Abstract
Background
Residual renal function (RRF) provides several benefits to patients on dialysis. Monocyte chemoattractant protein-1 (MCP-1) plays an important role in atherosclerotic lesions. We considered the relationship between RRF and cardiovascular morbidity and the significant role of MCP-1 serum concentrations in hemodiafiltration (HDF) patients.
Methods
We enrolled 76 patients on on-line HDF. RRF was defined by interdialytic urine output, and we studied the patients in two groups according to the preservation or not of urine output. MCP-1 levels were measured using enzyme-linked immunosorbent assay. χ2 tests were applied for the association between RRF and left ventricular hypertrophy (LVH), coronary artery disease (CAD), peripheral artery disease (PAD), and systolic and diastolic cardiac dysfunction. We built an adjusted model using logistic regression analysis for the factors which might impact on the loss of urine output.
Results
χ2 tests showed a significant association between the loss of urine output and LVH, diastolic dysfunction, and PAD (χ2 = 7.4, p = 0.007; χ2 = 14.3, p = 0.001; χ2 = 4.2, p = 0.03, respectively), although the association with CAD and systolic dysfunction was found to be nonsignificant. The patients without RRF had significantly higher MCP-1, and the urine volume was inversely associated with MCP-1 (r = −465, p = 0.03). In the built adjusted model, the elevated MCP-1 was found to be a significant predictor for the loss of RRF.
Conclusion
The loss of RRF was significantly associated with LVH, diastolic dysfunction, and PAD in HDF patients. The increased MCP-1, affected by the lack of urine, may act as an additional underlying factor on this relationship, reflecting a progressive inflammation/oxidative stress condition.
Keywords: Cardiovascular disease, Hemodiafiltration, Inflammation, Monocyte chemoattractant protein-1, Residual renal function
Introduction
Residual renal function (RRF) may provide many benefits to patients on permanent renal replacement therapy. These benefits are related to better volume control and greater solute clearance, playing a crucial role in the achievement of a sufficient dialysis treatment [1, 2]. Moreover, RRF is accompanied by more phosphate urinary excretion, better control of secondary hyperparathyroidism, improved nutritional markers, and better endogenous vitamin D and erythropoietin production [3]. Therefore, RRF preservation contributes considerably to the improvement of quality of life, cardiovascular protection, and even better survival in this patient population [4].
Previous studies have reported the impact of RRF on patient outcomes in peritoneal dialysis (PD), even though most patients with end-stage renal disease worldwide are treated with hemodialysis (HD) [5, 6]. However, few studies examined the role of RRF on outcomes in HD patients, due mainly to difficulties with accurate interdialytic urine collection from HD patients. Moreover, a faster decline in RRF is noticed in thrice weekly conventional HD compared with PD, which is attributable to common intradialytic hypotension and abrupt volume depletion in HD [7]. However, the use of on-line hemodiafiltration (HDF) with high-flux biocompatible membranes and ultrapure water for dialysate may preserve RRF longer, due partly to less sodium removal compared with conventional HD, resulting in less intradialytic hypotension and improved intradialytic cardiovascular stability [8, 9].
In spite of the advances in HD procedure, mortality remains high in patients undergoing HD compared with the general population due to accelerated atherosclerosis, resulting in a higher risk of cardiovascular death [10]. Atherosclerosis and cardiovascular disease in this patient population are mainly affected by nontraditional risk factors, including persistent low-grade inflammation, metabolic acidosis, volume overload, and comorbidities. The causes of inflammation are multifactorial and include imbalance between increased production and decreased removal of pro-inflammatory cytokines, oxidative stress, factors linked to dialysis treatment, and chronic and recurrent infections related to dialysis access [11].
Monocyte chemoattractant protein-1 (MCP-1), a monomeric polypeptide, is the main representative of chemokines, which are cytokines whose main function is the direction of circulating leukocytes to sites of inflammation. MCP-1, which may be related to the low-grade inflammation in patients undergoing HD, acts as a chemoattractant specific for monocytes and may promote migration of monocytes into the atherosclerotic plaque after their initial adhesion to the endothelium, playing a particular role in the lesions of atherogenesis [12].
In this study, we considered the association of RRF with cardiovascular morbidity in relation to the potential role of MCP-1 serum concentrations in permanent HDF treatment patients.
Subjects and Methods
Subjects
This was a dual-center observational cross-sectional study of a cohort of 76 patients on permanent dialysis therapy. We enrolled 47 men and 29 women, mean age 62.2 ±15 years, permanently treated with predilution on-line HDF. The median time on HDF treatment was 5 years (interquartile range 3–10 years).
The HDF treatment was prescribed 3 times weekly for 4 h per session in all enrolled subjects. Similar dialysis conditions were applied in all participants, including a dialysis high-flux filter of 1.5 m2 surface area defined by a ultrafiltration coefficient >20 mL/h [13] and of the same synthetic membrane material. The blood flow was 400 mL/min during HDF sessions and the dialysate flow rate was 500–600 mL/min. The volume of replacement liquids during HDF was 20 L per session and the sodium concentration was 140 mmol/L. A bicarbonate-based ultrapure buffer solution was used, with a final concentration of bicarbonate in dialysate of 32 mmol/L. The used calcium dialysate concentration was 1.50–1.75 mmol/L. Dialysis sufficiency was defined by spKt/V/session (sp, single pool; K, dialyzer clearance; t, time; V, urea distribution volume) [14]. Patients whose calculated spKt/V/session was <1.2 were excluded from the study.
