Abstract
Objective
The aim of this study was to determine the levels of serum pro-brain natriuretic peptide (pro-BNP) and interleukin (IL)-6 in patients with stable chronic obstructive pulmonary disease (COPD) and to correlate these markers with health-related quality of life using the COPD assessment test (CAT).
Materials and Methods
Serum pro-BNP and IL-6 levels were measured in 82 patients with stable COPD. Serum pro-BNP and serum IL-6 levels, pulmonary function, and oxygen saturation (SpO2) were measured according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage and CAT score. Also, the associations of both pro-BNP and IL-6 with the clinical parameters of patients were tested.
Results
The serum levels of IL-6 (7.57 [5–11.16] pg/mL) and pro-BNP (120.55 [92.89–144.20] pg/mL) were higher with enhancing disease severity based on the GOLD classification (p = 0.034 and 0.068, respectively). Also, serum levels of pro-BNP (120.55 [89.50–147.90] pg/mL) and IL-6 (6.68 [4.40–11.97] pg/mL) were increased in patients with high CAT scores (p = 0.004 and 0.017, respectively). There was a significant positive correlation between plasma pro-BNP and IL-6 levels (r = 0.332, p = 0.002).
Conclusion
The results demonstrated that with increased severity of obstruction based on the GOLD criteria both IL-6 and pro-BNP were elevated. This increase in inflammatory markers was associated with a reduced quality of life and the severity of hypoxia. These findings indicated that lowering IL-6 and pro-BNP could be useful in the management of COPD patients.
Keywords: Chronic obstructive pulmonary disease, Pro-brain natriuretic peptide, Interleukin-6, COPD assessment test
Significance of the Study
This study revealed that with increasing severity of obstruction in patients with stable chronic obstructive pulmonary disease (COPD), both IL-6 and pro-brain natriuretic peptide (pro-BNP) increased. Of equal importance, a significant positive correlation was observed between IL-6 and pro-BNP serum levels. This increase in inflammatory markers was associated with a reduced quality of life and the severity of hypoxia. These findings indicate that lowering IL-6 and pro-BNP could be useful in the management of COPD patients.
Introduction
The main characteristic of chronic obstructive pulmonary disease (COPD) is a limitation of airflow that is not fully reversible but preventable and treatable with significant extrapulmonary effects [1]. Usually, the limitation of airflow in COPD is progressive and related to an abnormal inflammatory condition in response to gases and noxious particles [1]. Obstruction of airflow can lead to dyspnea, inactivity, and poor health-related quality of life [2]. Moreover, in patients with COPD, systemic inflammatory conditions might result from the spill-over of cytokines, mediators, or activated inflammatory cells from the lung to the systemic circulation or, alternatively, from peripheral tissues to the lung [3]. Several studies indicated elevated circulating levels of cytokines such as tumor necrosis factor-α (TNF-α) [4], interleukin (IL)-6 [5], and IL-8 [6] in patients with COPD. The repetitive release of cytokines in COPD probably affects the systemic consequence of COPD and health-related quality of life [3].
One of the most common problems associated with reduced quality of life in COPD patients is cardiac comorbidities [7]. Pulmonary vascular disease, coronary-artery disease, and heart failure are common comorbidities associated with COPD [7, 8]. It was shown that chronic comorbidities in COPD could affect health outcomes and that COPD patients could die from cardiac disorders [9].
One of the cardiac problems in COPD patients is right-sided heart distention that results in an increased level of serum brain natriuretic peptide (BNP). The BNP is a 32-amino acid polypeptide, and is synthesized and secreted predominantly from the right and left cardiac ventricles [10]. The main stimulus for BNP secretion is ventricular stress with pressure or volume overload. Other triggers of BNP secretion are proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 [10]. Increased levels of proinflammatory cytokines could be due to the increase in BNP in patients with a normal left ventricular function. Therefore, the purpose of this study was to evaluate serum levels of pro-BNP and IL-6 in stable COPD patients and investigate the relationship between these factors and health status using the COPD assessment test (CAT).
