Abstract
Objective
To determine the effect of supplementation with n-3 polyunsaturated fatty acids (PUFAs) on circulatory resistin and monocyte chemoattractant protein 1 (MCP-1) levels in type 2 diabetes mellitus (T2DM) patients.
Subjects and Methods
This was a 10-week, placebo-controlled, double-blind, randomized trial of n-3 PUFAs (2,700 mg/day) versus placebo (soft gels containing 900 mg of edible paraffin). Forty-four T2DM patients were supplemented with n-3 PUFAs and another 44 patients received placebo (3 patients discontinued the trial). Serum resistin, MCP-1, and the lipid profile were measured before and after supplementation. The adiponectin-resistin index (1 + log10 [resistin] – log10 [adiponectin]) and atherogenic index (log10 triglyceride/high-density lipoprotein cholesterol) of plasma (an indicator of cardiovascular complications) were assessed. The independent Student t test was used to assess the differences between the supplement and placebo groups and the paired t test to analyze the before/after changes.
Results
In this study, n-3 PUFAs reduced serum MCP-1 levels (from 260.5 to 230.5 pg/mL; p = 0.002), but they remained unchanged in the placebo group. n-3 PUFAs could not decrease serum resistin levels. The adiponectin-resistin index was significantly reduced after supplementation with n-3 PUFAs when compared to the placebo. The atherogenic index was also significantly improved after supplementation with n-3 PUFAs (from 1.459 to 1.412; p = 0.006).
Conclusions
The MCP-1 levels and lipid profile were improved after supplementation with n-3 PUFAs, but resistin serum levels were not changed. Hence, the anti-inflammatory effects of n-3 PUFAs might be mediated by targeting MCP-1.
Keywords: n-3 Polyunsaturated fatty acids, Type 2 diabetes mellitus, Resistin, Inflammatory cytokines, Monocyte chemoattractant protein 1
Significance of the Study
During this 10-week, placebo-controlled, double-blind, randomized trial involving patients with type 2 diabetes mellitus (T2DM), supplementation with n-3 polyunsaturated fatty acids (PUFAs) reduced the serum levels of monocyte chemoattractant protein 1 and the overexpression of chemokines that occur in T2DM. n-3 PUFA supplementation could thus improve the lipid profile of T2DM patients.
Introduction
The prevalence of both type 2 diabetes mellitus (T2DM) and obesity are increasing worldwide, supporting the possibility of their close relationship [1]. Abnormalities in inflammatory pathways and dysfunctions in adipose tissue are linked [2]. Therefore, improving inflammatory situations could be a new approach for the management of T2DM and its complications [3].
Long-chain n-3 polyunsaturated fatty acids (PUFAs), namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), decrease plasma triglyceride (TG) levels, arrhythmias, and inflammation, thereby ameliorating endothelial dysfunction [2, 4]. The beneficial effect of n-3 PUFA intake on the metabolic profile of patients with T2DM and obesity has been reported [5]. Clinical trials have shown that the consumption of n-3 PUFAs is predictive of a decrease in the cardiometabolic complications of T2DM [5]. n-3 PUFAs accumulate in adipose tissue [4], so it could be hypothesized that some of their effects might partly be associated with improvements in adipose tissue function. Moreover, n-3 PUFAs have been found to ameliorate the low-grade inflammation in adipose tissue and regulate adipokine gene expression [4, 6].
Currently, adipose tissue is recognized as a metabolically active organ [7]. The interwoven network of adipose-derived polypeptide hormones [4, 8] (e.g., adiponectin and resistin) and inflammatory cytokines (e.g., TNF-α, IL-6, and monocyte chemoattractant protein 1 [MCP- 1]) mainly contribute to the development of obesity-associated conditions (e.g., hypertension, T2DM, and atherosclerosis) [7]. Resistin is one of the important adipokines overexpressed in obesity, insulin-resistant diabetes, and T2DM via an unknown molecular mechanism [8]. Interestingly, both in vitro and in vivo studies show that administration of exogenous recombinant resistin impairs glucose tolerance and insulin action in normal-weight and glycemic-controlled animals, and anti-resistin antibody improves insulin sensitivity in obese and insulin-resistant animals [9, 10].