Moreover, we excluded patients younger than 18 years of age at initiation of HD treatment, those with less than 6 months of follow-up, and patients with autoimmune diseases and infections as well as those without regular vascular HD access. We also excluded patients with multiple intradialytic hypotensive episodes, atrial fibrillation, and interdialytic weight gain >5$ of total body weight. Interdialytic weight gain was calculated as the mean of 12–13 HDF sessions during a treatment month. The included patients did not have interdialytic peripheral edema or interdialytic orthostatic hypotension.
Patients with a predialysis blood pressure ≥140/90 mm Hg (n = 29, 38.2$) were considered hypertensive and were receiving antihypertensive drugs, including calcium channel blockers, beta-blockers, or inhibitors of angiotensin II receptors. Only calcium-free phosphate binders were prescribed. None of our patients was receiving statin or NaHCO3 per os. The total of enrolled subjects were regularly treated with an erythropoietin-α or erythropoietin-β agent in combination with oral trivalent iron (ferric hydroxide polymaltose complex).
Nineteen (25$) of the studied patients were current tobacco smokers. Twenty subjects (26.3$) presented RRF (defined by an interdialytic urine volume >100 mL).
Cardiovascular disease was defined as the prevalence of left ventricular hypertrophy (LVH; n = 46, 60.5$), coronary artery disease (CAD; n = 25, 32.9$), and congestive heart failure (CHF). Coronary syndrome was documented by clinical signs of angina pectoris as well as a history of myocardial infarction and coronary artery angioplasty or bypass surgery. CHF was defined by systolic (n = 22, 28.9$) and diastolic dysfunction (n = 46, 60.5$). Prevalence of peripheral artery disease (PAD; n = 31, 40.8$) was documented by both measurement of ankle-brachial index (ABI) and clinical criteria including reduced or lost ankle pulses on physical examination, intermittent claudication, and a history of past ischemic amputation or limb artery revascularization. The first and the current cardiovascular events during the study were recorded as one event.
In our data, the primary renal disease included hypertensive nephrosclerosis (32.9$), chronic glomerulonephritis (28.9$), polycystic disease (11.8$), diabetic nephropathy (9.2$), and other causes (17.1$).
Blood Collection
Before the start of the weekly HDF sessions, blood samples were obtained from the enrolled patients in a 12-h fasting state from the vascular access. Serum was separated and processed for various assays. At the end of the sessions, blood samples was obtained at 2 min postdialysis from the arterial dialysis tubing after reduction of the blood pump speed to <80 ml/min for the calculation of spKt/V/session. The mean of 12–13 calculations of spKt/V during a treatment month was used for statistical analysis.
Laboratory Measurements
Albumin, calcium (Ca) corrected for the albumin levels, phosphate (P), glucose, and the ratio of low-density lipoproteins to high-density lipoproteins were measured by spectrophotometric technique using Chemistry Analyzer (MINDRAY BS-200; Diamond Diagnostics, USA). The products of Ca × P were calculated. Hematological analyzer (Sysmex, xt-4000i; Roche) was used for hemoglobin measurement.
High-sensitivity C-reactive protein (hsCRP) and MCP-1 serum concentrations were measured using enzyme-linked immunosorbent assay (Immundiagnostik AG, Germany, and Alpco Diagnostics, USA, respectively) according to the manufacturer's specifications.
MCP-1 values were measured in the enrolled subjects and in 24 healthy subjects, who had similar age and body mass index (BMI) to those of the group of patients.
The concentrations of intact parathormone (iPTH) and insulin were measured by radioimmunoassays (CIS bio international, France and BioSource Europe SA, Belgium, respectively).
Insulin resistance was calculated using the homeostatic model assessment of insulin resistance (HOMA-IR) [15].
The normalized protein catabolic rate for dry body mass was calculated from the urea generation rate [16]. The BMI was obtained from height and postdialysis body weight.
Hemodynamic Measurements
Predialysis peripheral systolic (SBP) and diastolic blood pressure (DBP) were calculated as the mean of 12–13 measurements during a treatment month using an automatic sphygmomanometer (Omron M4-I; Omron Co Ltd, Kyoto, Japan). Mean peripheral predialysis BP (MBP) was calculated as follows: MBP = DBP + 1/3 (SBP – DBP).
Before the midweek HDF session, hemodynamic measurements were performed in the enrolled subjects after resting for at least 10 min. Arterial stiffness was measured as carotid-femoral pulse wave velocity (cfPWV) and carotid augmentation index using the SphygmoCor system® (AtCor Medical Pty Ltd, Sydney, Australia) according to the manufacturer's specifications. In each subject, two sequences of measurements were performed, and their mean was used for statistical analysis. Pulse pressure (PP) was derived. The ABI was also calculated as the lower values of ankle systolic pressure (pre- or posttibial artery) divided by stabilized arm systolic pressure. ABI values <0.9 were rated as low, indicating PAD, and values >1.4 were rated as high.
Echocardiographic Assessment
Echocardiographic assessment was conducted using a Hewlett Packard SONOS 2500 device with a 2.25-MHz transducer. The patients were examined by two cardiologists with the method of conventional M-mode and two-dimensional echocardiography for LVH estimation, systolic and diastolic cardiac function assessment, and consideration of the ischemic findings according to the recommendations of the American Society of Echocardiography [17]. LVH was defined by a thickness of the interventricular septum >11.5 mm. The systolic function of the left ventricle (LV) was assessed by measurement of the ejection fraction, and systolic dysfunction was defined as an ejection fraction <50$. The diastolic function of the LV was assessed by determination of the maximum velocity of the early (E) and late (A) phase of ventricular filling and the calculation of the E/A ratio (ratio of early to late transmitral flow velocity). Diastolic dysfunction of the LV was defined as an E/A ratio ≤1.
