Abstract
Objective
To determine the effect of glucose, sucrose, and saccharin on growth, adhesion, and biofilm formation of Candida albicans and Candida tropicalis.
Materials and Methods
The growth rates of mono-cultures of planktonic C. albicans and C. tropicalis and 1:1 mixed co-cultures were determined in yeast nitrogen broth supplemented with 5$ (30 mM) and 10$ (60 mM) glucose, sucrose, and saccharin, using optical density measurements at 2-h intervals over a 14-h period. Adhesion and biofilm growth were performed and the growth quantified using a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The biofilm architecture was visualized using scanning electron microscopy. One- and two-way analysis of variance (ANOVA) was performed to analyse the differences among multiple means.
Results
The highest planktonic growth was noted in 5$ glucose after 14 h (p < 0.05). No significant planktonic growth was observed in either concentration of saccharin. Both the concentrations of glucose and sucrose elicited significantly increased adhesion from MTT activity of 0.017 to >0.019 in mono- as well as co-cultures (p < 0.05), whilst the lower concentration of saccharin significantly dampened the adhesion. Maximal biofilm growth was observed in both species with the lower concentration of sucrose (5$), although a similar concentration of saccharin abrogated biofilm development: the highest MTT value (>0.35) was obtained for glucose and the lowest (>0.15) for saccharin.
Conclusion
In this study, glucose and sucrose accelerated the growth, adhesion, and biofilm formation of Candida species. However, the non-nutritive sweetener saccharin appeared to dampen, and in some instances suppress, these virulent attributes of Candida.
Keywords: Candida species, Biofilms, Adhesion, Growth sucrose, Glucose, Saccharin
Significance of the Study
In this study, the non-nutritive sweetener saccharin dampened the in vitro biofilm growth of both Candida albicans and Candida tropicalis. The finding revealed that saccharin could suppress yeast growth and biofilm formation within the oral milieu and hence indirectly contribute to oral health.
Introduction
Candida species are opportunistic fungal pathogens that inhabit 50–60$ of the oral cavities of humans [1]. Although Candida albicans is the predominant human oral commensal, other species such as Candida tropicalis are also commonly isolated from the oral niche [2, 3]. Indeed, it had been reported that 10–15$ of oral candidal infections are due to mixed Candida species, in particular C. albicans and C. tropicalis[4]. These fungi cause frequent recalcitrant oral and systemic infections particularly in compromised patient populations such as those with HIV infection and patients on immunosuppressive therapy [5].
Although a yeast of low virulence, Candida species possess a range of pathogenic mechanisms, including the ability to form complex biofilms either with other communal bacteria or yeasts on mucosal and tooth surfaces or on acrylic denture surfaces [6, 7]. Both C. albicans and C. tropicalis are known to produce extensive oral biofilms both in vitro and in vivo, and some are macroscopically visible as white, pseudo-membranes in disease such as oral thrush [8, 9, 10]. Furthermore, it is known that fermentable dietary sugars including sucrose and glucose promote Candida growth, adhesion, and biofilm development [11] and could aggravate oral diseases, in particular Candida-associated denture stomatitis and dental caries [12].
Sucrose is a naturally occurring, universally available, and widely consumed, yet highly cariogenic, dietary sugar [13]. It serves as a substrate for the synthesis of extracellular and intracellular polysaccharides of plaque biofilms [13]. The monosaccharide glucose, a by-product of sucrose fermentation, is also known to contribute equally to the development of oral biofilms [13]. Due to the diverse health concerns associated with rampant and worldwide sucrose consumption, an array of non-nutritive sweeteners such as saccharin, sucralose, aspartame, acesulfame-K, and neotame [14, 15] have been introduced in the food industry and are widely available. Non-nutritive sweeteners are known as chemosensory signalling compounds that influence digestive processes and behaviour [16]. Saccharin is by far the most widely consumed non-nutritive sweetener and is commonly recommended for patients with diabetes as an alternative to the fermentable sugar sucrose [16]. Most of the commercial toothpastes and mouth washes also contain a percentage of non-nutritive sweeteners, especially saccharine [17, 18].