MCP-1 is another adipokine which is expressed by a variety of activated cells, such as in the adipose tissue, exerting a critical role in inflammatory pathways [2]. MCP-1 is overexpressed in the insulin-resistant state, obesity, and T2DM [11].
Little is known about the effect of n-3 PUFAs on the interwoven network of the metabolic-inflammatory pathway in patients with T2DM, which could potentially benefit from supplementation with n-3 PUFAs. The majority of the available studies were mostly carried out on experimental animals and provided inconsistent findings [12]. Therefore, the aim of this trial was to determine the effect of n-3 PUFAs on plasma resistin, MCP-1, and the adiponectin-resistin index.
Subjects and Methods
Trial Design
This was a single-center, randomized, double-blind, placebo-controlled clinical trial with a parallel-group design. Forty-four T2DM patients were supplemented with n-3 PUFAs and another 44 patients received placebo (3 patients discontinued the trial). The diabetic individuals were randomly allocated to 2 groups to receive n-3 PUFAs (supplement group) or placebo (placebo group). The Ethics Committee of the Tehran University of Medical Sciences approved the protocols of the trial.
Participants
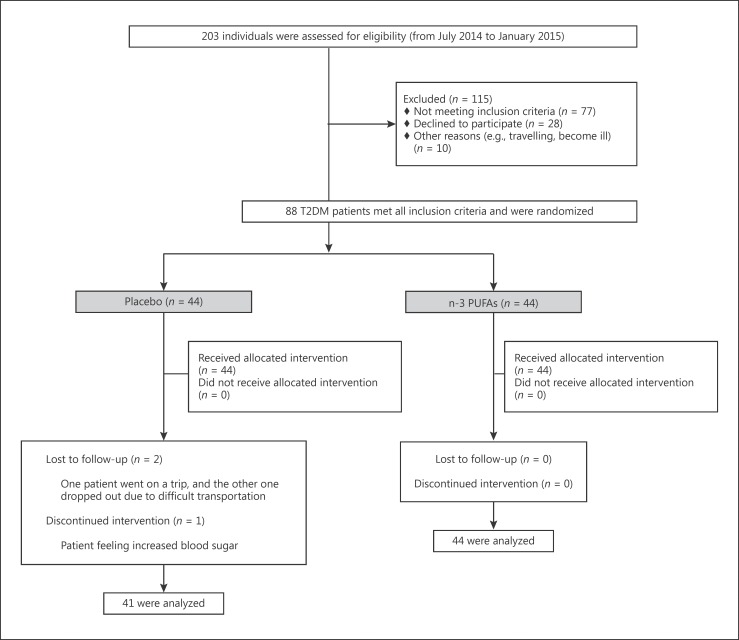
Two-hundred and three T2DM patients were initially recruited into the study from July 2014 to January 2015. All the patients were diagnosed and referred by an endocrinologist based on the criteria of the American Diabetes Association [13]. Inclusion criteria were: men aged 30–65 years and premenopausal women aged >30 years, a willingness to participate, a BMI of 25–40, and a willingness to adhere to the diet and maintain physical activity during the 10-week period of the study. Exclusion criteria were: any kind of interfering medications (such as insulin or thiazolidinediones), pregnancy or lactation, comorbidities (e.g., renal, hepatic, thyroid, and hematological disorders and malignancies), allergies to fish or fish oil, and any changes in the type or dosage of medications during the study. A total of 115 patients were excluded due to lacking eligibility (n = 77), unwillingness (n = 28), and other reasons (n = 10). The details of the characterization of the trial are shown in Figure 1. The remaining 88 T2DM patients were randomly allocated (1:1 ratio) to the supplement or placebo group, and were followed for 10 weeks until May 2015. All the enrolled individuals were informed about the purpose of the study and completed a written informed consent form. Patients were free to discontinue the study whenever they wanted.
Fig. 1.
Participants' trial profile during the study as a flow diagram.
Randomization
The permutated-block randomization method was used to allocate the patients into the 2 groups. Sequentially numbered containers were generated for concealment by a blinded statistician. Clinicians as well as participants were blinded to the randomization procedure.