Data Analysis
Data were analyzed using the SPSS 15.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA) and expressed as mean ± standard deviation or as median value ± interquartile range for data that showed skewed distribution. Differences between mean values were assessed by using the unpaired t test for two groups, and data that showed skewed distributions were compared with the Mann-Whitney U test. Correlations between variables were defined by Spearman coefficient and the relationships between categorical variables were defined by χ2 tests. p values <0.05 were considered significant. We built a model using logistic regression analysis for the factors which might impact on the loss of RRF in our data. We also built a model by linear regression analysis examining the factors which might influence the levels of MCP-1, making a control for multi-collinearity.
Results
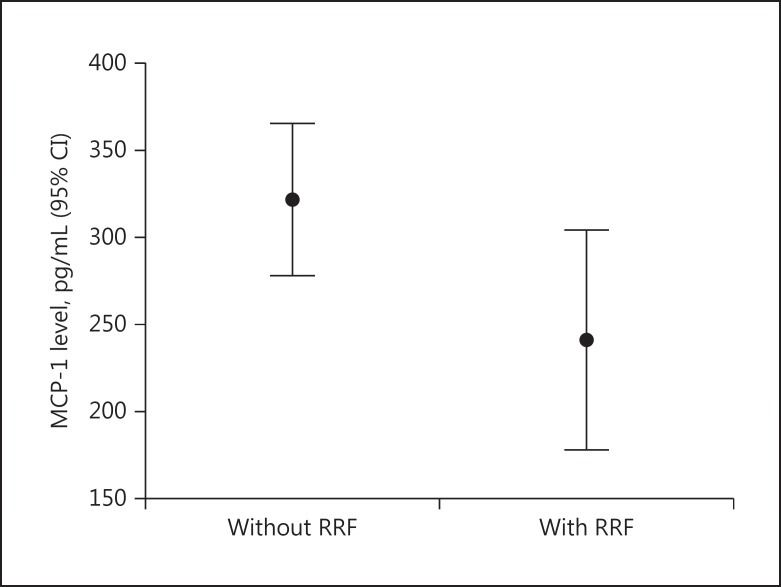
In Table 1, the differences between the groups of patients with preserved RRF (n = 20) and without preserved RRF (n = 56) are shown. The subjects in both groups had similar age, dialysis treatment duration, dialysis sufficiency defined by spKt/V for urea, and interdialytic weight gain. However, the patients without preserved RRF had significantly higher interventricular septum thickness and MCP-1 serum concentrations (Fig. 1) and a significantly lower E/A ratio than the patients with preserved RRF. The same group of subjects also had higher insulin levels, HOMA-IR, hsCRP, iPTH, Ca × P products, cfPWV, and PP compared to the group with preserved urine volume.
Table 1.
Differences between groups of patients according to preserved residual renal function in a total of 76 patients enrolled in the study
| Patients with preserved RRF (n = 20), n (%), mean ± SD, or mean rank | Patients without preserved RRF (n = 56), n (%), mean ± SD, or mean rank | p value | |
|---|---|---|---|
| Sex, males/females | 9 (45%)/11 (55%) | 38 (68%)/18 (32%) | 0.07 |
| Age, years | 59.8 ± 13.4 | 64.1 ± 14.3 | 0.2 |
| Dialysis vintage, years | 37.92 | 38.71 | 0.9 |
| Kt/V for urea | 41.48 | 37.44 | 0.5 |
| nPCR, g/kg/day | 2.2 ± 0.5 | 2.4 ± 0.5 | 0.1 |
| Interdialytic urine volume, mL | 211 ± 143 | – | – |
| Body mass index | 24.5 ± 3.0 | 24.4 ± 3.1 | 0.9 |
| Interdialytic weight gain, kg | 2.1 ± 1.1 | 2.2 ± 0.9 | 0.8 |
| Intact parathormone, pg/mL | 36.82 | 43.20 | 0.3 |
| Calcium corrected for albumin, mg/dL | 9.5 ± 0.8 | 9.4 ± 0.6 | 0.5 |
| Phosphate, mg/dL | 5.3 ± 1.5 | 5.7 ± 2.1 | 0.3 |
| Calcium × phosphate product | 50.5 ± 17.4 | 54.1 ± 19.4 | 0.4 |
| Hemoglobin, g/dL | 11.9 ± 1.3 | 11.8 ± 1.3 | 0.8 |
| Albumin, g/dL | 40.2 | 37.9 | 0.7 |
| Low-density lipoprotein/high-density lipoprotein | 2.2 ± 0.8 | 2.3 ± 0.9 | 0.5 |
| Glucose, mg/dL | 93.9 ± 15.8 | 96.5 ± 28.3 | 0.6 |
| Insulin, µU/mL | 36.5 | 39.3 | 0.6 |
| HOMA-IR, mmol/L | 37.5 | 38.8 | 0.8 |
| Monocyte chemoattractant protein-1, pg/mL | 30.1* | 41.5 | 0.04 |
| High-sensitivity C-reactive protein, mg/L | 6.8 ± 4.7 | 8.4 ± 6.2 | 0.3 |
| Carotid-femoral pulse wave velocity, m/s | 11.2 ± 1.8 | 11.3 ± 1.9 | 0.9 |
| Augmentation index, % | 24.1 ± 2.08 | 24.4 ± 2.5 | 0.6 |
| Pulse pressure, mm Hg | 56.5 ± 18.6 | 62.9 ± 20.9 | 0.2 |
| Mean peripheral predialysis BP, mm Hg | 97.7 ± 12.5 | 100.5 ± 13.2 | 0.4 |
| Ankle-brachial index | 1.1 ± 0.3 | 1.1 ± 0.5 | 0.9 |
| Interventricular septum thickness, mm | 18.4* | 45.7 | 0.001 |
| EF, % | 42.7 | 37.01 | 0.3 |
| E/A ratio | 57.03* | 31.9 | 0.001 |
| Diabetics/nondiabetics | 1 (5%)/19 (95%) | 6 (10.7%)/50 (89.3%) | 0.3 |
| Smoking, yes/no | 6 (30%)/14 (70%) | 13 (23.2%)/43 (76.8%) | 0.8 |
| Left ventricular hypertrophy, yes/no | 7 (35%)/13 (65%)* | 39 (70%)/17 (30%) | 0.007 |
| Coronary artery disease, yes/no | 9 (45%)/11 (55%) | 16 (29%)/40 (71%) | 0.5 |
| Diastolic dysfunction, yes/no | 5 (25%)/15 (75%)* | 41 (73%)/15 (27%) | 0.001 |
| Systolic dysfunction (EF <50%), yes/no | 4 (20%)/16 (80%) | 18 (32%)/38 (68%) | 0.4 |
| Peripheral artery disease, yes/no | 12 (60%)/8 (40%)* | 19 (34%)/37 (66%) | 0.03 |
| Hypertension, yes/no | 10 (50%)/10 (50%) | 19 (34%)/37 (66%) | 0.6 |
BP, blood pressure; EF, ejection fraction; HOMA-IR, homeostatic model assessment of insulin resistance; nPCR, normalized protein catabolic rate for dry body mass; RRF, residual renal function; SD, standard deviation.