Both nutritive and non-nutritive sweeteners are extensively consumed worldwide [15, 16]. Different environmental conditions such as nutritional sources can influence the activities of micro-organisms. The type of sugar and the concentration are two of the major determinants of biofilm activity of Candida species in the oral cavity [11]. The effect of both fermentable natural and non-fermentable synthetic sugars on oral biofilms should be investigated because of the increased consumption of these sugars and artificial sweeteners. Their relative impact on the growth, adhesion, and biofilm development of the opportunistic oral fungal pathogen Candida has not been studied. Hence, the objective of this study was to investigate in vitro the effect of 5 and 10$ sucrose, glucose, and saccharin on biofilm formation of C. albicans and C. tropicalis.
Materials and Methods
Candida Strains and Planktonic Growth
C. albicans and C. tropicalis (ATCC® 10231 and 13803, respectively) derived from the American Type Culture Collection [19] were used in this study. After confirmation of their identity by using a germ tube test (culture on CHROM agar and cornmeal Tween 80 agar), the yeasts were subcultured on Sabouraud dextrose agar (Sigma-Aldrich, USA) and incubated at 35°C for 48 h. The resultant growth was harvested and subcultured in yeast nitrogen broth (YNB; Sigma-Aldrich) supplemented with either 30 mM (5$) or 60 mM (10$) glucose, 15 mM (5$) or 30 mM (10$) sucrose, or 25 mM (5$) or 50 mM (10$) sodium saccharin (all Sigma Aldrich). Controls were prepared in YNB medium without adding sugars.
Planktonic growth rates of the yeasts were determined using the method initially described by Jin et al., [4, 11] with minor modifications. Briefly, suspensions of C. albicans and C. tropicalis (106 cells/mL) mono-cultures, and a co-culture of the yeasts (1:1 suspension; 106 cells/mL) were prepared in YNB and supplemented with glucose, sucrose, or saccharin. A 100-µL sample of prepared Candida cell suspensions was inoculated in triplicate into a 96-well, flat-bottom, microtiter plate. The growth rates were then determined by optical density (OD492) measurements in each well at 492 nm at 2-h intervals for 14 h using a microtiter plate reader (SpectraMax Plus 384; Molecular Devices, Inc., USA) and growth curves were prepared [8, 11].
Candidal Adhesion Studies
Standard cell suspensions (107 cells/mL) of C. albicans and C. tropicalis were prepared in sterile YNB laced with the different concentrations of glucose, sucrose, or saccharin. Both mono-species suspensions of C. albicans and C. tropicalis and a 1:1 mixture of C. albicans and C. tropicalis suspensions were prepared.
To examine the initial adhesion phase, 100 µL of the prepared standard cell suspensions were inoculated in triplicate wells of a microtiter plate and incubated for 90 min at 37°C [6, 8]. After 90 min of incubation, the plates were carefully washed twice with 200 µL sterile PBS to remove non-adherent cells, and the adherent cells were quantified using a tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich) [20].
For the MTT assay, 1 mg/mL aqueous solution of MTT was prepared. To quantify the adherent viable cell mass, 50 µL of the latter solution was added to each well and incubated at 37°C for 4 h. Afterwards, the MTT solution was carefully removed, 100 µL of dimethyl sulfoxide (DMSO; Sigma-Aldrich) was added to solubilize the produced product, and absorbance was measured at wavelengths of 570 and 630 nm.
Candidal Biofilm Growth
The growth rates of the biofilms were determined using the method described previously by Jin et al. [4, 11] with modifications. Standard cell suspensions (107 cells/mL) of C. albicans, C. tropicalis, and mixed species were prepared in sterile PBS, as described above, and 100 µL was inoculated into wells of a microtiter plate in triplicate. The plates were incubated for 90 min at 37°C to initiate the adhesion phase. Then, the plates were washed twice with 200 µL of sterile PBS and filled with 100 µL of sterile YNB supplemented with the appropriate concentrations of the sweeteners. Subsequently, the plates were incubated at 37°C for 96 h and the culture medium was replenished daily. The biofilm biomass was quantified at 24, 48, 72, and 96 h using the MTT assay, and growth curves were constructed. In addition, pH values of the biofilms were measured before and after the addition of sugars.
Scanning Electron Microscopy
In order to examine the ultrastructural features, Candida biofilms were developed on 10-mm diameter glass coverslips using the identical procedure described above. The biofilms were fixed with 2.5$ glutaraldehyde solution for 2 h and serially dehydrated with ethanol. After overnight drying in a desiccator, gold-coated biofilms were examined with scanning electron microscopy (SEM) (Hitachi SU 6600, Japan). The matured 1:1 mixed species (C. albicans and C. tropicalis) biofilm (72 h old) grown with the presence of 5$ sucrose and C. albicans biofilm grown with the presence of 5$ sucrose and 5$ saccharin were visualized using SEM [8, 21, 22]. These procedures were done in triplicate.