Interventions
The supplement group received 3 soft gels of n-3 PUFAs with their meals for 10 weeks (each gel contained 600 mg EPA and 300 mg DHA; NUTRALAB, Canada; prepared at Zahravi Pharmaceutical Co, Tabriz, Iran). The placebo group also received 3 soft gels with the same appearance (containing 900 mg of edible paraffin; Minoo Pharmaceutical, Cosmetic and Hygienic Co., Iran). The participants were asked to maintain their routine diets and physical activity levels. Their adherence to the interventions was checked by weekly phone calls.
Outcomes and Measurements
The measurements were performed before and after supplementation. A blood sample (10 mL) was collected from all the participants after 12 h of fasting. The samples were centrifuged to separate the serum. The sera were stored at −80°C for further analyses.
Serum resistin (Mediagnost, Reutlingen, Germany) and MCP-1 (Diaclone, France) were measured using the ELISA method according to the instructions provided by the manufacturer.
Anthropometric, metabolic, and biochemical parameters were measured at baseline and after 10 weeks of supplementation with either n-3 PUFAS or placebo. A form was used to record the demographic data and medical history of each patient. Body weight and height were measured. BMI was then calculated. Serum total cholesterol, triglycerides (TG), and high-density and low-density lipoprotein cholesterol (HDL-c and LDL-c) were measured using the standard enzymatic methods with commercial kits (Pars Azmoon, Tehran, Iran). Serum adiponectin was measured using the ELISA method (Mediagnost). The detailed method was reported previously [14]. The atherogenic index of plasma is considered as: log10 TG/HDL-c [15], and the adiponectin-resistin index as: 1 + log10 (resistin) – log10 (adiponectin) [16].
Statistical Analysis
Data are presented as mean ± standard deviation (SD). The differences between the supplement and placebo groups were tested by the independent t test and the before/after changes were analyzed using the paired t test. Statistical analyses were performed using SPSS v20.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA). p values <0.05 were regarded as statistically significant.
Results
The mean age of the patients was 50.93 ± 7.27 years, and the mean BMI was 28.7 ± 4.1. There was no statistically significant difference in demographic, anthropometric, or laboratory measurements between the intervention groups before the intervention (Table 1). Overall, 85 T2DM patients (44 in the supplement group and 41 in the placebo group) successfully finished the trial. Loss to follow-up is shown in Figure 1.
Table 1.
Baseline characteristics of the type 2 diabetes mellitus patients
| Baseline characteristics | n-3 PUFAs (n = 44) | Placebo (n = 41) | p valuea |
|---|---|---|---|
| Sex, n | 0.483 | ||
| Male | 29 | 24 | |
| Female | 15 | 17 | |
| Disease duration, years | 6.93 (1.82) | 7.57 (2.2) | 0.147 |
| Age, years | 51.15 (7.45) | 50.56 (7.21) | 0.7 |
| Weight, kg | 82.56 (15.87) | 79.57 (12.94) | 0.34 |
| Height, cm | 1.67 (0.10) | 1.64 (0.09) | 0.18 |
| BMI | 29.22 (3.58) | 29.21 (2.9) | 0.99 |
| Waist-to-hip ratio | 0.95 (0.05) | 0.96 (0.07) | 0.56 |
| FBS, mg/dL | 172.11 (39.55) | 182.26 (52.48) | 0.315 |
| HbA1c, % | 7.53 (1.10) | 7.84 (1.12) | 0.207 |
All continuous values are expressed as means (SD).
Significances are based on the independent t test. FBS, fasting blood sugar; HbA1c, glycated hemoglobin.