p < 0.05.
Fig. 1.
Monocyte chemoattractant protein-1 (MCP-1) levels in patients without and with residual renal function (RRF) (p = 0.04). CI, confidence interval.
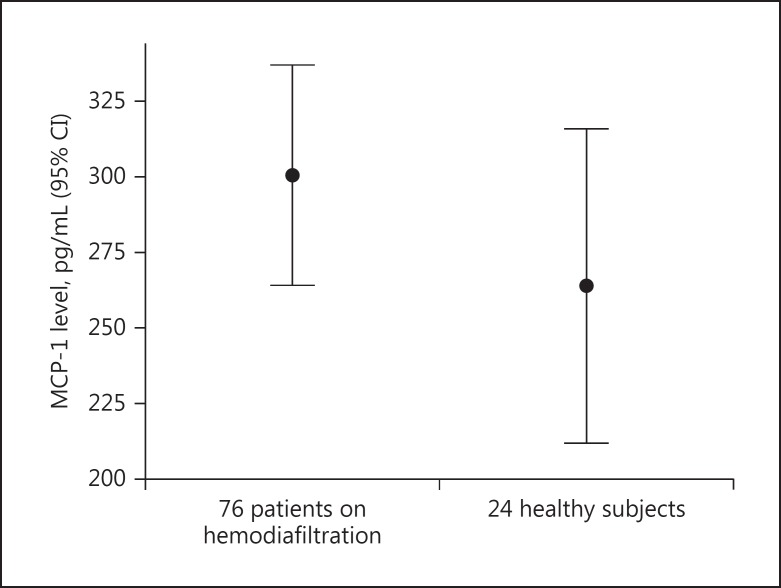
The difference in MCP-1 levels between patients and healthy subjects was found to be nonsignificant by the Mann-Whitney U test, as MCP-1 values showed skewed distribution (mean rank 52.2 vs. 45.04, p = not significant; Fig. 2).
Fig. 2.
Monocyte chemoattractant protein-1 (MCP-1) levels in the enrolled patients (n = 76) and in the healthy subjects (n = 24) (p = not significant).
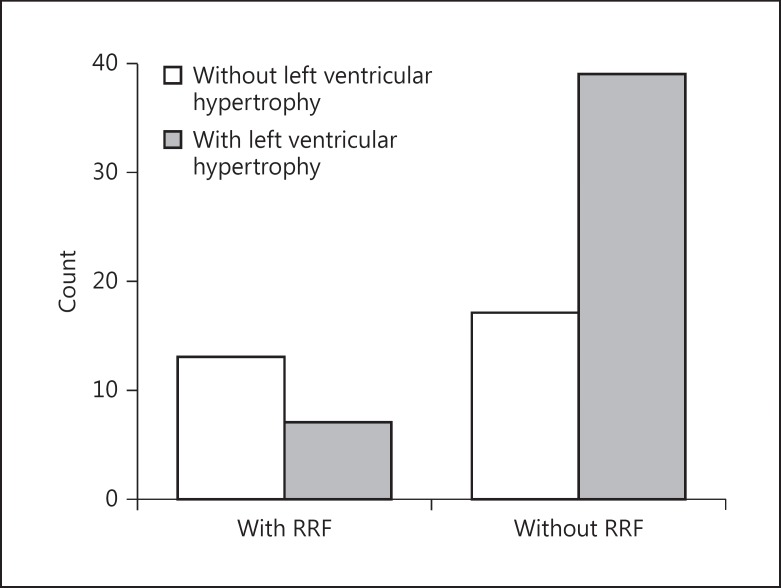
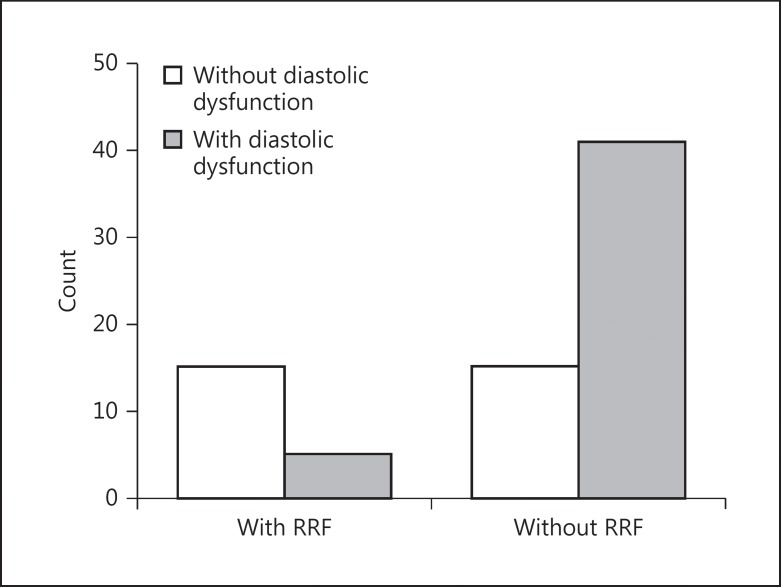
χ2 tests showed significant association between loss of urine output and LVH, diastolic cardiac dysfunction, and PAD (χ2 = 7.4, p = 0.007 [Fig. 3]; χ2 = 14.3, p = 0.001 [Fig. 4]; and χ2 = 4.2, p = 0.03, respectively). The association between the RRF preservation or not and CAD, systolic cardiac dysfunction, and hypertension was found to be nonsignificant.