Statistics
One- and two-way analysis of variance (ANOVA) was performed to analyse the differences among multiple means using the Statistical Package for Social Sciences (SPSS) for windows, version 16.0 (SPSS Inc., Chicago, IL, USA) The p value was used to determine the significant differences of adhesion among sugars. p values <0.05 were considered statistically significant.
Results
Candidal Growth
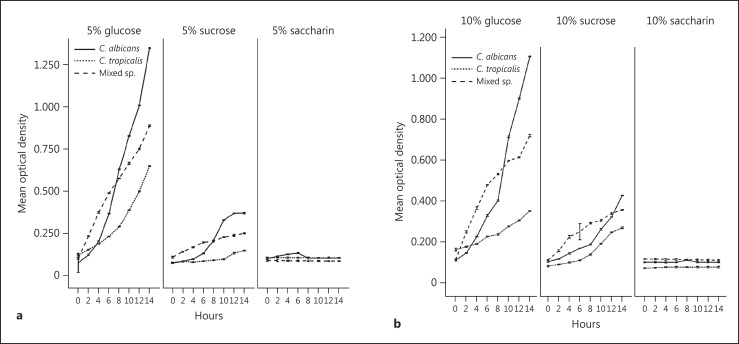
The planktonic cultures of C. albicans and C. tropicalis exhibited copious growth in the two concentrations of both sugars (glucose and sucrose), although much more profuse growth was discernible in glucose. With 5$ glucose, C. albicans exhibited a maximum growth (1.350 OD490) after 14 h followed by 5$ sucrose (0.370 OD490) and 5$ saccharine (0.104 OD490). With 10$ glucose, sucrose, and saccharin after 14 h the growth was recorded as 1.105, 0.425, and 0.100 (OD490) respectively. C. tropicalis and mixes species also exhibited a similar growth pattern.
Saccharin did not support the planktonic growth of either the mono- or co-cultures of the two species. Both species showed continuous, uninterrupted growth and achieved a maximal cell density at 14 h. In relative terms, the mono-culture growth of C. albicans was more intense than that of C. tropicalis, whilst the co-cultures demonstrated an intermediate growth profile (Fig. 1).
Fig. 1.
a Growth curves of planktonic Candida albicans and Candida tropicalis and 1:1 mixed co-culture grown in glucose, sucrose, and saccharin 5$ (mean ± 2 SD of 3 independent experiments performed in triplicate). b Growth curves of planktonic C. albicans and C. tropicalis and 1:1 mixed co-culture grown in glucose, sucrose, and saccharin 10$ (mean ± 2 SD of 3 independent experiments performed in triplicate).
Candidal Adhesion
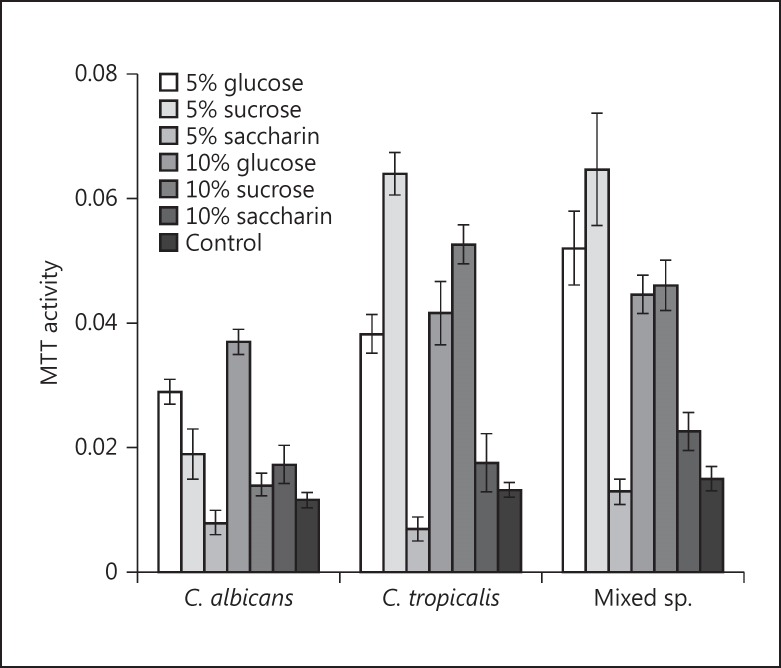
Compared to the sugar-free controls, 5$ glucose and 5$ sucrose (Fig. 2) exposure, either in mono- or co-culture, promoted the adhesion of both Candida species (p < 0.001). However, 5$ saccharin exposure significantly reduced the adhesion of C. albicans and C. tropicalis compared to the saccharin-free control (p < 0.05), although no effect was noted in the co-cultures. An increased concentration of saccharin (10$) promoted the adhesion in both the mono- and co-cultures of C. albicans (p < 0.005), but not in those of C. tropicalis (p > 0.05).