Subjects and Methods
Participants
In this study, 82 patients with stable COPD in Ardabil University of Medical Sciences Respiratory Clinic were consecutively enrolled from November 2015 to September 2016. The diagnosis of COPD was established based on American Thoracic Society (ATS) guidelines [11]. Inclusion criteria were a chronic obstructive pulmonary disease with a forced expiratory volume in 1 s (FEV1):forced vital capacity (FVC) ratio <70$. Based on previous studies [12, 13, 14], the exclusion criteria were potential factors that increase the level of pro-BNP, such as sepsis, pulmonary and renal dysfunction, infectious disease, recent surgery, left ventricular dysfunction, a history of hospitalization 1 month before this study, cardiac ischemia or pulmonary disorders other than COPD, and exacerbation based on a pulmonary examination. Also, for all patients, cardiac history and physical examination were performed by a cardiologist to exclude heart comorbidities.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines were used to categorize the severity of COPD as follows: stage I, mild (FEV1 ≥80$ predicted); stage II, moderate (50$ ≤ FEV1 < 80$ predicted); stage III, severe (30$ ≤ FEV1 < 50$ predicted); and stage IV, very severe (FEV1 <30$ predicted or FEV1 <50$ predicted plus chronic respiratory failure).
A history review and a physical examination were carried out and all patients completed the Persian version of the CAT respiratory questionnaire [15]. The total CAT score was calculated for each individual by summing the points of each variable. The CAT was scored from 0 to 40 and classified into 4 groups based on the impact level of the disease on health status: low, <10; medium, 10–20; high, 21–30; and very high, >30 [16]. This study was approved by the Institutional Ethics Committee and all patients provided written informed consent.
Pulmonary Function Test
A spirometer (Chest Inc., Tokyo, Japan) was used to perform the standard pulmonary function test based on the ATS standards. For each patient, 3 acceptable tests were performed and the one with the highest score was used for the analysis. Pulmonary function tests and biochemical tests were conducted on the same day.
Biochemical Measurements
Peripheral blood samples (3–5 mL) were collected in tubes containing EDTA for measuring plasma pro-BNP and IL-6. Plasma pro-BNP and IL-6 concentrations were measured using a commercial kit (Roche Diagnostics, USA) and an electrochemiluminescence method with an Elecsys 2010 Automated Analyzer (Roche Diagnostics). The results are presented in picograms per milliliter (pg/mL).
Statistical Analysis
Data are presented as the median with 25th to 75th percentiles. To examine the differences among the 3 or 4 groups, a Kruskal-Wallis test was performed. Correlation coefficients were assessed with the Spearman rank test. A p value <0.05 was considered significant.
Result
COPD Severity and Baseline Characteristics of the Study Population
Baseline parameters of the study population for the severity of COPD based on GOLD criteria are shown in Table 1. Of the 82 patients, 4.87$ had mild COPD, 40.24$ had moderate COPD, 37.80$ had severe COPD, and 17.09$ had very severe COPD. No significant differences were found for gender (p = 0.46) or smoking history (p = 0.44) with the stages of COPD.
Table 1.
COPD stage and baseline characteristics of the study population
| Variables | Stage I | Stage II | Stage III | Stage IV | P value |
|---|---|---|---|---|---|
| n | 4 | 33 | 31 | 14 | |
| Female/male | 0/4 | 10/23 | 12/19 | 5/9 | 0.466 |
| Smoking, packs per year | 30 (27.50–40) | 30 (22.50–42.5) | 35 (20–40) | 40 (30–50) | 0.444 |
| FEV1, % predicted | 92.50 (87–93) | 58 (54–70) | 39 (32–46) | 27 (25–28) | 0.000 |
| FVC, % predicted | 112.50 (103–123.50) | 75 (65–85) | 61 (53.50–69) | 44 (40–49) | 0.000 |
| FEV1/FVC, % | 65 (62.50–68) | 60 (53–67) | 50 (43–56) | 45 (39–47) | 0.000 |
| SpO2, % | 95.50 (94.50–96.50) | 93 (91–95) | 92 (88.50–94) | 87.50 (76–92) | 0.001 |
| IL-6, pg/mL | 3.17 (1.705–4.590) | 4.16 (1.50–7.79) | 6.68 (4.405–11.975) | 6.015 (3.48–8.92) | 0.034 |
| Pro-BNP, pg/mL | 69.64 (21.54–114.45) | 60.87 (32.45–131.50) | 87.01 (42.19–123.30) | 120.55 (89.50–147.90) | 0.065 |
Data are depicted as the median (25th to 75th percentiles), unless otherwise specified. FEV1, forced expiratory volume in 1 s; FVC, forced volume capacity; SpO2, O2 saturation; IL-6, interlukin-6; pro-BNP, pro-brain natriuretic peptide.