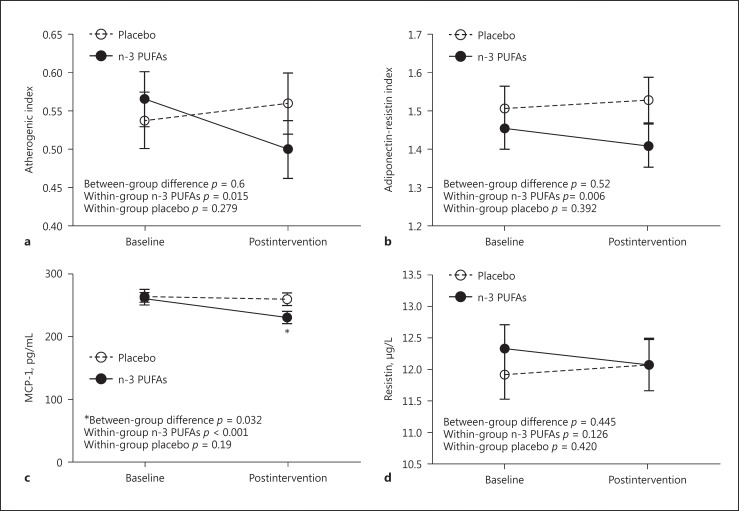
Supplementation with n-3 PUFAs significantly reduced serum MCP-1 levels (from 260.46 to 230.513 pg/mL; p < 0.001) but remained unchanged in the placebo group (Fig. 2a). The postintervention difference between the supplement and placebo groups was statistically significant (p = 0.032). However, n-3 PUFAs could not significantly decrease the serum resistin level (Fig. 2b; p = 0.126).
Fig. 2.
Postintervention/baseline of atherogenic index (a), adiponectin-resistin index (b), MCP-1 (c), and resistin (d) values in patients with type 2 diabetes mellitus treated with n-3 polyunsaturated fatty acids (n-3 PUFAs) in comparison with the placebo group. * p < 0.05.
A statistically significant reduction was observed in the adiponectin-resistin index after supplementation with n-3 PUFAs (Fig. 2c). The atherogenic index was also statistically significantly improved after supplementation with n-3 PUFAs, with no change in the placebo group (Fig. 2d).
The changes in lipid profile after intervention are shown in Table 2. A statistically significant postintervention difference (p = 0.039) was observed only for TG, but the other lipid markers remained unchanged in both groups.
Table 2.
Lipid profile of patients with type 2 diabetes mellitus before and after the 10-week period of supplementation with n-3 PUFAs or placebo
| Time point | Placebo | n-3 PUFAs | p value | |
|---|---|---|---|---|
| TG, mg/dL | baseline | 166 (71) | 172 (68) | 0.685 |
| after intervention | 171 (78) | 141 (54) | 0.039* | |
| TC, mg/dL | baseline | 203.95 (45.24) | 195.41 (63.37) | 0.479 |
| after intervention | 187.76 (46.99) | 187.39 (41.90) | 0.970 | |
| LDL-c, mg/dL | baseline | 93.803 (2.69) | 94.95 (35.47) | 0.877 |
| after intervention | 95.383 (5.25) | 99.10 (30.85) | 0.605 | |
| HDL-c, mg/dL | baseline | 45.02 (9.26) | 44.25 (11.91) | 0.740 |
| after intervention | 43.32 (10.10) | 42.55 (9.79) | 0.722 |
All values are means (SD). TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol.
p < 0.05.
Discussion
This clinical trial on T2DM patients showed that n-3 PUFA supplementation reduced the serum concentrations of MCP-1 significantly, with no effect on resistin. It also found that n-3 PUFAs significantly improved the plasma atherogenic index, which is a predictor of cardiovascular complications.
The finding that the consumption of n-3 PUFAs had a cardioprotective effect in T2DM, was safe, and was without adverse metabolic effects is consistent with another study which found that the blood pressure was lower, platelet aggregability was reduced, the risk of primary cardiac arrest was lowered, cardiovascular mortality and TG were levels decreased, HDL-c levels were increased, and there was improved endothelial function [5]. The finding that the serum concentration of MCP-1 was reduced after supplementation with n-3 PUFAs could be due to the beneficial effects of n-3 PUFAs (as outlined above) and their correlation with anti-inflammatory effects [2].