Fig. 2.
Results of the MTT assay. Adhesion of C. albicans, C. tropicalis, and mixed species in yeast nitrogen broth (YNB) supplemented with 5 and 10$ glucose, sucrose, and saccharin. In this experiment YNB without sugar was used as negative control. All error bars represent the mean ± 2 SD.
Candidal Biofilm Development
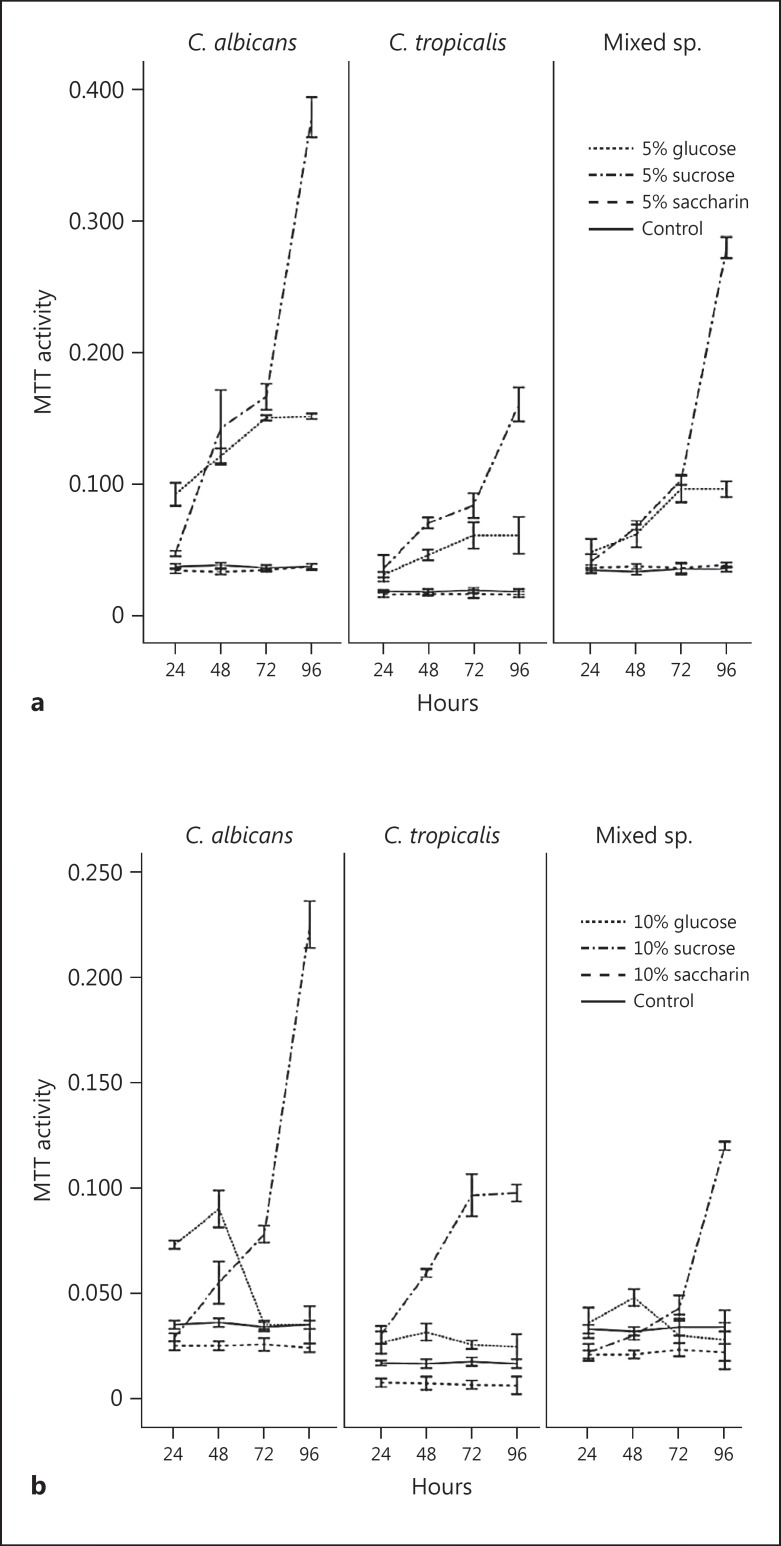
In 5$ sucrose suspensions, biofilm development in both the mono- and co-cultures of Candida was rapid with peak growth at 96 h. The sweetener-free, control suspension was devoid of biofilm growth (Fig. 3a). A similar growth pattern was noted in 10$ sucrose, except for C. tropicalis biofilm activity which appeared to plateau after 48 h (Fig. 3b). Saccharin did not support candidal biofilm development.
