fide in vitro model for further ALS studies and highlights additional genome engineering and cell survival challenges that must be solved before these cells can be used as a form of regenerative medicine.
Traditional neuronal transdifferentiation techniques utilize one input cell to directly generate one output neuronal cell. To become an effective therapy for neurodegeneration, the impact of transdifferentiation on glial populations and the surrounding microenvironment must be minimized. Therefore, a targeted in vivo reprogramming strategy that generates multiple output neuronal cells for each input glial cell was designed by directly transforming resident astrocytes of the adult mouse spinal cord into proliferative neuroblasts (Su et al., 2014). Lentiviral expression of the transcription factor SOX2 induced astrocytes to adopt a doublecortin-positive neuroblast identity. Cell lineage tracing, MKI67 immunostaining, and transplantation of fluorescence-sorted in vitro SOX2-reprogrammed mouse spinal astrocytes confirmed proliferative astrocytes as the source of induced neuroblasts (Su et al., 2014). Astrocyte-derived neuroblasts were generated in young, aged, and injured spinal cords indicating a broad utility for this reprogramming model (Su et al., 2014).
Importantly, treatment with the histone deacetylase inhibitor valproic acid induced neuroblast differentiation toward a morphologically mature RBFOX3-positive interneuron-like fate. Heterogeneous glutamatergic and GABAergic induced neurons were detectable eight weeks after reprogramming and persisted more than 30 weeks post induction (Su et al., 2014). These neurons formed functional SYN1-positive synaptic connections with endogenous cholinergic neurons indicating a capacity for integration into local neural circuitry (Su et al., 2014). As a start point for in vivo generation of proliferative neuroblast cells, this model demonstrates the feasibility of directly reprogramming a small population of glia toward a non-pluripotent progenitor state and subsequent differentiation into a larger population of functional neurons. With adaptation toward a motor neuron fate, these techniques might enable partial functional recovery from ALS-induced neurodegeneration.
3.5. Huntington disease
3.5.1. Neuropathology of Huntington disease
HD is progressive neurodegenerative disorder with average onset ranging from 35 to 42 years of age (Martin and Gusella, 1986). Initial changes in cognitive function progress into physical instability, involuntary movements, emotional outbursts, and impairments of abstract thinking (Martin and Gusella, 1986). Histological analyses indicate that there are at least five types of striatal neurons (DiFiglia et al., 1976) and medium-sized type I spiny neurons (MSN) are the most significantly affected cell type during disease progression (Graveland et al., 1985). These neurons undergo pathological changes such as dendrite retraction, curling, branching, and arborization, which might indicate the activation of pro-survival mechanisms prior to neuron death (Graveland et al., 1985). Although striatal neurons are most severely affected, widespread neuron death is also observed in the cortex, globus pallidus, and thalamic nuclei.
The molecular etiology of HD has been well established. An autosomal dominant mutation in the Huntingtin gene (HTT), resulting from the expansion of a CAG trinucleotide repeat, encodes a polyglutamine repeat in HTT protein (Gusella et al., 1983; The Huntington’s collaborative research group, 1993). Disease-unaffected populations carry 11–34 trinucleotide repeats, while affected individuals typically encode 42–66 trinculeotide repeats with higher repeat copies correlating to earlier disease onset (The Huntington’s collaborative research group, 1993). The proteolytic cleavage of mutant HTT generates short peptides with high aggregation potential (Scherzinger et al., 1997) and when localized to the nucleus these polyglutamine-rich aggregates become cytotoxic (Yang et al., 2002). However, causative roles for the misfolding, proteolytic cleavage, and aggregation of short HTT peptides in the onset of HD have yet to be conclusively demonstrated. Numerous studies have also identified potential roles for caspase activation (Gervais et al., 2002), gene transcription (Steffan et al., 2000), and dysregulated intracellular transport (Gauthier et al., 2004) in disease onset.
3.5.2. Pharmacological treatments and drawbacks
No therapeutic treatment has been discovered that can slow or cure the progression of HD. Pharmacological interventions can temporarily improve motor control and HD-associated psychiatric disorders; however, these short-lived gains do not affect the underlying mechanisms of neurodegeneration (Kumar et al., 2015). Tetrabenazine, a catecholamine-depleting compound, has been shown to improve motor control in HD-afflicted individuals and, unlike dopamine-depleting compounds, tetrabenazine does not induce tardive dyskinesia (Diana, 2007). Ongoing clinical trials will evaluate the efficacy of compounds targeting neuronal excitotoxicity, mitochondrial dysfunction, and HTT proteolysis, aggregation, and clearance (Kumar et al., 2015). As an alternative to molecule-based therapeutics, human fetal neuroblasts were transplanted into the striatal regions of five HD-afflicted individuals. While three patients exhibited initial clinical improvement, these benefits persisted only four to six years post engraftment (Kumar et al., 2015). This suggests that modification of the striatal microenvironment and improved survival of transplanted neurons might be required to promote further functional improvement.
3.5.3. Huntington-relevant induced pluripotent stem cell models
The derivation of iPSCs from somatic cells of HD-afflicted individuals demonstrated the feasibility of generating patient-specific cells for disease modeling and genetic analyses (Park et al., 2008). The neural differentiation of iPSC lines with CAG-repeat expansions in HTT enabled in vitro modeling of cytoskeletal, cell adhesion, and energetic changes related to HD onset (The HD iPSC Consortium, 2012). iPSCs restricted to an NSC lineage were differentiated to striatal PPP1R1B-positive neurons and withdrawal of brain-derived neurotrophic factor following differentiation increased cell death in HD-mutant neurons (The HD iPSC Consortium, 2012). This collection of HD-specific iPSC lines represents a valuable resource for investigating the genetic and molecular mechanisms underlying disease progression, as well as, a novel system for screen therapeutic compounds.
3.5.4. Huntington-relevant regeneration strategies
The in vitro transdifferentiation of adult human fibroblasts to functional neurons by miR-124, miR-9/9*, NEUROD2, ASCL1, and MYT1L revealed a novel and essential role for microRNAs in cell fate specification (Yoo et al., 2011). Building on this discovery, miR-124, miR-9/9*, MYT1L, BCL11B, DLX1, and DLX2 were combined to directly induce subtype-specific striatal MSNs from postnatal and adult human fibroblasts in vitro (Victor et al., 2014). These induced neurons expressed MAP2, GABA, and GAD1 with 70% of the total population also immunoreactive for PPP1R1B (Victor et al., 2014).
The clinical relevance of neural transdifferentiation technologies relies on the ability to induce functional subtype-specific neurons closely representative of an equivalent endogenous neuron. The single-cell transcriptome of postmortem adult human MSNs isolated by laser capture microdissection was compared with RNA transcripts isolated from fibroblast-derived MSNs (Victor et al., 2014). A pairwise comparison indicated strong correlation in gene expression between endogenous and reprogrammed MSNs that segregated from untreated adult fibroblasts (Victor et al., 2014). Further, induced MSNs exhibited membrane polarization and functional properties characteristic of endogenous human MSNs (Victor et al., 2014).
In vitro reprogrammed MSNs transplanted into the mouse striatum survived more than six months post engraftment and functionally integrated into local striatal circuitry (Victor et al., 2014). The transplanted MSNs projected axons into the substantia nigra and globus pallidus indicating these neurons acquired an ability to decipher axon guidance cues utilized by endogenous striatal neurons (Victor et al., 2014). In addition to the generation of fibroblast-derived MSNs, DLX2 is sufficient to induce mouse postnatal cortical astrocytes toward a functional GABAergic neuron fate in vitro (Heinrich et al., 2010). These foundational in vitro discoveries have laid the groundwork for further investigation into the in vivo transdifferentiation of striatal glia and the induction of functional MSNs for the reconstruction of neuronal circuits damaged by the onset of HD.