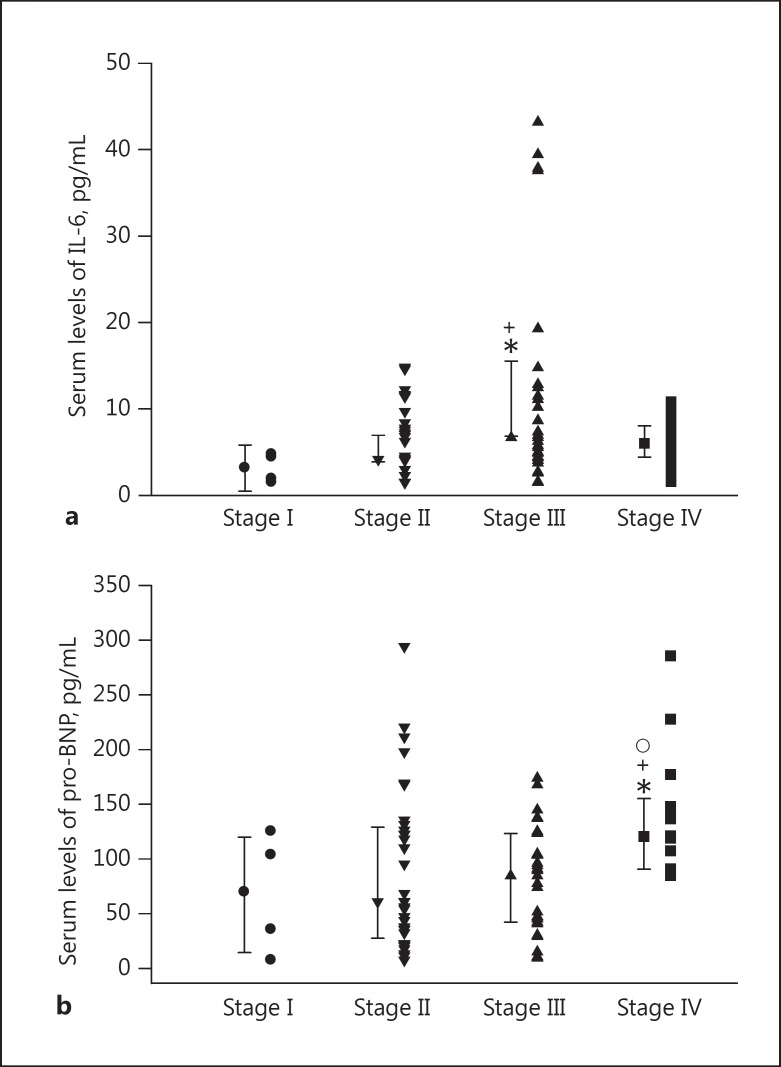
The plasma IL-6 levels were significantly higher in patients with stage III (6.68 [4.40–11.97] pg/mL) than those with stage I (3.17 [1.70–4.59] pg/mL) and stage II scores (4.16 [1.50–7.79] pg/mL, p = 0.038 and 0.015, respectively). In addition, there was no statistically significant difference in plasma IL-6 levels between stage I (3.17 [1.70–4.59] pg/mL, p = 0.078) and stage IV (6.015 [3.48–8.92] pg/mL). There was also no statistical difference between stage IV (6.015 [3.48–8.92] pg/mL) and stage II (4.16 [1.50–7.79] pg/mL, p = 0.180) or stage III (6.68 [4.405–11.975] pg/mL, p = 0.418; Fig. 1a).
Fig. 1.
Individual values and median ± 1 interquartile range of plasma IL-6 levels (a) and plasma pro-BNP levels (b) in GOLD guidelines for the severity of COPD. Statistical differences: stage I versus other stages, * p < 0.05; stage II versus stages III and IV, +p < 0.05; stage III versus stage IV, ° p < 0.05.
The plasma pro-BNP concentrations were significantly higher in stage IV (120.55 [89.50–147.90] pg/mL) compared with stage II (60.87 [32.45–131.50] pg/mL) and stage III patients (87.01 [42.19–123.30] pg/mL, p = 0.027 and 0.012, respectively). There was no statistically significant difference between the other stages in plasma pro-BNP levels (Fig. 1b).