Fig. 3.
Relationship between loss of residual renal function (RRF) and left ventricular hypertrophy (χ2 = 7.4, p = 0.007).
Fig. 4.
Relationship between loss of residual renal function (RRF) and diastolic dysfunction (χ2 = 14.3, p = 0.001).
The bivariate correlation between urine volume and MCP-1 serum concentration was found to be significantly inverse (r = −465, p = 0.03).
In the built logistic regression analysis model for the risk factors related to the loss of RRF, elevated MCP-1 serum concentration was found to be a significant predictor adjusting for confounders such as age, BMI, smoking, dialysis vintage, and primary renal disease (Table 2).
Table 2.
Logistic regression analysis for the factors which impact on the loss of residual renal function in hemodiafiltration patients
| Characteristic | p value | OR | CI |
|---|---|---|---|
| Age | 0.1 | 1.03 | 0.9–1.08 |
| Smoking | 0.4 | 0.5 | 0.1–2.1 |
| Dialysis vintage | 0.3 | 1.06 | 0.9–1.2 |
| Body mass index | 0.6 | 0.9 | 0.7–1.1 |
| Primary renal disease | 0.7 | 1.1 | 0.5–2.5 |
| MCP-1 | 0.04 | 1.005 | 1.001–1.012 |
CI, confidence interval; MCP-1, monocyte chemoattractant protein-1; OR, odds ratio.
In the built linear regression analysis concerning the prediction of high MCP-1 levels, we observed that loss of urine, current tobacco smoking, and hypertensive nephrosclerosis as primary renal disease were significant risk factors after adjusting for age, Kt/V for urea, and BMI, without the existence of multi-collinearity between these variables (Table 3).
Table 3.
Linear regression analysis showing the factors which influence the levels of MCP-1 in hemodiafiltration patients
| Characteristic | St. beta | P value | 95% CI for B (lower/upper) | Collinearity (tolerance/VIF) |
|---|---|---|---|---|
| Age | –0.1 | 0.4 | –3.8 to 1.5 | 0.7/1.3 |
| Body mass index | 0.2 | 0.1 | –2.3 to 20.8 | 0.9/1.09 |
| spKt/V for urea | –0.04 | 0.7 | –231.4 to 150.8 | 0.8/1.1 |
| Loss of residual renal function | 0.2 | 0.03 | 4.3 to 160.7 | 0.9/1.05 |
| Tobacco smoking | 0.2 | 0.04 | –1.3 to 162.2 | 0.8/1.1 |
| Primary renal disease (hypertensive nephrosclerosis) | 0.2 | 0.04 | 1.5 to 89.4 | 0.8/1.1 |
CI, confidence interval; MCP-1, monocyte chemoattractant protein-1; VIF, variance inflation factor.
Discussion
Previous studies have highlighted the importance of preserving RRF at the initiation of HD therapy. Dialysis is an intermittent method of treatment, whereas native kidney function is continuous [3, 4, 18].
There are several methods to measure RRF in dialysis patients, such as estimated glomerular filtration rate, residual renal urea clearance, urine volume, and newer biomarkers including serum β2 microglobulin and serum cystatin C [19, 20]. In this study, preservation of RRF was defined as a urine volume >100 mL during the interdialytic interval, similar to another previous study [21], despite another studies defining RRF as a urine output >200 or >250 mL/day [20, 22].
Previous prospective studies have strongly documented a lower risk of mortality as a benefit of RRF preservation in dialysis patients [23, 24]. The survival benefit is likely connected to advantages in fluid management, because frequently volume-overloaded HD patients are at high risk of hypertension, LVH, and CHF [25].
In agreement with this, we noted in this study a significant association between the loss of RRF and LVH, diastolic dysfunction, and PAD manifestation, even though the association with CAD, systolic cardiac dysfunction, and hypertension was found to be nonsignificant.