3.6. Parkinson disease
3.6.1. Neuropathology of Parkinson disease
PD is an adult onset neurodegenerative disorder characterized by bradykinesia, rigidity, gait disturbance, and resting tremor (Olanow and Tatton, 1999). These motor deficits result from dopaminergic neuron death and disruptions in dopamine synaptic transmission in the pars compacta region of the substantia nigra (Lang and Lozano, 1998). In later stages of the disease, pathological cell death occurs in the midbrain, basal forebrain, and neocortex (Lang and Lozano, 1998). This progressive brain-wide degeneration is associated with the onset of dementia, autonomic dysfunction, and severe postural instability (Olanow and Tatton, 1999).
The molecular etiology of PD is complex with genetic, physiologic, and environmental factors each contributing to disease onset. A defined mechanism for sporadic PD has yet to be elucidated; however, environmental toxins, neuronal excitotoxicity, mitochondrial dysfunction, and glial immune modulators have each been implicated in disease onset (Lang and Lozano, 1998). Familial PD has been linked to both heritable autosomal dominant and recessive mutations in more than ten genes. Autosomal-dominant mutations in SNCA (Polymeropoulos et al., 1997), PARK3 (Gasser et al., 1998), UCHL1 (Liu et al., 2002), LRRK2 (Funayama et al., 2002), and NR4A2 (Le et al., 2003), as well as, autosomal-recessive mutations in PARK2 (Kitada et al., 1998), PINK1 (Pankratz and Foroud, 2004), and PARK7 (Pankratz and Foroud, 2004) have been implicated in the onset and progression of PD.
Lewy body inclusions, a cytological hallmark of PD, develop in the soma and neurites of degenerating dopaminergic neurons (Lang and Lozano, 1998). The dense protein-rich core of Lewy bodies consists of aggregated cytosolic proteins and 200–600 nm length α-synuclein fibrils that emanate into the cytosol (Spillantini et al., 1997; Crowther et al., 2000). These aggregates containing HSPA chaperone proteins (Auluck et al., 2002), regulators of ubiquitination (Shimura et al., 2001), and protein degradation complexes broadly disrupt proteomic equilibrium resulting in cytotoxicity and dopaminergic neuron loss (Leverenz et al., 2007).
3.6.2. Pharmacological treatments and drawbacks
Levodopa (l-3,4-dihydroxyphenylalanine) is the most effective pharmacological treatment for PD (The Parkinson Study Group, 2004). As precursor to the neurotransmitter dopamine, levodopa is metabolized by aromatic l-amino acid decarboxylase to increase dopamine concentration at degenerating synapses. Although initially beneficial, the effectiveness of levodopa declines throughout disease progression. Therefore, an alternative cell-based strategy utilized human embryonic dopamine-producing neurons transplanted directly into the adult substantia nigra to enhance dopamine production (Freed et al., 2001). Embryonic neurons survived transplantation and modestly improved motor function in young PD-afflicted individuals (Freed et al., 2001). As no therapeutic treatment has been identified that can halt or repair the damage imparted by PD, the generation of functional patient-specific striatal neurons in situ might offer a mechanism for the recovery of dopamine signaling in damaged neural circuitry.
3.6.3. Parkinson-relevant induced pluripotent stem cell models
An autosomal dominant G2019S missense mutation in LRRK2 has been identified in familial and sporadic cases of PD (Nguyen et al., 2011). The generation, functional characterization, and dopaminergic differentiation of iPSCs with this common mutation enabled genetic modeling of PD onset in living cells (Nguyen et al., 2011; Liu et al., 2012a,b). Single-cell gene expression analyses revealed increased expression of oxidative stress-response genes and SNCA (Nguyen et al., 2011). LRRK2-mutant neurons exhibit sensitivity to in vitro cell stress assays and significantly increased SNCA expression in differentiated mature neurons (Nguyen et al., 2011). This increase SNCA transcription results in higher monomeric α-synuclein protein levels, a pathological hallmark in neural tissues of some PD-afflicted individuals (Nguyen et al., 2011).
Another genetic model of PD, Parkin-mutant iPSCs derived from adult human dermal fibroblasts were differentiated into midbrain dopaminergic neurons for functional characterization (Jiang et al., 2012). These iPSC-derived neurons exhibited reduced dopamine reuptake at inter-neuronal synapses and oxidative stress response mediated by increased transcription of monoamine oxidases. Importantly, these phenotypes were rescued by lentiviral expression of Parkin (Jiang et al., 2012). In addition to genetic and molecular analyses of PD, cynomolgus monkey iPSC-derived dopaminergic neurons were transplanted into the putamen of a non-human primate Parkinsonian brain (Hallett et al., 2015). The reprogrammed neurons survived and underwent extensive outgrowth into transplantation site and surrounding putamen (Hallett et al., 2015). Further, the engraftment of these neurons improved motor function and increased motor activity without immune suppression (Hallett et al., 2015). These in vitro and in vivo transplantation studies demonstrate the value of pre-clinical modeling with iPSCs and highlight the feasibility of therapeutic neuron transplantation (Hallett et al., 2015).
3.6.4. Parkinson-relevant regeneration strategies
The transdifferentiation of fibroblasts into functional dopaminergic neurons has been achieved in vitro by viral overexpression of varied combinations of pro-neuronal and dopamine-specifying transcription factors (Caiazzo et al., 2011; Kim et al., 2011; Liu et al., 2012a,b, 2014; Pfisterer et al., 2011; Torper et al., 2013). Human embryonic and fetal fibroblasts transduced with lentiviruses encoding doxycycline-regulated ASCL1, POU3F2, MYT1L, LMX1A, and FOXA2 reprogrammed into functional tyrosine hydroxylase-positive dopaminergic neurons (Pfisterer et al., 2011). The withdrawal of doxycycline from neuronal culture media three days following lentiviral infection did not affect the efficiency of reprogramming or the morphological and functional complexity of induced neurons (Pfisterer et al., 2011). This indicates that the transient expression of pro-dopaminergic factors in vitro is sufficient to drive fibroblasts toward a neuronal fate.
Further refinement of these neuron induction strategies revealed that the minimal set of ASCL1, NR4A2, and LMX1A is sufficient to induce functional midbrain dopaminergic neuron-like cells from mouse and human fibroblasts (Caiazzo et al., 2011). The expression of tyrosine hydroxylase, a dopamine processing enzyme, and vesicular transporters of dopamine indicate these cells acquired functional dopamine synthesis and processing mechanisms. Moreover, differential gene expression analyses confirmed broad upregulation of neurogenic- and dopamine-related RNA transcripts (Caiazzo et al., 2011). Expanding this core set of transcription factors to include PITX3, FOXA2, and EN1 combined with the signaling molecules sonic hedgehog and fibroblast growth factor 8, adult mouse tail tip fibroblasts were directly reprogrammed to dopaminergic neurons with functional properties that mimic endogenous midbrain dopaminergic neurons (Kim et al., 2011). The transplantation of these neurons into the rat striatum lesioned by 6-OHDA, a functional model of PD, successfully demonstrated improvements in motor function post neuron engraftment (Kim et al., 2011). Histological analyses revealed the functional integration of 350–1900 reprogrammed neurons and significant improvements in amphetamine-induced rotation scores (Kim et al., 2011). These findings suggest that the striatal implantation of engineered dopaminergic neurons might be a feasible therapeutic option for PD-afflicted individuals.
One significant drawback to most transdifferentiation techniques is a low efficiency of functional reprogramming. Remarkably, the co-transduction of a dominant negative TP53 lentiviral construct with neuron reprogramming factors improves dopaminergic neuron transdifferentiation efficiency at least 4-fold (Liu et al., 2014). This functional inhibition of TP53 improves cell survival and does not induce proliferation indicating TP53 repression in vitro does not promote tumorigenesis (Liu et al., 2014). Dopaminergic neurons induced from adult human fibroblasts by ASCL1, NR4A2, PITX3, NEUROG2, and SOX2, with or without TP53 repression, improved rotary motor behavior when transplanted into 6-OHDA lesioned rat brains (Liu et al., 2012a,b, 2014).