Fig. 3.
a Biofilm development of C. albicans, C. tropicalis, and mixed species in yeast nitrogen broth (YNB) supplemented with 5$ glucose, sucrose, and saccharin as determined by MTT assay (mean ± 2 SD of 3 independent experiments performed in triplicate). b Biofilm development of C. albicans, C. tropicalis, and mixed species in YNB supplemented with 10$ glucose, sucrose, and saccharin as determined by MTT assay (mean ± 2 SD of 3 independent experiments performed in triplicate).
Scanning Electron Microscopy
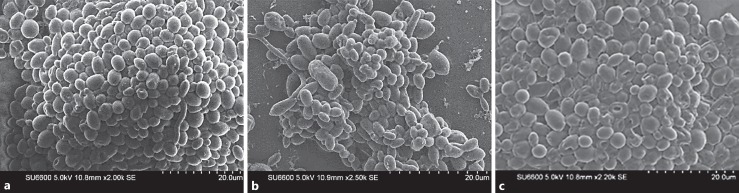
The 72-h-old biofilms of C. albicans and the 1:1 mixed species biofilms of C. albicans and C. tropicalis grown with the presence of 5$ sucrose were subjected to SEM to visualize the ultrastructure, as the highest growth was obtained with the respective concentration of sucrose. Further Candida biofilms grown for 72 h with the presence of 5$ saccharin were also subjected to SEM as it showed a reduced growth. In a comparison of biofilm formation of C. albicans and C. tropicalis, C. albicans was the most prolific biofilm former based on the SEM observations.
The mature biofilm of C. albicans demonstrated a heavy aggregate of almost equal-sized yeast blastospores with very few hyphal elements (Fig. 4a). The co-culture mixed species biofilms had a similar structure, and the species were distinguishable by virtue of the marginally larger-sized C. tropicalis blastospores (Fig. 4b). In contrast, the biofilm of C. albicans in 5$ saccharin was less dense and devoid of either hyphal or budding blastospores. Extracellular polymeric material which is characteristic of biofilms was not visualized in any of the biofilms analysed (Fig. 4c).
Fig. 4.
a Electron microscopic appearance of 72-h mature biofilms of C. albicans with 5$ sucrose. Scale, 20 µm. b Electron microscopic appearance of 72-h mature biofilms of C. albicans and C. tropicalis mixed species co-culture with 5$ sucrose. Scale, 20 µm. c Electron microscopic appearance of 72-h mature biofilms of C. albicans with 5$ saccharin. Scale, 20 µm.
Discussion
In this study, C. tropicalis adhesion was facilitated in both 5 and 10$ sucrose concentration, while C. albicans adhesion was promoted with both 5 and 10$ glucose concentrations. Although these sugars facilitate the adhesion of the two species, the overall biofilm growth of C. albicans and C. tropicalis did not get promoted with 10$ sucrose, glucose, or saccharine. In high concentrations of sugar, the water concentration is less, and this fact could act as a limiting factor for the biofilm growth of C. albicans and C. tropicalis. With the increase of sugar concentration in the medium, biofilm/micro-organism respiration and bio-synthesis rates will increase. However, in this situation water became a limiting factor due to the high sugar concentration in the surrounding media. These data are similar to previous findings of a number of researchers [23, 24, 25]. Both sugars are known to promote the production of a floccular, fibrillar layer, especially in C. albicans, which is thought to mediate adhesion and subsequent biofilm formation [26]. The adhesion of suspended, planktonic cells onto either a biotic surface such as the oral mucosa or an abiotic surface such as an acrylic denture is the initial step in biofilm formation.