We observed that supplementation with n-3 PUFAs decreased the MCP-1 level. Since n-3 PUFAs are agonists of peroxisome proliferator-activated receptor (PPAR)-γ and PPAR-α [17], it could be reasonable to hypothesize that supplementation with n-3 PUFAs might induce the PPAR-γ/PPAR-α-dependent pathway to ameliorate the inflammatory characteristics of T2DM. Consumption of n-3 PUFAs was found to reduce early chemokines, chemokine ligand 5 (CCL5, also known as RANTES [regulated on activation normal T-cell expressed and secreted]), and MCP-1 in an animal study [17], and also in randomized controlled trials of human insulin-resistant individuals [18, 19]. However, Dewell et al. [20] showed that various doses of n-3 PUFAs do not affect MCP-1 and some other inflammatory markers in metabolic syndrome.
Resistin is a cysteine-rich protein which is mainly secreted by macrophages [8, 9]. Some studies [7, 21, 22, 23] have demonstrated the close relationship of obesity, insulin resistance, and inflammation. They showed that adipokines, such as resistin, are molecular links of the action of adipocytes and macrophages [24]. Therefore, resistin might provide a unique link between the risk of obesity, inflammation, and metabolic syndrome in humans [21]. It has been reported that serum resistin levels are significantly higher in diabetic patients than in healthy individuals, and that this is negatively correlated with insulin sensitivity [25, 26]. Moreover, positive associations have been demonstrated between serum resistin and BMI, insulin resistance, and diabetes [26]. Numerous inflammatory factors upregulated by resistin are well-recognized mediators involved in the development of insulin resistance. Hence, it seems reasonable that resistin could play a role in the pathogenesis of T2DM [26].
The finding of a nonsignificant reduction in resistin level after n-3 PUFA supplementation is contrary to the previous in vitro and animal studies that indicated the efficacy of n-3 PUFAs in reducing resistin levels [27, 28]. The finding regarding the significantly reduced MCP-1 and the nonsignificant amelioration of resistin after the intervention could be due to the fact that these cytokines are secreted from various sources. The majority of secreted MCP-1 is from the macrophages, although resistin is predominantly produced by adipocytes. Therefore, we hypothesized that following patients for a few additional weeks may also reveal the effect of n-3 PUFAs on resistin; this necessitates long-term trials.
The plasma atherogenic index was also significantly improved after supplementation with n-3 PUFAs, but there was no change in the placebo group. It has been demonstrated that n-3 PUFAs can significantly improve the plasma atherogenic index of T2DM patients [15]. The high plasma level of the atherogenic index is linked with the small LDL particle size, which is itself is a predictor of conditions like obesity, hyperinsulinemia, and inflammatory status. Coronary artery disease, T2DM, hypertension, and metabolic syndrome have all been linked with a high plasma atherogenic index [15].
Moreover, we observed that n-3 PUFAs could significantly ameliorate the adiponectin-resistin index, which is a better marker than the individual adiponectin and resistin level [16]. Adiponectin and resistin are endocrine hormones that play principal roles in the regulation of energy, glycemic status, and lipid homeostasis. The overall similarity of resistin and adiponectin regarding their multimeric assembly structure, along with their opposing physiological effects, indicates a common regulatory mechanism in metabolic homeostasis [16]. This mechanism is represented by the adiponectin-resistin index, which was able to show the modest beneficial change exerted by n-3PUFAs in our T2DM patients. The limitation of this study was the short follow-up period.
Conclusion
In this study, n-3 PUFA supplementation for 10 weeks reduced the serum level of MCP-1 in T2DM patients but had no effect on the resistin level. We revealed that the anti-inflammatory effects of n-3 PUFAs might be mediated by targeting MCP-1. More studies are needed to explain the exact molecular mechanisms involved in such beneficial roles of n-3 PUFAs, focusing particularly on their effects on the expression of the peroxisome proliferator-activated receptor (PPAR) genes as well as experimenting with different doses and durations of supplementation.
Disclosure Statement
There were no conflicts of interest.