The survival, integration, and function of induced neurons transplanted to the striatum demonstrate that fully differentiated neurons can thrive in the neural microenvironment. In an effort to demonstrate that non-neuronal cells in the striatum can be targeted for reprogramming in vivo, human fibroblasts expressing doxycycline-regulated ASCL1, POU3F2, MYT1L, LMX1A, LMX1B, FOXA2, and OTX2 were implanted within 6-OHDA lesioned rat brains and in vivo transdifferentiated using doxycycline administration (Torper et al., 2013). This generation of fibroblast-derived tyrosine hydroxylase-positive neurons within the striatal microenvironment lends support to the feasibility of in vivo reprogramming non-neuronal cells toward a subtype-specific neuronal fate.
In pursuit of this goal, a targeted in vivo reprogramming strategy designed to minimize the impact of reprogramming on endogenous glial populations was developed by directly transforming resident astrocytes of the adult mouse striatum into proliferative neuroblasts (Niu et al., 2013). Lentiviral-mediated expression of SOX2, specifically targeted to astrocytes using the GFAP promoter, induced astrocytes to adopt a proliferative doublecortin-positive neuroblast identity. One critical advantage of GFAP-regulated transgene expression relative to constitutive promoters is a fate-dependent reduction in SOX2 expression during the acquisition of neuroblast identity. Lentivirus encoding constitutively expressed SOX2 resulted in a 37-fold reduction in the population of induced neuroblasts (Niu et al., 2013). Additionally, cell lineage-specific tracing validated astrocytes as the cell-of-origin (Niu et al., 2013).
The clinical relevance of in vivo reprogramming strategies hinges on the induction of long surviving, targeted cell types in aged and degenerating tissues. Remarkably, SOX2-induced neuroblasts survive 14 weeks beyond induction and can be generated in the brains of 24-months-old mice (Niu et al., 2013). Furthermore, treatment with valproic acid or a combination of brain-derived neurotrophic factor and noggin coaxed these neuroblasts to differentiate into functional RBFOX3-positive neurons (Niu et al., 2013). The functional integration of these induced neurons with endogenous striatal neurons highlights the promising potential of in vivo reprogramming in the treatment of PD and other age-related neurodegenerative disorders (Fig. 1).
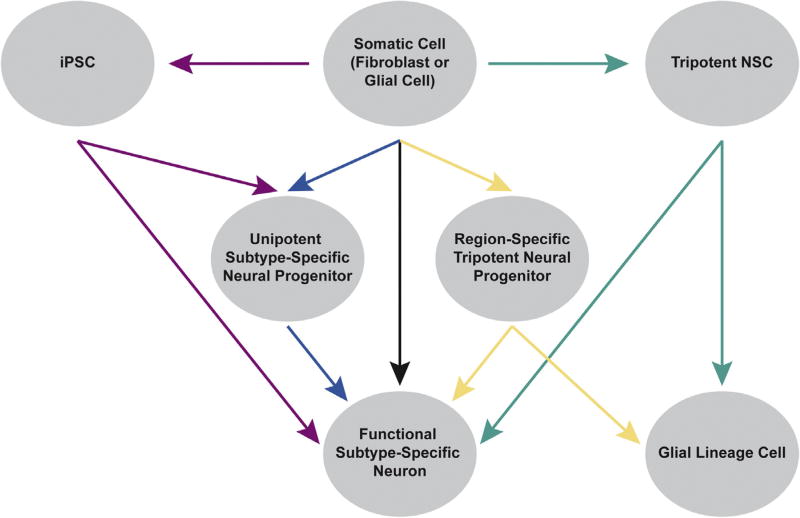
Fig. 1.
Neuron induction by diverse cell identity reprogramming strategies. Indirect reprogramming through an iPSC (red): somatic or glial cells adopt a pluripotent state before differentiation into functional subtype-specific neurons or neural progenitor cells that differentiate to yield neurons. Direct reprogramming through a neural progenitor (blue and yellow): somatic or glial cells adopt a unipotent subtype-specific neural progenitor state or tripotent region-specific neural progenitor state. These progenitor cells then differentiate into functional subtype-specific neurons. Direct reprogramming through a tripotent NSC (green): somatic or glial cells adopt a tripotent NSC fate then directly differentiate to neurons, glia or regional neural progenitor cells. Direct reprogramming by transdifferentiation (black): somatic or glial cells adopt a specific neuronal fate without passing through a pluripotent or neural progenitor stage.
4. Clinical translation: challenges and goals
The clinical application of cell identity reprogramming technologies to neurodegenerative disorders will require advances in cell targeting, refined mechanisms for delivery of reprogramming factors, improved neuron induction efficiency, and effective genetic engineering strategies (Fig. 2). While patient-specific induced neurons possess immense therapeutic potential, these challenges currently hinder the widespread use of reprogramming technologies in the clinical setting.
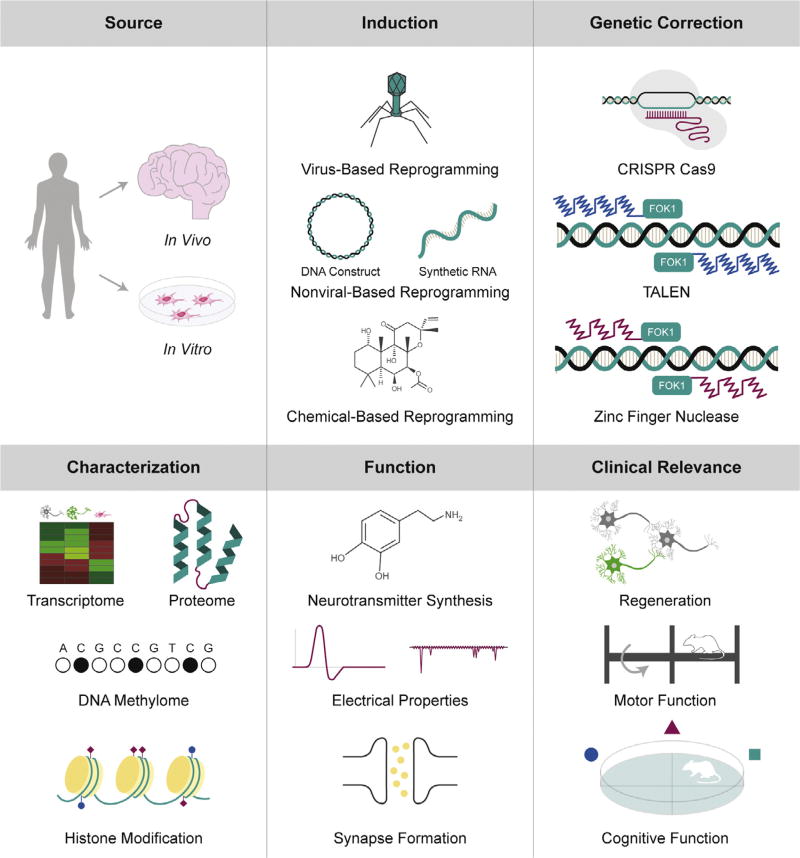
Fig. 2.
Challenges to clinical translation. Numerous challenges face the clinical application of cell identity reprogramming technologies. In vitro generation of neurons requires a large population of patient-specific source cells for reprogramming and transplantation. Alternatively, in vivo reprogramming strategies typically target large glial cell populations for neuronal induction. The refinement of reprogramming factor delivery, genetic correction of disease-causative mutations, and characterization of induced neurons will be essential to the clinical evaluation of these technologies.