Saccharin 5$ reduced adhesion of both C. albicans and C. tropicalis. However, 10$ saccharin facilitated the adhesion of both Candida species. Saccharin had a paradoxical effect on yeast adhesions, as lower concentrations (5$) of this non-nutritive sweetener reduced the adhesion of both yeast species whereas higher concentrations (10$) facilitated the adhesion of both Candida species. The attachment of Candida species to the surface is mediated by electrostatic forces and certain other non-specific interactions. Further, the contribution of a high concentration of sodium in 10$ saccharin could influence the electrostatic interactions between the cell surface and the polystyrene plate surface compared to other non-polar sugar molecules which promote candidal adhesion.
Fermentable sugars such as sucrose and glucose play a critical role in the initiation and development of major oral diseases such as caries and Candida-associated denture stomatitis. In an early study it was demonstrated that dietary carbohydrates, also termed nutritive sweeteners, including glucose and sucrose promoted adhesion and colonization of Candida species on both biotic and abiotic surfaces of the oral cavity [27]. Available data revealed that there is a strong relationship between quorum sensing, quorum sensing inhibition/quorum quenchers, and environmental and nutritional conditions including carbon source (type of sugar) [28]. Quorum sensing is a key contributory factor of microbial social behaviours and biofilm development. As regards planktonic growth of the yeasts, our data confirm the pervious findings that both glucose and sucrose promote yeast growth, and glucose is better at fostering such growth. Noteworthy, however, was the fact that saccharin did not support the planktonic growth of either the mono- or co-cultures of the two species, implying that the yeasts are incapable of metabolizing this substrate.
The finding that both glucose and sucrose facilitated candidal biofilm development confirmed the findings of a previous study [12]. Equally, the finding that C. albicans was a superior biofilm former to C. tropicalis corroborated previous findings [10] because Candida is a yeast which prefers to grow under low pH conditions, but pH 5 created by 10$ sucrose did not foster its growth. Akin to planktonic cultures, saccharin did not support biofilm growth of either Candida species.
The co-culture studies of the two yeast species confirmed that they grew well together and had the ability to form multi-species biofilms. Clinical findings have also indicated that both C. albicans and C. tropicalis could cause poly-fungal infections in the oral cavity and elsewhere [29].
The ultrastructural findings of the biofilms confirmed the classic architecture of candidal biofilms. Extracellular polysaccharide material covering the biofilms (an integral biofilm component) was not visualized probably due to a processing artefact such as high vacuum and to the electron beam of SEM. Most importantly, the candidal cells were crenate, some demonstrating pockmarks with the presence of 5$ saccharine, possibly indicating cell death due to loss of contents.
Clinically, these findings could imply that saccharin might also suppress yeast growth and biofilm formation within the oral milieu, and indirectly contribute to oral health.
Conclusion
Both glucose and sucrose accelerated the growth, adhesion, and biofilm formation of Candida species, either in mono- or co-cultures, to varying degrees. However, the widely used artificial, non-nutritive sweetener saccharin appeared to dampen, and in some instances suppress, these virulent attributes of Candida. Further studies are recommended to clarify the mechanism underlying this phenomenon, which has not been previously reported.