Features related to cardiovascular disease in this population of patients include inflammation, secondary hyperparathyroidism, and metabolic disorders. Indeed, in this study we observed that the patients without urine output, in combination with a higher prevalence of LVH, diastolic cardiac dysfunction, and PAD manifestation, had also higher iPTH and Ca × P products linked to higher arterial stiffness markers including cfPWV, augmentation index, and PP in a similar dialysis sufficiency and dialysis vintage. Moreover, we noted higher glucose, insulin, HOMA-IR, and hsCRP as well as significantly higher MCP-1 serum concentrations in the group of patients without urine output than in the patients with urine output. We also found a significant inverse association between urine volume and MCP-1 serum concentrations.
During HD modalities, the circulating leukocytes of blood are activated and the production of pro-inflammatory cytokines is elevated due to the activation of complement (C3a, C5a) and anaphylatoxin generation, resulting in stimulation of inflammation, progression of oxidative stress, and metabolic disorders [26, 27]. Furthermore, it has been shown that HD induces phenotypic changes in the expression of adhesion molecules on monocytes, which continues during the interdialytic period and influences the adherence to endothelial cells [28]. However, convective dialysis, such as HDF, may reduce the inflammatory procedure due to the convective removal of medium-molecular-weight uremic toxins and the use of biocompatible dialysis membranes [29].
Controversial results have been reported for whether the uremic state affects MCP-1 concentrations mainly depending on dialysis membranes. Previous studies observed a significant increase in circulating MCP-1 levels in hemodialyzed patients, treated with either cellulosic or synthetic membranes, compared with control subjects [26, 30]. On the contrary, another study using an in vitro method reported significantly lower spontaneous production of MCP-1 from mononuclear cells of uremic patients on cuprophane treatment compared to healthy subjects and that HD with synthetic membranes normalized MCP-1 release [31]. In the present study enrolling diabetic subjects and in our previous study excluding diabetic nephropathy and using exclusively synthetic high-flux dialysis membranes, the serum concentrations of circulating MCP-1 were similar in patients compared to healthy subjects [32].
Comparable MCP-1 levels have been reported in HD, PD, and predialysis patients, and this finding probably shows that dialysis itself does not significantly affect their elimination [33]. On the other hand, the increase in molecules as MCP-1 is mainly observed in increased oxidative stress conditions and inflammatory diseases [34]. In this study, the enrolled patients were in a good status, excluding the patients with active infections. Furthermore, many factors have been reported to be involved in the elevated MCP-1 levels in these patients, including erythropoietin and parenteral iron treatment due to the stimulation of oxidative stress causing increased MCP-1 production [35, 36]. In this study, the total of enrolled patients was on long-term erythropoietin treatment, and none had received parenteral iron during the last period before the start of the study.
However, in our data we observed that loss of urine output, current smoking, and hypertensive nephrosclerosis as primary renal disease were significant predictors for high MCP-1 serum concentrations adjusting to age, dialysis sufficiency, and BMI. Previously, high urine MCP-1 has been reported in lupus nephritis, macroalbuminuric diabetic nephropathy, and primary glomerulonephritis [37, 38, 39].
A relationship between tobacco smoking and MCP-1 levels has been also shown. Tobacco smoking leads to the development of endothelial dysfunction, which is characterized by an imbalance between vasodilation and vasoconstriction, a pro-inflammatory phenotype of the endothelial cells, and increased adhesion of monocytes in combination with increased MCP-1 levels [40, 41]. The endothelial dysfunction caused by hypertension may explain our finding that hypertensive nephrosclerosis as primary renal disease was found to be a significant predictor for high MCP-1 levels.
However, although endothelial dysfunction has prognostic value for cardiovascular disease, the association between primary renal disease and events of cardiovascular disease was found to be nonsignificant in our data. We also observed a nonsignificant relationship between primary renal disease and loss of RRF.
Regarding the significant influence of urine loss on the increased MCP-1 levels in our model, it has already been supported that the kidney plays an important role in the catabolism and removal of molecules such as MCP-1. Indeed, we noted in this study lower MCP-1 serum concentrations in our subjects with preserved RRF than in patients without urine output, and an inverse correlation between urine volume and MCP-1 serum concentrations. However, we could hypothesize that, apart from increased MCP-1 catabolism and removal, the preservation of RRF itself may contribute to the reduction of inflammation/oxidative stress and to an improvement of cellular function, resulting in a reduced production of MCP-1 as an additional benefit of RRF preservation.
On the other hand, it seems that elevated MCP-1 serum values could act as a significant predictor for the loss of RRF in this population of patients, reflecting the stimulation of multiple routes including inflammation, oxidative stress, and metabolism. Such a finding was found in our built adjusted multifactorial model about the prediction of the loss of RRF, including traditional and specific covariates for these patients. The question arises whether the high MCP-1 could be the result of or the cause for the continuous loss of RRF in this population of patients. Even though renal disease itself is definitely considered an inflammatory condition, taking into account the exclusion criteria of this study and the similar levels of MCP-1 in patients and healthy subjects, we could suggest that the elevated MCP-1 serum concentrations may be the result of the lack of RRF rather than the cause for the loss of RRF.
In the meantime, in our previous study excluding diabetic nephropathy, we observed a significant role of MCP-1 serum concentrations on LVH, mainly attributed to the relationship between MCP-1 and sodium retention [32]. Therefore, elevated MCP-1 serum concentrations may be closely connected particularly to LVH, due to their relationship with fluid imbalance including both sodium retention and loss of RRF in HD therapy patients. Vice versa, the liquid imbalance in these patients, defined by both sodium retention and loss of RRF, promotes endothelial dysfunction and progression of inflammation/oxidative stress, contributing to an increased MCP-1 production resulting in more adverse effects on the cardiovascular system. Indeed, fluid overload is included in promoting the inflammation factors [11].