4.1. Neuron induction: cell source and reprogramming strategy
An easily accessible, large population of starting cells is essential for in vitro reprogramming and transplantation strategies. Therefore, adult human dermal fibroblasts are frequently targeted for iPSC-to-neuron reprogramming (Takahashi et al., 2007) and direct transdifferentiation (Ambasudhan et al., 2011; Yoo et al., 2011). One pitfall to the stereotactic transplantation of these reprogrammed fibroblasts is mechanical damage to existing cortical and striatal neural networks. Less invasive in vivo transdifferentiation strategies have been used to target large populations of proliferating glia for conversion into neuroblasts (Niu et al., 2013; Su et al., 2014) and mature neurons (Guo et al., 2014; Heinrich et al., 2014). While these methods target endogenous populations of glia that can proliferate to replace neuron-converted cells, these strategies rely on disruptive lentiviral or retroviral integration of reprogramming factors into the genome.
Diverging from established gene-based viral transdifferentiation strategies, diverse clinic-friendly nonviral and chemical-based reprogramming methods have been developed (Al-Dosari and Gao, 2009). For instance, the expression of neurogenic transcription factors using an integration-free bioreducible linear poly(amido amine) vehicle enabled the lineage-specific induction of functional neurons from mouse fibroblasts (Adler et al., 2012). Additionally, nucleofection of mouse and human fibroblasts with a nonviral polycistronic construct of four transcription factors successfully generated iPSCs in vitro (Kaji et al., 2009; Gonzalez et al., 2009). Mouse and human fibroblast-derived iPSCs were likewise induced in vitro using nonviral gene-based DNA constructs (Okita et al., 2008; Narsinh et al., 2011) and synthetic RNAs (Warren et al., 2010). The system-level in vivo generation of iPSCs in the adult mouse liver by hydrodynamic tail-vein injection of gene-based DNA constructs highlights the functional applications of nonviral gene-based cell identity conversion tools in living tissue (Yilmazer et al., 2013).
Cell identity is a high-level description of the transcriptional and translational mechanisms that confer specific physiological functions to a cell. The targeted modulation of these mechanisms can functionally alter cell identity. Small molecule cocktails that promote or inhibit intracellular signaling often dramatically modulate transcription and control properties such as cell division. Taking advantage of these properties, a cocktail of seven small molecules was used to induce a pluripotent state in cultured mouse fibroblasts (Hou et al., 2013). Interestingly, other combinations of small molecules have been identified that transdifferentiate cultured mouse and human fibroblasts into functional neurons (Li et al., 2015; Hu et al., 2015). The governing roles for signal transduction pathways in cell fate maintenance enable multiple points of entry for therapeutic reprogramming. Demonstrating this principle, two small molecules were combined with NEUROG2 and SOX11 overexpression to convert adult human fibroblasts into functional subtype-specific cholinergic neurons with greater than 90% efficiency in vitro (Liu et al., 2013). With numerous in vitro and in vivo reprogramming protocols established, the focus of much regenerative research is the induction of disease-relevant neurons with subtype-specific identities and functions.
4.2. Neuron subtype: induction of subtype-specific neurons and loss of cell-of-origin identity
Neuron induction strategies aim to generate neurons that mimic the molecular profile and functional properties of endogenous neurons. Direct conversion of a somatic cell to a specialized neuron requires broad shifts in the transcriptome, proteome, DNA methylome, histone modifications, high-order chromatin structure, membrane polarization properties, and cytoskeletal remodeling.
Global gene expression analysis by RNA sequencing provided insight into the early pro-neuronal changes in transcription triggered by the overexpression of ASCL1, POU3F2, and MYT1L in mouse fibroblasts during reprogramming (Wapinski et al., 2013). ASCL1 strongly activated a pro-neural transcription program within 48 h of expression and synergized with POU3F2 and MYT1L to endow transduced fibroblasts with a neuronal transcriptome in 22 days (Wapinski et al., 2013). This direct conversion between cellular identities generated neurons with a heterogeneous genetic and epigenetic background that resembled both the cell-of-origin fibroblast and desired endogenous neuron. A computational transcriptome-based analysis of origin fibroblasts, endogenous neurons, and induced neurons illustrated how induced neurons adopt a functioning neuron identity while retaining non-neuronal gene expression and epigenetic hallmarks specific to fibroblasts (Cahan et al., 2014).
In turn, these transcriptional mechanisms define the cellular proteome and must be fine-tuned for directed differentiation into dopaminergic neurons (Pfisterer et al., 2011), medium spiny GABAergic neurons (Victor et al., 2014), cholinergic neurons (Liu et al., 2013), and motor neurons (Son et al., 2011). Posttranslational histone acetylation and methylation marks encode an additional layer of gene regulation that defines the proteome of an induced neuron (Wapinski et al., 2013). These genome-wide histone marks control chromatin accessibility, which affects the efficiency and specificity of developmental transdifferentiation (Zuryn et al., 2014).
Interestingly, a single-cell analysis of gene expression in fibroblasts pushed toward a pluripotent state revealed that long non-coding RNAs perform gene regulatory functions critical to the suppression of lineage-specific differentiation and iPSC reprogramming (Kim et al., 2015). Detailed mechanistic studies identified several intermediate stages of iPSC reprogramming defined by waves of transcription and biphasic changes in microRNA expression and histone marks (Polo et al., 2012). Moreover, genome-wide DNA methylation and gene expression studies in human ESC and iPSC lines have enabled quantitative comparisons of differentiation potential (Bock et al., 2011). A similar genome-wide methylation study demonstrated that somatic cell-derived iPSCs retain methylation signatures characteristic of the donor cell, which suggests that reprogrammed iPSCs are not fully reset from the cell-of-origin identity (Kim et al., 2010).
The focused depletion of H3K27 and enhanced H3K4 histone methylation signatures in gene enhancer regions precede transcriptional activation during iPSC conversion (Koche et al., 2011). Further, the repression of histone-modifying methyltransferases such as SUV39H1, YY1, and DOT1L enhance the efficiency of iPSC generation (Onder et al., 2012). Cytosine methyl-oxidation products also enhance DNA accessibility to promote transcription factor binding and early iPSC reprogramming (Doege et al., 2012; Bhutani et al., 2010; Hon et al., 2014). Targeted exploitation of these multi-layered molecular mechanisms will be essential to the efficient induction of subtype-specific neurons without cell-of-origin genetic contamination.
4.3. Neuron maturation: genetic and functional maturation
The genetic and epigenetic maturation of induced neurons requires these cells to adopt the RNA and protein expression pattern, epigenetic signatures, chromatin condensation structure, and cytoskeletal features of endogenous neurons (Vierbuchen and Wernig, 2012; Apostolou and Hochedlinger, 2013). However, the therapeutic relevance of these cells is most directly related to cell function and circuit integration rather than precise genetic identity. Neurotransmitter synthesis and transport, synapse formation, action potential kinetics, responsive membrane potential, and secretion of survival factors within a neural microenvironment are the defining features of a medically relevant neuron induction protocol.
The in vivo conversion of astrocytes to calretinin-positive interneurons by SOX2 and valproic acid is an ASCL1-dependent process that generates functionally heterogeneous neurons (Niu et al., 2015). The histone deacetylase inhibitor valproic acid drives a pro-neurogenic epigenetic program that consistently induces four predominant types of neurons responsive to neurotransmitter stimulation and capable of firing repetitive action potentials (Niu et al., 2015). These neurons exhibit diverse resting membrane potentials, action potential firing rates, and broad spontaneous postsynaptic current frequencies (Niu et al., 2015). This functional heterogeneity underscores the genetic diversity of astroglia in the adult brain and the complex mechanisms regulating the in vivo differentiation of reprogrammed cells.
In vivo reprogrammed cortical and striatal neurons exhibit dendritic branching and outgrow axons that form synaptic connections with endogenous interneurons (Guo et al., 2014; Niu et al., 2013). Whether these newly integrated neurons function to improve or disrupt network signaling has yet to be investigated. Encouragingly, the transplantation of fibroblast-derived dopaminergic neurons into a mouse model of PD improved basic motor function suggesting that reprogrammed neurons promote functional recovery of degenerated neural circuits (Kim et al., 2011).