COPD Assessment Test and Baseline Characteristics of the Study Population
The CAT data are summarized in Table 2. There were statistically significant differences for gender (p = 0.022), history of smoking (p = 0.026), FEV1 (p = 0.001), FVC (p = 0.001), oxygen saturation (SpO2; p = 0.001), IL-6 (p = 0.017), and pro-BNP (p = 0.004) with different CAT scores.
Table 2.
COPD assessment test and baseline characteristics of the study population
| Parameter | CAT II | CAT III | CAT IV | p value |
|---|---|---|---|---|
| n | 17 | 41 | 24 | |
| Female/male | 1/16 | 15/26 | 11/13 | 0.02 |
| Smoking, packs per year | 30 (20–35) | 40 (25–40) | 35 (30–0) | 0.03 |
| FEV1, % predicted | 58 (49–78) | 50 (39–60) | 30 (27–36) | 0.00 |
| FVC, % predicted | 84 (72–105) | 68 (58–78) | 49 (42–62) | 0.00 |
| FEV1/FVC, % | 62 (50–67) | 54 (49–62) | 46.50 (41–55.50) | 0.00 |
| SpO2, % | 94 (94–95) | 92 (90–94) | 88.50 (78.50–92) | 0.00 |
| IL-6, pg/mL | 4.43 (1.50–5.01) | 4.54 (1.59–8.64) | 7.575 (5–11.16) | 0.02 |
| Pro-BNP, pg/mL | 51.57 (35.58–118.20) | 68.49 (32.45–122.40) | 120.55 (92.89–144.20) | 0.00 |
Data are depicted as the median (25th to 75th percentiles), unless otherwise specified. CAT, COPD assessment test; FEV1, forced expiratory volume in 1 s; FVC, forced volume capacity; SpO2, O2 saturation; IL-6, interleukin-6; pro-BNP, pro-brain natriuretic peptide.
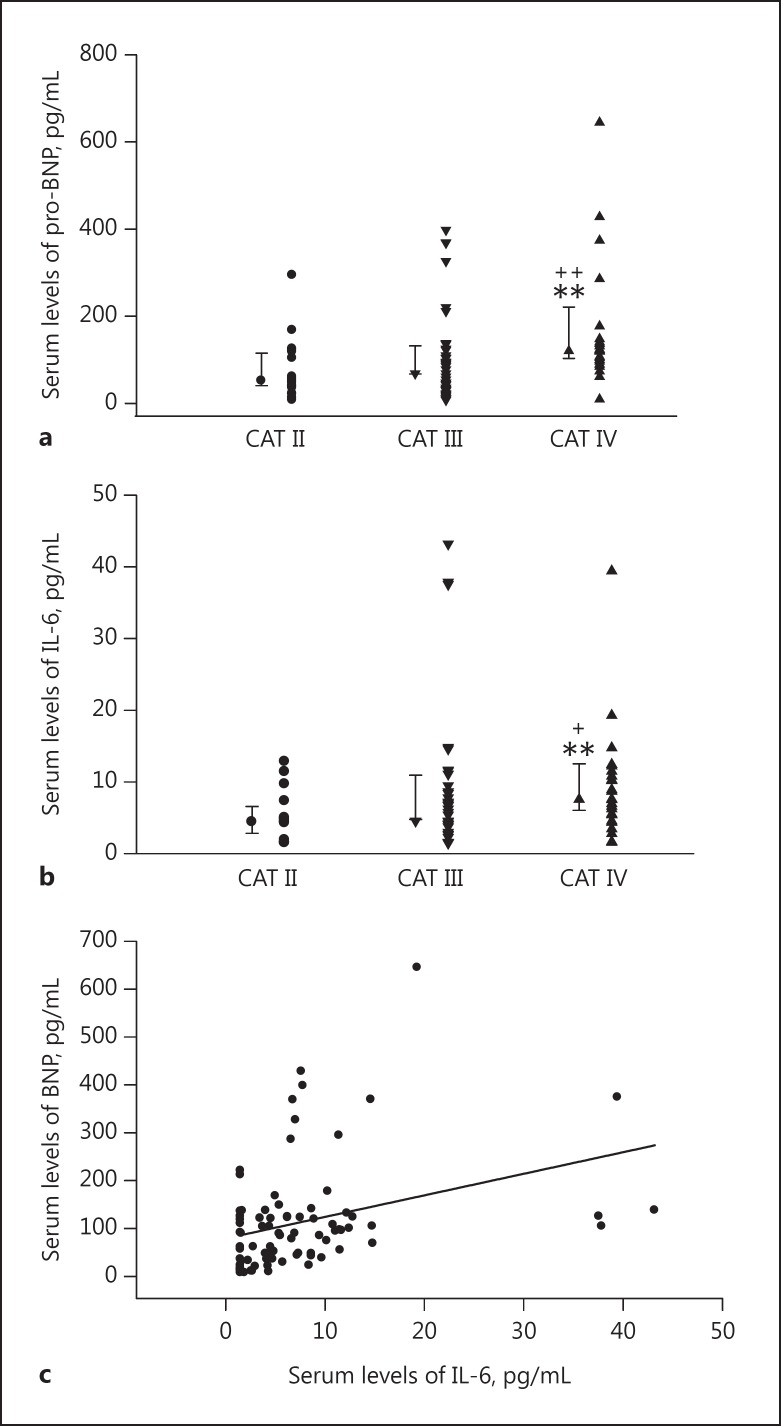
The plasma pro-BNP levels in the very high CAT score group (120.55 [92.89–144.20] pg/mL) were higher than those in the medium CAT (51.57 [35.58–118.20] pg/mL, p = 0.005) and high CAT groups (68.49 [32.45–122.40] pg/mL, p = 0.004; Fig. 2a). However, the difference between the plasma pro-BNP levels with a high or medium CAT score was not statistically significant (p = 0.657).
Fig. 2.