However, due to the small number of enrolled patients, we cannot not safely conclude from the findings of this study that the high MCP-1 level affected by the loss of RRF plays a potential role in cardiovascular complications in dialysis patients. Bigger future studies need to clarify such a conclusion.
Conclusion
The loss of RRF was significantly associated with LVH, diastolic cardiac dysfunction, and PAD in HDF patients. The increased MCP-1 serum concentrations affected by urine loss may be an additional underlying pathophysiological factor in this relationship, reflecting a progressive inflammatory and oxidative stress condition due to the lack of RRF.
Limitations
The main limitation of our study is the small number of included subjects. In addition, the proper calculation of LV mass, including left ventricular mass index, was unavailable in this study.
Statement of Ethics
The study was approved by the General Hospital of Athens “Laïko” and the Renal Unit of the Diagnostic and Therapeutic Center of Athens Hygeia SA Institutional Review Board. All study participants provided oral informed consent prior to study enrollment. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of Interest Statement
All authors declare that they have no conflict of interest.
Author Contributions
V.D. Raikou: research plan, data collection, statistics, and manuscript writing. V. Kardalinos: hemodynamic measurements and echocardiographic assessment. D. Kyriaki: biochemical analyses, enzyme-linked immunosorbent assay, and radioimmunoassays.
References
- 1.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT, NECOSAD Study Group Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Unruh M, Zager PG, Kovesdy CP, Bargman JM, Chen J, Sankarasubbaiyan S, Shah G, Golper T, Sherman RA, Goldfarb DS. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64:181–186. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brener ZZ, Kotanko P, Thijssen S, Winchester JF, Bergman M. Clinical benefit of preserving residual renal function in dialysis patients: an update for clinicians. Am J Med Sci. 2010;339:453–456. doi: 10.1097/MAJ.0b013e3181cf7d5b. [DOI] [PubMed] [Google Scholar]
- 4.Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69:1726–1732. doi: 10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- 5.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O'Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC. US Renal Data System 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66((1 suppl 1)):S1–S305. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Hare AM, Wong SP, Yu MK, Wynar B, Perkins M, Liu CF, Lemon JM, Hebert PL. Trends in the timing and clinical context of maintenance dialysis initiation. J Am Soc Nephrol. 2015;26:1975–1981. doi: 10.1681/ASN.2013050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, NECOSAD Study Group Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 8.Schiffl H, Lang SM, Fischer R. Effects of high efficiency post-dilution on-line hemodiafiltration or conventional hemodialysis on residual renal function and left ventricular hypertrophy. Int Urol Nephrol. 2013;45:1389–1396. doi: 10.1007/s11255-012-0336-4. [DOI] [PubMed] [Google Scholar]
- 9.Raikou VD, Tentolouris N, Kyriaki D, Evaggelatou A, Tzanatou H. β2-Microglobulin, pulse pressure and metabolic alterations in hemodialysis patients. Nephron Clin Pract. 2011;117:c237–c245. doi: 10.1159/000320193. [DOI] [PubMed] [Google Scholar]
- 10.Vanholder R, Laecke SV, Verbeke F, Glorieux G, Biesen WV. Uraemic toxins and cardiovascular disease: in vitro research versus clinical studies. Nephrol Dial Transplant Plus. 2008;1:2–10. doi: 10.1093/ndtplus/sfm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. doi: 10.1159/000479254. [DOI] [PubMed] [Google Scholar]
- 12.Coll B, Alonso-Villaverde C, Joven J. Monocyte chemoattractant protein-1 and atherosclerosis: is there room for an additional biomarker? Clin Chim Acta. 2007;383:21–29. doi: 10.1016/j.cca.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Chauveau P, Nguyen H, Combe C, Chêne G, Azar R, Cano N, Canaud B, Fouque D, Laville M, Leverve X, Roth H, Aparicio M, French Study Group for Nutrition in Dialysis Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis. 2005;45:565–571. doi: 10.1053/j.ajkd.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Sternby J, Daugirdas JT. Theoretical basis for and improvement of Daugirdas' second generation formula for single-pool Kt/V. Int J Artif Organs. 2015;38:632–637. doi: 10.5301/ijao.5000456. [DOI] [PubMed] [Google Scholar]
- 15.Silva EA, Flexa F, Zanella MT. Impact of abdominal fat and insulin resistance on arterial hypertension in non-obese women. Arq Bras Endocrinol Metabol. 2009;53:340–343. doi: 10.1590/s0004-27302009000300007. [DOI] [PubMed] [Google Scholar]
- 16.Luman M, Jerotskaja J, Lauri K, Fridolin I. Dialysis dose and nutrition assessment by optical on-line dialysis adequacy monitor. Clin Nephrol. 2009;72:303–311. doi: 10.5414/cnp72303. [DOI] [PubMed] [Google Scholar]