4.4. Neuron longevity: acquisition of stable identity, efficient induction, and long-term survival
Transient exposure of non-neuronal cells to pro-neurogenic reprogramming factors must induce stable neuronal identity with high efficiency to be clinically effective. The restriction of doxycycline-dependent transgene expression three days following transduction with dopaminergic factors does not affect the in vitro conversion efficiency or survival of induced dopaminergic neurons (Pfisterer et al., 2011). Further, induced cholinergic neurons intrinsically downregulate the expression of exogenous NEUROG2 seven days following initial overexpression (Liu et al., 2013). When applied in vivo, doxycycline-induced expression of ASCL1, POU3F2, and MYT1L induced a stable neuronal identity in cells that functionally integrate into the local microenvironment and survive after doxycycline withdrawal (Torper et al., 2013). These results demonstrate that even brief exposure to pro-neural reprogramming factors can catalyze commitment to a lineage-specific identity.
Early transdifferentiation techniques generated neurons with extremely low efficiency and only modest improvements were obtained with other transgene-based methods. Adult human skin fibroblasts transduced with NEUROG2 and SOX11 encoding lentivirus convert with similarly low efficiency; however, treatment with the small molecules forskolin and dorsomorphin dramatically boost conversion to greater than 90% efficiency (Liu et al., 2013). This breakthrough in efficiency will enable the large-scale production of patient-specific neurons for transplantation or in vitro pharmaceutical screens.
Improvements in reprogramming efficiency can also be obtained by increasing the total number of reprogrammed neurons that survive into functional maturity. Proliferating astrocyte-derived neuroblasts yield multiple neurons and have been shown to persist in vivo at least 14 weeks post induction (Niu et al., 2013). Single-factor transdifferentiation in the cortex generates mature neurons that survive at least two months in the injured mouse cortex (Guo et al., 2014). Importantly, NEUROG2-induced neurons mature with starkly different efficiency in the cortex relative to striatum (Grande et al., 2013). This difference indicates that the neural microenvironment has a substantial role in facilitating the induction and survival of new neurons during in vivo reprogramming.
4.5. Neuron engineering: genetic correction and modulation of cellular processes
The direct cause of cognitive and motor deficits in age-related neurodegenerative disorders is synapse degeneration and neuron loss. However, the diverse factors responsible for these losses such as inherited and acquired genetic mutation remain unresolved in neurons induced from patient-specific somatic or glial cells. These causative mutations often result in the neurotoxic aggregation of disordered peptides to which induced neurons remain susceptible. Therefore, targeted genetic editing of these defects in induced neurons is essential to remodeling the neural microenvironment in disease-affected tissues.
Three genomic DNA editing technologies predominate: CRISPRCas9 (Hsu et al., 2014), transcription activator-like effector nucleases (Kim et al., 2014), and zinc finger nucleases (Kim et al., 2014). Recent advances in high-fidelity genome editing have enabled specific targeting of disease-related genes such as HTT and SOD1 in reprogrammed cells (An et al., 2012; Kiskinis et al., 2014). Mutation-containing fibroblasts were in vitro reprogrammed to pluripotency and genetically engineered to remove either an expanded CAG repeat in HTT or dominant acting A4V mutation in SOD1 (An et al., 2012; Kiskinis et al., 2014). Gene corrected-iPSCs were then differentiated to NSCs or functional motor neurons, respectively. Further gene replacement studies targeting PD-related LRRK2 and SNCA mutations in human-derived iPSCs provided mechanistic insight into the progression of PD and established testable in vitro diseased-state models (Reinhardt et al., 2013; Soldner et al., 2011). These studies highlight the breakout potential for in vitro reprogramming with gene editing as a tool for disease modeling and pharmaceutical discovery.
Drawing from the known mechanisms of neurogenesis and neural development, gene replacement and exogenous overexpression of pro-neurogenic genome-encoded factors have been widely used to generate functional neurons from non-neuronal cells. However, the next iteration of cellular engineering might utilize non-physiological synthetic factors that can target complex intracellular networks to modulate identity with high precision. For instance, synthetic RNA aptamers responsive to endogenous proteins can be used to modulate alternative splicing events and redefine complex signaling mechanisms that regulate the cellular transcriptome (Culler et al., 2010). These and similar synthetic tools might drive the next generation of tailored neuronal reprogramming strategies.
4.6. Safety
The clinical relevance of cellular reprogramming hinges upon the safe induction of large numbers of new neurons that halt or reverse neurodegeneration. In vivo reprogramming therapies must ensure that the identity and total number of cells targeted for conversion are tightly controlled and the function of endogenous neural networks is unaffected. One approach is the use of titrated lentivirus engineered to express a fusogenic protein and cell surface marker-specific antibody that catalyzes membrane fusion only with antigen-expressing cells (Yang et al., 2006). Although potentially useful if adapted to non-integrating adenoviral delivery of reprogramming factors (Stadtfeld et al., 2008), virus-based reprogramming methods still face significant hurdles to implementation and are not likely to be widely adopted.
Active cell division is a defining property of pluripotent stem cells and neural progenitors. Tightly regulated molecular signals control the rate of this proliferation to suppress tumor formation. In vivo reprogramming to pluripotency suggests deficits in these mechanisms as transitory expression of POU5F1, SOX2, KLF4, and MYC result in teratoma formation in multiple organ tissues (Abad et al., 2013). The ectopic expression of Oct4 itself can induce dysplasia in mouse epithelial tissue (Hochedlinger et al., 2005). However, in vivo reprogramming to neural-specific progenitor state or direct glial cell-to-neuron transdifferentiation is not limited by this tumorigenic potential (Niu et al., 2013; Su et al., 2014; Guo et al., 2014). In addition to the inherent proliferative potential of iPSCs, genome sequencing of 22 iPSC lines revealed numerous acquired non-synonymous, nonsense, splice variant, and epigenetic mutations in genes causative to cancer (Gore et al., 2011). These uncontrolled genetic modifications suggest broad genetic screening measures will be required for in vivo transplantation of patient-derived cell lines.
Although multiple engineering and safety issues must be resolved before cell fate reprogramming methods find therapeutic application, the rapid pace of discovery and refinement within this field has laid a promising groundwork for future development.
5. Conclusions
Recent advances in cell identity reprogramming have enabled researchers to investigate the mechanisms of neuron fate plasticity and degeneration to gain insight into the biological mechanisms that govern age-related neurodegenerative disorders. As early-stage biomedical technologies, stem cell-based reprogramming and transdifferentiation each face numerous challenges that limit clinical utility. As these limitations are overcome through refinement and innovation, diverse safe and efficient reprogramming methodologies will emerge to establish a new paradigm for patient-specific treatment of neurodegenerative disorders.
Acknowledgments
We thank Changhai Tian for assistance with outlining Fig. 1 and manuscript comments. We thank Huong Le (huongledesign@gmail.com) for assistance with Table 1 and Fig. 2 illustrations.
Funding
Zhang, C.-L.: Zhang, C-L is a W. W. Caruth Jr. Scholar in Biomedical Research. Research in the Zhang laboratory is supported by the Welch Foundation (I-1724), the Ellison Medical Foundation (AG-NS-0753-11), Texas Institute for Brain Injury and Repair, the Decherd Foundation, The Michael J. Fox Foundation, and National Institutes of Health (NS070981 and NS088095). Zheng, J.: Research in the Zheng laboratory is supported by the National Key Basic Research Program of China (973 Program Grant No. 2014CB965000 and project 1 No. 2014CB965001), National Natural Science Foundation of China (#81271419), Innovative Research Groups of the National Natural Science Foundation of China (#81221001), Joint Research Fund for Overseas Chinese, Hong Kong, and Macao Young Scientists of the National Natural Science Foundation of China (#81329002), National Institutes of Health (R01 NS41858-01, R01 NS061642-01, 2R56NS041858-15A1), and the State of Nebraska (DHHS-LB606 Stem Cell 2009-10, LB606 Stem Cell-2010-10).