Individual values and median ± 1 interquartile range of plasma BNP levels (a) and plasma IL-6 levels (b) for the CAT score. Statistical differences: CAT II versus CAT III and IV, ** p < 0.01; CAT III versus CAT IV, +p < 0.05, ++p < 0.01. c Spearman correlation analysis of IL-6 and pro-BNP serum levels, showing a significant positive correlation (r = 0.332, p = 0.002).
Equally, plasma IL-6 concentrations were significantly higher in CAT group IV (7.575 [5–11.16], pg/mL) compared with CAT group II (4.43 [1.50–5.01] pg/mL, p = 0.005) and CAT group III (4.54 [1.59–8.64] pg/mL, p = 0.034). There was no significant difference between CAT group II and CAT group III (p = 0.338; Fig. 2b).
Relationship between Plasma pro-BNP and IL-6 Levels with Study Parameters
The plasma pro-BNP levels were significantly associated with pulmonary function parameters such as $FEV1 (r = −0.238, p = 0.031), $FVC (r = −0.291, p = 0.008), and GOLD stages (r = 0.230, p = 0.038). There were also significant positive correlations between plasma pro-BNP levels and age (r = 0.372, p = 0.001), smoking history (r = 0.343, p = 0.007), SpO2 (r = −0.333, p = 0.002), and CAT groups (r = 0.335, p = 0.002; Table 3).
Table 3.
Spearman correlation analysis of the study parameters with pro-BNP
| Pro-BNP |
||
|---|---|---|
| r | p value | |
| Age | 0.372 | 0.001 |
| FEV1 (% predicted) | −0.238 | 0.031 |
| FVC (% predicted) | −0.291 | 0.008 |
| FEV1/FVC | −0.218 | 0.049 |
| GOLD stage | 0.230 | 0.038 |
| SpO2 | −0.333 | 0.002 |
| CAT score | 0.354 | 0.001 |
| Smoking history | 0.343 | 0.007 |
| CAT group | 0.335 | 0.002 |
FEV1, forced expiratory volume in 1 s; FVC, forced volume capacity; SpO2, O2 saturation; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test.
In addition, we found a significant positive association between plasma pro-BNP and plasma IL-6 levels (r = 0.332, p = 0.002; Fig. 2c). There were correlations between IL-6 and FEV1 (r = −0.210, p = 0.058), GOLD stages (r = 0.244, p = 0.027), and CAT groups (r = 0.287, p = 0.009; Table 4).
Table 4.