- 17.Han SS, Cho GY, Park YS, Baek SH, Ahn SY, Kim S, Chin HJ, Chae DW, Na KY. Predictive value of echocardiographic parameters for clinical events in patients starting hemodialysis. J Korean Med Sci. 2015;30:44–53. doi: 10.3346/jkms.2015.30.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquez IO, Tambra S, Luo FY, Li Y, Plummer NS, Hostetter TH, Meyer TW. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6:290–296. doi: 10.2215/CJN.06100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SH, Filler G, Lindsay RM. Residual renal function calculated from serum cystatin C measurements and knowledge of the weekly standard Kt/V urea. Perit Dial Int. 2012;32:102–104. doi: 10.3747/pdi.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang HS, Hong YA, Yoon HE, Chang YK, Kim SY, Kim YO, Jin DC, Kim SH, Kim YL, Kim YS, Kang SW, Kim NH, Yang CW. Comparison of clinical outcome between twice-weekly and thrice-weekly hemodialysis in patients with residual kidney function. Medicine (Baltimore) 2016;95:e2767. doi: 10.1097/MD.0000000000002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nechita AM, Rădulescu D, Peride I, Niculae A, Bratu O, Ferechide D, Ciocâlteu A, Checheriță IA, Mischianu D. Determining factors of diuresis in chronic kidney disease patients initiating hemodialysis. J Med Life. 2015;8:371–377. [PMC free article] [PubMed] [Google Scholar]
- 23.van der Wal WM, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT, Korevaar JC, Geskus RB, Netherlands Cooperative Study on the Adequacy of Dialysis Study Group (NECOSAD) Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26:2978–2983. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 24.Obi Y, Streja E, Rhee CM, Ravel V, Amin AN, Cupisti A, Chen J, Mathew AT, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis. 2016;68:256–265. doi: 10.1053/j.ajkd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voroneanu L, Cusai C, Hogas S, Ardeleanu S, Onofriescu M, Nistor I, Prisada O, Sascau R, Goldsmith D, Covic A. The relationship between chronic volume overload and elevated blood pressure in hemodialysis patients: use of bioimpedance provides a different perspective from echocardiography and biomarker methodologies. Int Urol Nephrol. 2010;42:789–797. doi: 10.1007/s11255-010-9767-y. [DOI] [PubMed] [Google Scholar]
- 26.Rousseau Y, Haeffner-Cavaillon N, Poignet JL, Meyrier A, Carreno MP. In vivo intracellular cytokine production by leukocytes during haemodialysis. Cytokine. 2000;12:506–517. doi: 10.1006/cyto.1999.0574. [DOI] [PubMed] [Google Scholar]
- 27.Okusawa S, Yancey KB, van der Meer JW, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med. 1988;168:443–448. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 29.Beerenhout CH, Luik AJ, Jeuken-Mertens SG, Bekers O, Menheere P, Hover L, Klaassen L, van der Sande FM, Cheriex EC, Meert N, Leunissen KM, Kooman JP. Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20:1155–1163. doi: 10.1093/ndt/gfh775. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SH, Thylén P, Lundahl J. Three monocyte-related determinants of atherosclerosis in haemodialysis. Nephrol Dial Transplant. 2000;15:1414–1419. doi: 10.1093/ndt/15.9.1414. [DOI] [PubMed] [Google Scholar]
- 31.Pertosa G, Grandaliano G, Gesualdo L, Ranieri E, Monno R, Schena FP. Interleukin-6, interleukin-8 and monocyte chemotactic peptide-1 gene expression and protein synthesis are independently modulated by hemodialysis membranes. Kidney Int. 1998;54:570–579. doi: 10.1046/j.1523-1755.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- 32.Raikou VD, Moisakis J, Kyriaki D. Correlation between MCP-1 (monocyte chemoattractant protein-1) and cardiovascular disease in patients on the end stage of renal disease. Am J Pharmacol Pharmacother. 2014;1:127–133. [Google Scholar]
- 33.Bonomini M, Reale M, Santarelli P, Stuard S, Settefrati N, Albertazzi A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79:399–407. doi: 10.1159/000045084. [DOI] [PubMed] [Google Scholar]
- 34.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 35.Marchi SD, Cecchin E, Falleti E, Giacomello R, Stel G, Sepiacci G, Bortolotti N, Zanello F, Gonano F, Bartoli E. Long-term effects of erythropoietin therapy on fistula stenosis and plasma concentrations of PDGF and MCP-1 in hemodialysis patients. J Am Soc Nephrol. 1997;8:1147–1156. doi: 10.1681/ASN.V871147. [DOI] [PubMed] [Google Scholar]
- 36.Pawlak K, Pawlak D, Mysliwiec M. Long-term erythropoietin therapy decreases CC-chemokine levels and intima-media thickness in hemodialyzed patients. Am J Nephrol. 2006;26:497–502. doi: 10.1159/000097269. [DOI] [PubMed] [Google Scholar]
- 37.Abulaban KM, Song H, Zhang X, Kimmel PL, Kusek JW, Nelson RG, Feldman HI, Vasan RS, Ying J, Mauer M, Nelsestuen GL, Bennett M, Brunner HI, Rovin BH. Predicting decline of kidney function in lupus nephritis using urine biomarkers. Lupus. 2016;25:1012–1018. doi: 10.1177/0961203316631629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titan SM, Vieira JM, Jr, Dominguez WV, Moreira SR, Pereira AB, Barros RT, Zatz R. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012;26:546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Worawichawong S, Worawichawong S, Radinahamed P, Muntham D, Sathirapongsasuti N, Nongnuch A, Assanatham M, Kitiyakara C. Urine epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as biomarkers for interstitial fibrosis and tubular atrophy in primary glomerulonephritis. Kidney Blood Press Res. 2016;41:997–1007. doi: 10.1159/000452595. [DOI] [PubMed] [Google Scholar]
- 40.Giebe S, Cockcroft N, Hewitt K, Brux M, Hofmann A, Morawietz H, Brunssen C. Cigarette smoke extract counteracts atheroprotective effects of high laminar flow on endothelial function. Redox Biol. 2017;12:776–786. doi: 10.1016/j.redox.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furie MB, Raffanello JA, Gergel EI, Lisinski TJ, Horb LD. Extracts of smokeless tobacco induce pro-inflammatory changes in cultured human vascular endothelial cells. Immunopharmacology. 2000;47:13–23. doi: 10.1016/s0162-3109(99)00181-2. [DOI] [PubMed] [Google Scholar]