Abbreviations
- 6-OHDA
6-hydroxydopamine
- Aβ
amyloid-β
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- APOE4
apolipoprotein E4
- APP
amyloid precursor protein
- ASCL1
achaete-scute family basic helix-loop-helix transcription factor 1
- ATP
adenosine triphosphate
- BCL11B
B-cell CLL/Lymphoma 11B
- CRISPR-Cas9
clustered regularly interspaced short palindromic repeats-Cas9
- DLX1
distal-less homeobox 1
- DLX2
distal-less homeobox 2
- DNA
deoxyribonucleic acid
- DOT1L
DOT1-like histone H3 methyltransferase
- EGF + FGF2
epidermal growth factor and fibroblast growth factor 2
- EN1
engrailed homeobox 1
- ESC
embryonic stem cell
- FOXA2
forkhead box A2
- FOXG1
forkhead box G1
- FUS
FUS ribonucleic acid binding protein
- GABA
gamma-aminobutyric acid
- GAD1
glutamate decarboxylase 1
- GFAP
glial fibrillary acidic protein
- GSK3B
glycogen synthase kinase 3 beta
- H3K27
histone 3 lysine 27
- H3K4
histone 3 lysine 4
- HD
Huntington disease
- HSPA
heat shock 70 kilodaltons protein family
- HTT
Huntingtin
- iPSC
induced pluripotent stem cell
- ISL1
ISL LIM homeobox 1
- KLF4
kruppel-like factor 4
- LHX3
LIM homeobox 3
- LIN28A
lin-28 homolog A
- LMX1A
LIM homeobox transcription factor 1 alpha
- LMX1B
LIM homeobox transcription factor 1 beta
- LRRK2
leucine-rich repeat kinase 2
- MAP2
microtubule-associated protein 2
- MAPT
microtubule-associated protein tau
- MKI67
marker of proliferation Ki-67
- MNX1
motor neuron and pancreas homeobox 1
- mRNA
messenger ribonucleic acid
- MSN
medium spiny neuron
- MYC
v-myc avian myelocytomatosis viral oncogene homolog
- MYRF
myelin regulatory factor
- MYT1
myelin transcription factor 1
- MTY1L
myelin transcription factor 1-like
- NEUROD1
neuronal differentiation 1
- NEUROD2
neuronal differentiation 2
- NEUROG2
neurogenin 2
- NKX2-2
NK2 homeobox 2
- NKX6-3
NK6 homeobox 3
- NR4A2
nuclear receptor subfamily 4 group A member 2
- NSC
neural stem cell
- OLIG1
oligodendrocyte transcription factor 1
- OLIG2
oligodendrocyte transcription factor 2
- OTX2
orthodenticle homeobox 2
- PARK2
parkin RBR E3 ubiquitin protein ligase
- PARK3
Parkinson disease 3
- PARK7
Parkinson protein 7
- PD
Parkinson disease
- PI3K
phosphatidylinositol 3-kinase
- PINK1
PTEN induced putative kinase 1
- PITX3
paired-like homeodomain 3
- PN
projection neuron
- POU3F2
POU class 3 homeobox 2
- POU3F4
POU class 3 homeobox 4
- POU5F1
POU class 5 homeobox 1
- PPP1R1B
protein phosphatase 1 regulatory subunit 1B
- RBFOX3
ribonucleic acid binding protein fox-1 homolog 3
- RNA
ribonucleic acid
- SNCA
synuclein alpha
- SOD1
superoxide dismutase 1
- SOX2
sex determining region Y-box 2
- SOX10
sex determining region Y-box 10
- SOX11
sex determining region Y-box 11
- ST18
suppression of tumorigenicity 18
- SUV39H1
suppressor of variegation 3–9 homolog 1
- SYN1
synapsin 1
- TALEN
transcription activator-like effector nuclease
- TARDBP
TAR deoxyribonucleic acid binding protein
- TCF3
transcription factor 3
- TP53
tumor protein p53
- TUBB3
tubulin beta 3 class III
- UCHL1
ubiquitin carboxyl-terminal esterase L1
- YY1
YY1 transcription factor
- ZNF536
zinc finger protein 536.
Footnotes
Authors’ contributions
Smith, D.K. performed literature research, authored and revised the manuscript text, and illustrated Table 1 and Figs. 1 and 2. He, M. performed literature research and outlined Fig. 1. Zhang, C.-L. and Zheng, J. commented on the manuscript.
Conflict of interest
None declared.
References
- Abad M, Mosteiro L, Pantoja C, Cañamero M, Rayon T, Ors I, Graña O, Megías D, Domínguez O, Martínez D, Manzanares M, Ortega S, Serrano M. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–345. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- Adler AF, Grigsby CL, Kulangara K, Wang H, Yasuda R, Leong KW. Nonviral direct conversion of primary mouse embryonic fibroblasts to neuronal cells. Mol. Ther. Nucleic Acids. 2012;1:e32. doi: 10.1038/mtna.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian EK, Jr, Walker BE. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J. Neuropathol. Exp. Neurol. 1962;21:597–609. doi: 10.1097/00005072-196210000-00007. [DOI] [PubMed] [Google Scholar]
- Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institutes on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An MC, Zhang N, Scott G, Montoro D, Wittkop T, Mooney S, Melov S, Ellerby LM. Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell. 2012;11:253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PM, Al-Chalabl A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat. Rev. Neurol. 2011;7:603–615. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462–471. doi: 10.1038/nature12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck PK, Chan HYE, Trojanowski JQ, Lee VM-Y, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 1995;168:842–857. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Götz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, Park I-H, Friedman BA, Daley GQ, Wyllie DJA, Hardingham GE, Wilmut I, Finkbeiner S, Maniatis T, Shaw CE, Chandran S. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5803–5808. doi: 10.1073/pnas.1202922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland LG, Sahai E, Kelly G, Golding M, Greensmith L, Schiavo G. Deficits in axonal transport precede ALS symptoms in vivo. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20523–20528. doi: 10.1073/pnas.1006869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, Gnirke A, Eggan K, Meissner A. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft G, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, Rodolfa CT, Dimos JT, Mikkilineni S, MacDermott AB, Woolf CJ, Henderson CE, Wichterle H, Eggan K. A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Briggs R, King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc. Natl. Acad. Sci. U. S. A. 1952;38:455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüstle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RDG. In vitro-generated neural precursors participate in mammalian brain development. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14809–14814. doi: 10.1073/pnas.94.26.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J. Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- Byrne S, Walsh C, Lynch C, Bede P, Elamin M, Kenna K, McLaughlin R, Hardlman O. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2011;82:623–627. doi: 10.1136/jnnp.2010.224501. [DOI] [PubMed] [Google Scholar]
- Cahan P, Li H, Morris SA, Lummertz da Rocha E, Daley GQ, Collins JJ. CellNet: network biology applied to stem cell engineering. Cell. 2014;158:903–915. doi: 10.1016/j.cell.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Carri AD, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, Toselli M, Martino G, Barker RA, Dunnett SB, Biella G, Cattaneo E. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP32+ medium-sized spiny neurons. Development. 2013;140:301–312. doi: 10.1242/dev.084608. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1372. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- Crowther RA, Daniel SE, Goedert M. Characterization of isolated alpha-synuclein filaments from substantia nigra of Parkinson’s disease brain. Neurosci. Lett. 2000;292:128–130. doi: 10.1016/s0304-3940(00)01440-3. [DOI] [PubMed] [Google Scholar]
- Culler SJ, Hoff KG, Smolke CD. Reprogramming cellular behavior with RNA controllers responsive to endogenous proteins. Science. 2010;330:1251–1255. doi: 10.1126/science.1192128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rossa A, Bellone C, Golding B, Vitali I, Moss J, Toni N, Lüscher C, Jabaudon D. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat. Neurosci. 2013;16:193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the anyloid precursor protein. J. Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- Deacon T, Dinsmore J, Costantini LC, Ratliff J, Isacson O. Blastula-stage stem cells can differentiate into dopaminergic and serotonergic neurons after transplantation. Exp. Neurol. 1998;149:28–41. doi: 10.1006/exnr.1997.6674. [DOI] [PubMed] [Google Scholar]