References
- 1.Samaranayake LP. Host factors and oral candidosis. In: Samaranayake LP, MacFarlane TW, editors. Oral Candidosis. London: Butterworth; 1990. pp. 66–103. [Google Scholar]
- 2.Sardi JC, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 3.Weerasekera MM, Sissons CH, Wong L, et al. Use of denaturing gradient gel electrophoresis for the identification of mixed oral yeasts in human saliva. J Med Microbiol. 2013;62:319–330. doi: 10.1099/jmm.0.050237-0. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y, Yip HK, Samaranayake YH, et al. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol. 2003;41:2961–2967. doi: 10.1128/JCM.41.7.2961-2967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazqhez JA. Optimal management of oropharyngeal and esophageal candidiasis in patients living with HIV infection. HIV AIDS (Aukl) 2010;2:89–101. doi: 10.2147/hiv.s6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thein ZM, Seneviratne CJ, Samaranayake YH, et al. Community lifestyle of Candida in mixed biofilms: a mini review. Mycoses. 2009;52:467–475. doi: 10.1111/j.1439-0507.2009.01719.x. [DOI] [PubMed] [Google Scholar]
- 7.Weerasekera MM, Sissons CH, Wong L, et al. Denaturing gradient gel electrophoresis profiles of bacteria from the saliva of twenty four different individuals form clusters that showed no relationship to the yeasts present. Arch Oral Biol. 2017;82:6–10. doi: 10.1016/j.archoralbio.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Weerasekera MM, Wijesinghe GK, Jayarathna TA, et al. Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion, and biofilm development. Mem Inst Oswaldo Cruz. 2016;111:697–702. doi: 10.1590/0074-02760160294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee PK, Zhou G, Munyon R, et al. Candida biofilm: a well-designed protected environment. Med Mycol. 2005;43:191–208. doi: 10.1080/13693780500107554. [DOI] [PubMed] [Google Scholar]
- 10.Bizerra FC, Nakamura CV, De Poersch C, et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8:442–450. doi: 10.1111/j.1567-1364.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y, Samaranayake LP, Samaranayake YH, et al. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789–798. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Parahitiyawa NB, Samaranayake YH, Samaranayake LP, et al. Interspecies variation in Candida biofilm formation studied using the Calgary biofilm device. APMIS. 2006;114:298–306. doi: 10.1111/j.1600-0463.2006.apm_394.x. [DOI] [PubMed] [Google Scholar]
- 13.Loesche WJ. Microbiology of dental decay and periodontal disease. In: Baron S, editor. Medical Microbiology. ed 4. Galveston: University of Texas Medical Branch at Galveston; 1996. p. 99. [PubMed] [Google Scholar]
- 14.Ibrahim OO. High intensity sweeteners chemicals structure, properties and applications. NSD. 2015;1:88–94. [Google Scholar]
- 15.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston CA, Stevens B, Foreyt JP. The role of low-calorie sweeteners in diabetes. US Endocrinol. 2013;9:96–98. doi: 10.17925/EE.2013.09.02.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okpalugo J, Ibrahim K, Inyang US. Toothpaste formulation efficacy in reducing oral flora. Trop J Pharm Res. 2009;8:71–77. [Google Scholar]
- 18.Marsh PD, Bradshaw DJ. Physiological approaches to the control of oral biofilms. Adv Dent Res. 1997;11:176–185. doi: 10.1177/08959374970110010901. [DOI] [PubMed] [Google Scholar]
- 19.American Type Culture Collection ATCC®: The essentials of life science research. 2017. https://www.atcc.org/Search_Results.aspx?dsNav=Ntk:PrimarySearch%7cmi%7c3%7c,Ny:True,Ro:0,N:1000552&searchTerms=mi&redir=115.10.2017
- 20.Tsang PW, Bandara HMHN, Fong W. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrano-Fujarte I, López-Romero E, Reyna-López GE, et al. Influence of culture media on biofilm formation by Candida species and response of sessile cells to antifungals and oxidative stress. Biomed Res Int. 2015;2015:783639. doi: 10.1155/2015/783639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forssten SD, Björklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients. 2010;2:290–298. doi: 10.3390/nu2030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCourtie J, Douglas LJ. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981;32:1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassar HM, Li M, Gregory RL. Effect of honey on Streptococcus mutans growth and biofilm formation. Appl Environ Microbiol. 2012;78:536–540. doi: 10.1128/AEM.05538-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldrop R, McLaren A, Calara F, et al. Biofilm growth has a threshold response to glucose in vitro. Clin Orthop Relat Res. 2014;471:3305–3310. doi: 10.1007/s11999-014-3538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Elteen KH. The influence of dietary carbohydrates on in vitro adherence of four Candida species to human buccal epithelial cells. Microb Ecol Health Dis. 2005;17:156–162. [Google Scholar]
- 27.Modrzewska B, Kurnatowski P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity features. Ann Parasitol. 2015;61:3–9. [PubMed] [Google Scholar]
- 28.Shrout JD, Chopp DL, Just CL, et al. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 29.Meurman JH, Siikala E, Richardson M, et al. Non-Candida albicans Candida yeasts of the oral cavity. In: Méndez-Vilas A, editor. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Badajoz: Formatex; 2007. 719 pp.731 pp. [Google Scholar]