References
- 1.Abranches MV, de Oliveira FCE, da Conceição LL, et al. Obesity and diabetes: the link between adipose tissue dysfunction and glucose homeostasis. Nutr Res Rev. 2015;28:121–132. doi: 10.1017/S0954422415000098. [DOI] [PubMed] [Google Scholar]
- 2.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal NK, Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5:697–710. doi: 10.4239/wjd.v5.i5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostowik M, Gajos G, Zalewski J, et al. Omega-3 polyunsaturated fatty acids increase plasma adiponectin to leptin ratio in stable coronary artery disease. Cardiovasc Drug Ther. 2013;27:289–295. doi: 10.1007/s10557-013-6457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nettleton JA, Katz R. n-3 Long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–440. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Moreno-Aliaga MJ, Lorente-Cebrián S, Martínez JA. Regulation of adipokine secretion by n-3 fatty acids. Proc Nutr Soc. 2010;69:324–332. doi: 10.1017/S0029665110001801. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Pronyuk K, Kuliesh O, et al. Adiponectin, resistin and leptin: possible markers of metabolic syndrome. Endocrinol Metab Syndr. 2015;4:212. [Google Scholar]
- 8.Gharibeh M, Al Tawallbeh G, Abboud M, et al. Correlation of plasma resistin with obesity and insulin resistance in type 2 diabetic patients. Diabetes Metab. 2010;36:443–449. doi: 10.1016/j.diabet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Jamaluddin MS, Weakley SM, Yao Q, et al. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pravenec M, Kazdová L, Landa V, et al. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem. 2003;278:45209–45215. doi: 10.1074/jbc.M304869200. [DOI] [PubMed] [Google Scholar]
- 11.Chow F, Nikolic-Paterson D, Ma F, et al. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 12.Wanders D, Plaisance EP, Judd RL. Pharmacological effects of lipid-lowering drugs on circulating adipokines. World J Diabetes. 2010;1:116–128. doi: 10.4239/wjd.v1.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazaherioun M, Saedisomeolia A, Javanbakht MH, et al. Beneficial effects of n-3 polyunsaturated fatty acids on adiponectin levels and AdipoR gene expression in patients with type 2 diabetes mellitus: a randomized, placebo-controlled, double-blind clinical trial. Arch Med Sci. 2017;13:716–724. doi: 10.5114/aoms.2016.62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onat A, Can G, Kaya H, et al. “Atherogenic index of plasma” (log10 triglyceride/high- density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4:89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Lau C-H, Muniandy S. Novel adiponectin-resistin (AR) and insulin resistance (IR AR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study. Cardiovasc Diabetol. 2011;10:8. doi: 10.1186/1475-2840-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-κB activation via a PPARα-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 18.Spencer M, Finlin BS, Unal R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62:1709–1717. doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung AM, Booker C, Ellis CD, et al. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol Dial Transpl. 2014:gfu283. doi: 10.1093/ndt/gfu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewell A, Marvasti FF, Harris WS, et al. Low-and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141:2166–2171. doi: 10.3945/jn.111.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocr Diab. 2006;114:544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 22.Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul. 2010;44:25–36. doi: 10.4149/endo_2010_01_25. [DOI] [PubMed] [Google Scholar]
- 23.Tokuyama Y, Osawa H, Ishizuka T, et al. Serum resistin level is associated with insulin sensitivity in Japanese patients with type 2 diabetes mellitus. Metabolism. 2007;56:693–698. doi: 10.1016/j.metabol.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, et al. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167:228–256. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 25.McTernan PG, Fisher FM, Valsamakis G, et al. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab. 2003;88:6098–6106. doi: 10.1210/jc.2003-030898. [DOI] [PubMed] [Google Scholar]
- 26.Park HK, Ahima RS. Resistin in rodents and humans. Diabetes Metab J. 2013;37:404–414. doi: 10.4093/dmj.2013.37.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding WJ, Wang Y, Fan JG. Regulation of adipokines by polyunsaturated fatty acids in a rat model of non-alcoholic steatohepatitis. Arch Iran Med. 2014;17:563–568. [PubMed] [Google Scholar]
- 28.Haugen F, Zahid N, Dalen KT, et al. Resistin expression in 3T3-L1 adipocytes is reduced by arachidonic acid. J Lipid Res. 2005;46:143–153. doi: 10.1194/jlr.M400348-JLR200. [DOI] [PubMed] [Google Scholar]