- Deane R, Yan SD, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-β peptide transport across the blood–brain barrier and accumulation in brain. Nat. Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deng HX, Zhai H, Bigio EH, Yan J, Fecto F, Ajroud K, Mishra M, Ajroud-Driss S, Heller S, Sufit R, Siddique N, Mugnaini E, Siddique T. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann. Neurol. 2010;67:739–748. doi: 10.1002/ana.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana P. Tetrabenazine in the treatment of Huntington’s disease. Neuropsychiatr. Dis. Treat. 2007;3:545–551. [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Pasik P, Pasik T. A golgi study of neuronal types in the neostriatum of monkeys. Brain Res. 1976;114:245–256. doi: 10.1016/0006-8993(76)90669-7. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, Guarnieri P, Bhagat G, Vanti WB, Shih A, Levine RL, Nik S, Chen EI, Abeliovich A. Early-stage epigenetic modification during somatic cell reprogramming. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Liu Y, Xi J, Zhang X, Yin Y, Lu J, Joers V, Swanson C, Holden JE, Zhang S-C. Induced pluripotent stem cell-derived neural cells survive and mature in the nonhuman primate brain. Cell Rep. 2013;3:646–650. doi: 10.1016/j.celrep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Ferraluolo L, Kirby J, Grlerson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- Finley MFA, Kulkarni N, Huettner JE. Synapse formation and establishment of neuronal polarity by P19 embryonic carcinoma cells and embryonic stem cells. J. Neurosci. 1996;16:1056–1065. doi: 10.1523/JNEUROSCI.16-03-01056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J. Cell Sci. 1995;108:3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai W-Y, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T, Müller-Myhsok B, Wszolek ZK, Oehlmann R, Calne DB, Bonifati V, Bereznai B, Fabrizio E, Vieregge P, Horstmann RD. A susceptibility locus for Parkinson’s disease maps to chromosome 2p13. Nat. Genet. 1998;18:262–265. doi: 10.1038/ng0398-262. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pagés M, Dompierre JP, Rangone H, Cordeliéres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Singaraja R, Xanthoudakis S, Gutekunst CA, Leavitt BR, Metzler M, Hackam AS, Tam J, Vaillancourt JP, Houtzager V, Rasper DM, Roy S, Hayden MR, Nicholson DW. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat. Cell Biol. 2002;4:95–105. doi: 10.1038/ncb735. [DOI] [PubMed] [Google Scholar]
- Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Belmonte JCI. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat. Protoc. 2010;5:811–820. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- Giorgio FPD, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F, Monasterio MB, Tiscornia G, Pulido NM, Vassena R, Morera LB, Piza IR, Belmonte JCI. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8918–8922. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung H-L, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Isreal MA, Kiskinis E, Lee J-H, Loh Y-H, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Blemonte JCI, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LSB, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. NSC. [DOI] [PubMed] [Google Scholar]
- Gossler A, Joyner AL, Rossant J, Skarnes WC. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989;244:463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grande A, Sumiyoshi K, López-Juárez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA, Williams RS, DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science. 1985;227:770–773. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Zheng W, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Laskey RA. The transplantation of nuclei from single cultured cells into enucleate frogs’ eggs. J. Embryol. Exp. Morphol. 1970;24:227–248. [PubMed] [Google Scholar]
- Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, Watkins PC, Ottina K, Wallace MR, Sakaguchi AY, Young AB, Shoulson I, Bonilla E, Martin JB. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306:234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell A-L, Schumacher JM, Spealman RD, Isacson O. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16:269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Schöler HR. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Hardlman O, van den Berg LH, Klernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7:639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Blum R, Gascón S, Masserdotti G, Tripathi P, Sánchez R, Tiedt S, Schroeder T, Götz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C, Bergami M, Gascón S, Lepier A, Viganó F, Dimou L, Sutor B, Berninger B, Götz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014;3:1–15. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon GC, Song C-X, Du T, Jin F, Selvaraj S, Lee AY, Yen C-A, Ye Z, Mao S-Q, Wang B-A, Kuan S, Edsall LE, Zhao BS, Xu G-L, He C, Ren B. 5mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol. Cell. 2014;56:286–297. doi: 10.1016/j.molcel.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan L-L, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B-Y, Weick JP, Yu J, Ma L-X, Zhang X-Q, Thomson JA, Zhang S-C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, Zhao H, Jin Y, Tang B, Yu Y, Zhao J, Pei G. Direct conversion of normal and Alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell. 2015;17:1–9. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Gorp SV, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LSB. Probling sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishma M, Lee H-J, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Aβ1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z, Feng J. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat. Commun. 2012;3:668. doi: 10.1038/ncomms1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautograpghs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J-Y, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- Kim DH, Marinov GK, Pepke S, Singer ZS, He P, Williams B, Schroth GP, Elowitz MB, Wold BJ. Single-cell transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. Cell Stem Cell. 2015;16:88–101. doi: 10.1016/j.stem.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-S, Kim J, Yeonju J, Jeon D, Cho YS. Direct lineage reprogramming of mouse fibroblasts to functional midbrain dopaminergic neuronal progenitors. Stem Cell Res. 2014;12:60–68. doi: 10.1016/j.scr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. U. S. A. 2011b;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung C-Y, Dawlaty MM, Tsai L-H, Jaenich R. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee S-H, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cell function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hübner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Schöler HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, Mckinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, Sandoe J, Williams LA, Boulting GL, Moccia R, Wainger BJ, Han S, Peng T, Thams S, Mikkilineni S, Mellin C, Merkle FT, Davis-Dusenbery BN, Ziller M, Oakley D, Ichida J, Costanzo SD, Atwater N, Maeder ML, Goodwin MJ, Nemesh J, Handsaker RE, Paull D, Noggle S, McCarroll SA, Joung JK, Woolf CJ, Brown RH, Eggan K. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell. 2014;14:781–795. doi: 10.1016/j.stem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Pierce GB., Jr Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964;24:1544–1551. [PubMed] [Google Scholar]
- Koche RP, Smith ZD, Adli M, Gu H, Ku M, Gnirke A, Bernstein BE, Meissner A. Reprogramming factor expression induces rapid and widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, Okita K, Takahashi K, Asaka I, Takashi A, Watanabe A, Watanabe K, Kadoya C, Nakano R, Watanabe D, Maruyama K, Hori O, Hibino S, Choshi T, Nakahata T, Hioki H, Kaneko T, Naitoh M, Yoshikawa K, Yamawaki S, Suzuki S, Hata R, Ueno S-I, Seki T, Kobayashi K, Toda T, Murakami K, Irie K, Klein WL, Mori H, Asada T, Takahashi R, Iwata N, Yamanaka S, Inoue H. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang Z-J, Zhang S-C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh SK, Kumar V, Kumar D, Agarwal S, Rana MK. Huntington’s disease: an update of therapeutic strategies. Gene. 2015;556:91–97. doi: 10.1016/j.gene.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. N. Engl. J. Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Le W-D, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson disease. Nat. Genet. 2003;33:85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J. Proteomic identification of novel proteins in cortical Lewy bodies. Brain Pathol. 2007;17:139–145. doi: 10.1111/j.1750-3639.2007.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu C-E, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y, Xu J, Xiao X, Wang J, Chai Z, Zhao Y, Deng H. Small-molecule-derived direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17:195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Linh T, Ha-Duong T. Exploring the Alzheimer amyloid-β peptide conformational ensemble: a review of molecular dynamics approaches. Peptides. 2015;69:86–91. doi: 10.1016/j.peptides.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Liu G-H, Qu J, Suzuki K, Nivet E, Li M, Montserrat N, Yi F, Xu X, Ruiz S, Zhang W, Wagner U, Kim A, Ren B, Li Y, Goebl A, Kim J, Soligalla RD, Dubova I, Thompson J, Yates J, III, Esteban CR, Sancho-Martinez I, Belmonte JCI. Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature. 2012a;491:603–607. doi: 10.1038/nature11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-L, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang C-L. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fang L, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan A-C, Zhang D, Benke TA, Sladek JR, Zahniser NR, Li C-Y. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2012b;22:321–332. doi: 10.1038/cr.