Spearman correlation analysis of the study parameters with IL-6
| IL-6 | p value | |
|---|---|---|
| Age | 0.077 | 0.493 |
| FEV1 (% predicted) | −0.210 | 0.058 |
| FVC (% predicted) | −0.107 | 0.337 |
| FEV1/FVC | −0.230 | 0.038 |
| GOLD stage | 0.244 | 0.027 |
| SpO2 | −0.196 | 0.078 |
| CAT score | 0.344 | 0.002 |
| Smoking history | 0.141 | 0.284 |
| CAT group | 0.287 | 0.009 |
FEV1, forced expiratory volume in 1 s; FVC, forced volume capacity; SpO2, O2 saturation; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test.
Discussion
In this study, serum pro-BNP and IL-6 levels were significantly increased with disease severity based on GOLD stages in stable COPD patients, and serum pro-BNP and IL-6 levels were higher in CAT group IV than the other CAT groups. In addition, there was a positive correlation between plasma IL-6 levels and pro-BNP concentrations.
The finding that the plasma concentration of pro-BNP was associated with the degree of reduced SpO2 could probably be due to the presence of hypoxia in patients with severe COPD that leads to an increased secretion of BNP [17]. The probable mechanism of the increased BNP level during exacerbation of COPD was the hypoxia-mediated contraction of small pulmonary arterioles. The arteriole contraction could lead to an increase in pulmonary arterial pressure and the subsequent cardiac stress could cause secretion of BNP [17]. Ishii et al. [18] reported a close association between pulmonary artery pressure and BNP concentrations. These findings showed that patients with stable COPD were under right heart tension and myocardial injury. Patients with BNP secreted in large amounts and impaired cardiac function due to myocardial wall stress as a result of pressure or volume overload were very susceptible to cardiovascular events and death [19]. On the other hand, elevated plasma BNP levels have previously been reported in patients with COPD [20]. However, the increase in serum BNP concentrations is lower in patients with COPD than with cardiac heart failure [21].
The levels of IL-6 enhanced with the severity of COPD and there was a significant correlation between IL-6 and BNP. This finding showed that patients with stable COPD were also prone to heart damage caused by inflammatory markers as it was reported that COPD is an intense inflammatory disorder in the airways, pulmonary vasculature, and parenchyma [22]. In patients with COPD, chronic inflammation leads to changes in the resistance of small airways and the destruction of the alveolar wall [22]. It was also reported that the inflammation was not limited to the airways and pulmonary parenchyma, but occurs as systemic inflammation [23]. During the exacerbation of COPD, the production of proinflammatory cytokines such as IL-1β, TNF-α, IL-6, and C-reactive protein is increased [24]. Other factors involved in the secretion of BNP are proinflammatory cytokines such as IL-1β, TNF-α, and IL-6. Ma et al. [25] showed that the increased production of proinflammatory cytokines during the inflammatory response is probably responsible for the elevated secretion of BNP. Indeed, enhanced IL-6 levels and other cytokines, together with the effect of hypoxia in severe COPD, result in pulmonary arterial changes and increased BNP levels. It was shown that excessive secretion of BNP occurs as a result of the synergistic effect of IL-1β, TNF-α, and IL-6 [25].
Also, in the present study, patients with severe and very severe COPD had a high CAT score, which was similar to previous findings [26]. In a study conducted by Watz et al. [27] it was shown that physical activity decreased significantly in patients with higher stages of COPD. With an increase in CAT score, the serum levels of pro-BNP and IL-6 were statistically increased, with a marked increase for pro-BNP. A significant positive correlation was also observed between the CAT score and IL-6 and pro-BNP levels. It was reported that the reduction of physical activity in COPD patients increases inflammatory factors such as C-reactive protein, IL-6, and fibrinogen [28]. Increased IL-6 can induce a catabolic response in muscle tissues and leads to proteolysis and the degradation of protein [29]. Nevertheless, it seems that systemic inflammation is a common feature of chronic diseases such as cardiovascular diseases, diabetes, and COPD [30]. Because these conditions may be seen in some patients with COPD, their comorbidities must be noted. In the present study, the increase in pro-BNP in GOLD stages and CAT score reflect, at least in part, the effect of comorbidities on health status.
The limitations of this study include a lack of cardiac imaging, especially echocardiography, to estimate pulmonary artery pressure. Also, right heart catheterization was not carried out to determine ventricular pressure.
Conclusion
In this study, the increased severity of obstruction in patients with stable COPD raised both IL-6 as a systemic inflammatory marker and pro-BNP as a marker of heart injury. These elevations of inflammatory markers were associated with a reduced quality of life and the severity of hypoxia in patients with stable COPD. These markers in COPD patients may be involved in the pathogenesis of disease and could be considered in the management of COPD patients.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgment
This study was financially supported by Ardabil University of Medical Sciences (95/0521).