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang Q, Li F, Li CY. Enhancing the efficiency of direct reprogramming of human primary fibroblasts into dopaminergic neuron-like cells through p53 suppression. Sci. China Life Sci. 2014;57:867–875. doi: 10.1007/s11427-014-4730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- Lois C, García-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzehimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Martin JB, Gusella JF. Huntington’s disease. N. Engl. J. Med. 1986;315:1267–1276. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimers disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Ruddle FH. Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell. 1976;9:45–55. doi: 10.1016/0092-8674(76)90051-9. [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi N, Nakashima H, Fehlings MG. Riluzole as a neuroprotective drug for spinal cord injury: from bench to bedside. Molecules. 2015;20:7775–7789. doi: 10.3390/molecules20057775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat. Biotechnol. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, Wu JC. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat. Protoc. 2011;6:78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Chang K-C, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J. Biol. Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, Palmer TD, Pera RR. LRRk2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang C-L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, Johnson JE, Zhang C-L. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 2015;4:780–794. doi: 10.1016/j.stemcr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noggle S, Fung H-L, Gore A, Martinez H, Satriani KC, Prosser R, Oum K, Paull D, Druckenmiller S, Freeby M, Greenberg E, Zhang K, Goland R, Sauer MV, Leibel RL, Egli D. Human oocytes reprogram somatic cells to a pluripotent state. Nature. 2011;478:70–75. doi: 10.1038/nature10397. [DOI] [PubMed] [Google Scholar]
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RDG. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech. Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Onder TT, Kara N, Cherry A, Sinha AU, Zhu N, Bernt KM, Cahan P, Mancarci BO, Unternaehrer J, Gupta PB, Lander ES, Armstrong SA, Daley GQ. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz N, Foroud T. Genetics of Parkinson disease. NeuroRx. 2004;1:235–242. doi: 10.1602/neurorx.1.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, Borkent M, Apostolou E, Alaei S, Cloutier J, Bar-Nur O, Cheloufi S, Stadtfeld M, Figueroa ME, Robinton D, Natesan S, Melnick A, Zhu J, Ramaswamy S, Hochedlinger K. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Park I-H, Arora N, Huo H, Maherali, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I-H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008b;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J. Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu G-Q, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–353. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Reinhardt P, Schmid B, Burbulla LF, Schöndorf DC, Wagner L, Glatza M, Höing S, Hargus G, Heck SA, Dhingra A, Wu G, Müller S, Brockmann K, Kluba T, Maisel M, Krüger R, Berg D, Tsytsyura Y, Thiel CS, Psathaki O-E, Klingauf J, Kuhlmann T, Klewin M, Müller H, Gasser T, Schöler HR, Sterneckert J. Genetic correction of a LRRK2 mutation in human iPSCs links Parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12:354–367. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu G-Q, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat. Neurosci. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat. Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions from amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- Shah S, Reichman WE. Treatment of Alzheimer’s disease across the spectrum of severity. Clin. Interv. Aging. 2006;1:131–142. doi: 10.2147/ciia.2006.1.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J. Clin. Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cell generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp. Neurol. 1997;148:577–586. doi: 10.1006/exnr.1997.6697. [DOI] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ. Ubiquitination of a new form of α-synuclein by parkin from human brain: implications for Parkinson’s disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- Soldner F, Laganiére J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, Zhang L, Guschin D, Fong LK, Vu BJ, Meng X, Urnov FD, Rebar EJ, Gregory PD, Zhang HS, Jaenisch R. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Kazantsev A, Spasic-Boskovic O, Greenwald M, Zhu YZ, Gohler H, Wanker EE, Bates GP, Housman DE, Thompson LM. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6763–6768. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. The biology of teratomas. Adv. Morphog. 1967;6:1–31. doi: 10.1016/b978-1-4831-9953-5.50005-6. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong L-M, Jakes R, Alberts MJ, Gilbert JR, Han S-H, Hulette C, Einstein G, Schmechel DE, Pericak-Vance MA, Roses AD. Hypothesis: microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp. Neurol. 1994;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Strübing C, Ahnert-Higler G, Shan J, Wiedenmann B, Hescheler J, Wobus AM. Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mech. Dev. 1995;53:275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu M-L, Zou Y, Zhang C-L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Van Dorpe J, Spittaeis K, Van den Haute C, Moechars D, Van Leuven F. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am. J. Pathol. 2000;156:951–964. doi: 10.1016/S0002-9440(10)64963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The HD iPSC Consortium. Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell. 2012;11:264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington’s collaborative research group. A novel gene containing a trinucleotide repeat that is expandable and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- The Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Their M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, Brüstle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tian C, Ambroz RJ, Sun L, Wang Y, Ma K, Chen Q, Zhu B, Zhang JC. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr. Mol. Med. 2012;12:126–137. doi: 10.2174/156652412798889018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Li Y, Huang Y, Wang Y, Chen D, Liu J, Deng X, Sun L, Anderson K, Qi X, Li Y, Mosley RL, Chen X, Huang J, Zheng JC. Selective generation of dopaminergic precursors from mouse fibroblasts by direct lineage conversion. Sci. Rep. 2015;5:12622. doi: 10.1038/srep12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Liu Q, Ma K, Wang Y, Chen Q, Ambroz R, Klinkebiel DL, Li Y, Huang Y, Ding J, Wu J, Zheng JC. Characterization of induced neural progenitors from skin fibroblasts by a novel combination of defined factors. Sci. Rep. 2013;3:1345. doi: 10.1038/srep01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Wang Y, Sun L, Ma K, Zheng JC. Reprogrammed mouse astrocytes retain a “memory” of tissue origin and possess more tendencies for neuronal differentiation than reprogrammed mouse embryonic fibroblasts. Protein Cell. 2011;2:128–140. doi: 10.1007/s13238-011-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Björklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal M, Bourassa P, Calon F. Can insulin signaling pathways be targeted to transport Aβ out of the brain? Front. Aging Neurosci. 2015;7:114. doi: 10.3389/fnagi.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng P-Y, Klyachko VA, Nerbonne JM, Yoo AS. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84:311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Wernig M. Molecular roadblocks for cellular reprogramming. Mol. Cell. 2012;47:827–838. doi: 10.1016/j.molcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ oligomers – a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, Giresi PG, Ng YH, Marro S, Neff NF, Drechsel D, Martynoga B, Castro DS, Webb AE, Südhof TC, Brunet A, Guillemot F, Chang HY, Wernig M. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155:621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh Y-H, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensible for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum. Mol. Genet. 2002;11:2905–2917. doi: 10.1093/hmg/11.23.2905. [DOI] [PubMed] [Google Scholar]
- Yilmazer A, de Lázaro I, Bussy C, Kostarelos K. In vivo cell reprogramming towards pluripotency by virus-free overexpression of defined factors. PLOS ONE. 2013;8:e54754. doi: 10.1371/journal.pone.0054754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow E-M. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S-C, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zuryn S, Ahier A, Portoso M, White ER, Morin M-C, Margueron R, Jarriault S. Sequential histone-modifying activities determine the robustness of transdifferentiation. Science. 2014;345:826–829. doi: 10.1126/science.1255885. [DOI] [PubMed] [Google Scholar]