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. 2017;3:575–601. doi: 10.1111/resp.13012. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghimlas F, Al-Omran M, Guyatt GH. Linguistic validation of the Chronic Respiratory Disease Questionnaire in an Arabic-speaking population with chronic respiratory diseases. Med Princ Pract. 2011;4:387–389. doi: 10.1159/000324874. [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early intervention. COPD. 2008;4:235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- 4.Broekhuizen R, Wouters EF, Creutzberg EC, et al. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;1:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;2:210–215. [PubMed] [Google Scholar]
- 6.Spruit M, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;9:752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agusti A. Thomas A. Neff lecture. Chronic obstructive pulmonary disease: a systemic disease. Proc Am Thorac Soc. 2006;6:478–481. doi: 10.1513/pats.200603-058MS. [DOI] [PubMed] [Google Scholar]
- 8.Mannino D, Watt G, Hole D, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;3:627–643. doi: 10.1183/09031936.06.00024605. [DOI] [PubMed] [Google Scholar]
- 9.McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;5:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderheyden M, Bartunek J, Goethals M. Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail. 2004;3:261–268. doi: 10.1016/j.ejheart.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;4:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 12.Loke I, Squire IB, Davies JE, et al. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail. 2003;5:599–606. doi: 10.1016/s1388-9842(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 13.Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;4:744–750. doi: 10.1016/j.ahj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Burke MA, Cotts WG. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev. 2007;1:23–36. doi: 10.1007/s10741-007-9002-9. [DOI] [PubMed] [Google Scholar]
- 15.Ghobadi H, Ahari SS, Kameli A, et al. The relationship between COPD assessment test (CAT) scores and severity of airflow obstruction in stable COPD patients. Tanaffos. 2012;2:22–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Lari SM, Ghobadi H, Attaran D, et al. COPD assessment test (CAT): simple tool for evaluating quality of life of chemical warfare patients with chronic obstructive pulmonary disease. Clin Respir J. 2014;1:116–123. doi: 10.1111/crj.12047. [DOI] [PubMed] [Google Scholar]
- 17.Stolz D, Breidthardt T, Christ-Crain M, et al. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008;5:1088–1094. doi: 10.1378/chest.07-1959. [DOI] [PubMed] [Google Scholar]
- 18.Ishii J, Nomura M, Ito M, et al. Plasma concentration of brain natriuretic peptide as a biochemical marker for the evaluation of right ventricular overload and mortality in chronic respiratory disease. Clin Chim Acta. 2000;1:19–30. doi: 10.1016/s0009-8981(00)00312-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;7:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 20.Lang CC, Coutie WJ, Struthers AD, et al. Elevated levels of brain natriuretic peptide in acute hypoxaemic chronic obstructive pulmonary disease. Clin Sci. 1992;5:529–533. doi: 10.1042/cs0830529. [DOI] [PubMed] [Google Scholar]
- 21.Salerno D, Marik PE. Brain natriuretic peptide measurement in pulmonary medicine. Respir Med. 2011;12:1770–1775. doi: 10.1016/j.rmed.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ, Shapiro S, and Pauwels R. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;4:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 23.Gan WQ, Man S, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;7:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;4:1058–1067. doi: 10.1378/chest.06-2336. [DOI] [PubMed] [Google Scholar]
- 25.Ma KK, Ogawa T, Adolfo J. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol. 2004;4:505–513. doi: 10.1016/j.yjmcc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Amani M, Ghadimi N, Aslani MR, et al. Correlation of serum vascular adhesion protein-1 with airflow limitation and quality of life in stable chronic obstructive pulmonary disease. Respir Med. 2017;132:149–153. doi: 10.1016/j.rmed.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J. 2009;2:262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 28.Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;4:1039–1046. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- 29.Wei J, Xiong X, Lin Y, et al. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: a systematic review and meta-analysis. Peer J. 2015;3:e1199. doi: 10.7717/peerj.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnussen H, Watz H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: relation with comorbidities. Proc Am Thorac Soc. 2009;8:648–651. doi: 10.1513/pats.200906-053DP. [DOI] [PubMed] [Google Scholar]