INTRODUCTION
The use of blood transfusions to augment hemodynamic status is common practice in the U.S., with over 11 million units red blood cells transfused annually in more than 3 million patients.1 Patients with major burn injury have major transfusion requirements (> one blood volume) from surgical blood loss, decreased red cell production, increased red cell destruction, and iatrogenic blood testing, making transfusions ubiquitous. In the intensive care unit approximately 25% of patients receive blood transfusions to ameliorate the effects of anemia, including decreased blood viscosity, increased oxygen extraction, and capillary/tissue alterations.2,3 Although critically ill patients may be predisposed to the adverse effects of anemia, they are also subject to the adverse consequences of blood transfusion, including infection, pulmonary edema, immune suppression, and microcirculatory alterations.4 Traditional liberal transfusion strategies administer blood at a hemoglobin of <10 g/dl. However, The Transfusion Requirements in Critical Care (TRICC) trial of intensive care unit (ICU) patients reported that a restrictive strategy was at least as effective as the liberal strategy in critically ill patients.5 Subsequent studies in other populations have supported these findings in stable non-bleeding patients.6–8
The TRICC trial and other transfusion study findings may not generalize to burn injured patients. First, patients with massive surgical blood loss, multiple operations, and prolonged ICU stays were excluded. Second, burn patients, due to their hypermetabolic state and prolonged critical illness, are physiologically distinct and excluded or underrepresented in transfusion studies. Finally, few studies to date have evaluated the effect of a restrictive strategy on infection or wound healing, which are major considerations in burn patients. The best transfusion strategy in burn care thus remains unknown.
Burn patients differ from other critically ill populations.9 The burn wound represents loss of the major infection barrier: the skin, and inhalation injury has systemic and local effects. Burn patient injury response is unique. Hypermetabolism (sustained increased temperature, tachycardia, cortisol), cardiac dysfunction, immunosuppression, and bone marrow suppression are ubiquitous. Burn treatment also differs due to multiple operations, frequent dressing changes, topical and systemic antibiotics, and prolonged stays with extensive rehabilitation needs. Data on a restrictive blood transfusion policy in adult burn patients vary. Most studies are either single center studies or retrospective observational multicenter studies.10–13 Previously, we assessed blood transfusion strategies by first surveying surgeons to determine transfusion practices and then reviewing those practices in 21 centers.14,15 These studies demonstrated that transfusion practices vary markedly among surgeons both in theory and in practice. ICU blood transfusions, in addition to age, total body surface area (TBSA) burn, and inhalation injury, were associated with increased mortality and infection (each transfusion increased the infection risk 11%). Although these studies suggest that blood transfusions influence burn injury outcomes, the retrospective nature of the studies precludes causation determination. Determining the role of transfusion in infection development is particularly important in burn patients, as infection is a leading contributor to morbidity and mortality in burns. The goal of TRIBE was to compare outcomes under a restrictive blood transfusion policy (maintaining a hemoglobin level 7–8 g/dl) to a traditional transfusion policy (maintaining hemoglobin 10–11 g/dl). Outcomes included incidence of blood stream infection, mortality, organ dysfunction, hospital length of stay, mechanical ventilation duration, and wound healing in adults with major burn injury.
METHODS
The trial was registered (Clintrials.gov identifier NCT01079247) and approved by the University of California Davis Human Subjects Review Board (Protocol #200816457), the Department of Defense Human Research Protection Official (Log# A-15003), and the Human Subjects Review Board of each site. Data were housed in a secure electronic database at the University of California Davis Clinical and Translational Science Center (CTSC) using Velos eResearch Electronic Data Management (eRDM). No study investigator had access to data during the trial. TRIBE was monitored by a Data Safety Monitoring Board with specific a priori safety stopping rules.
Trial design
This was a Phase III, multi-center, open label, investigator-initiated, randomized trial to compare patient outcomes as they related to transfusion strategy. Liberal transfusion practices, where hemoglobin levels were maintained at approximately 10–11 g/dl, were compared to a more restrictive transfusion strategy in which hemoglobin levels were maintained at 7–8 g/dl.
Participants
All patients admitted to a participating center were screened for enrollment. Patients were approached for enrollment if they were admitted to a participating burn center within 96 hours of injury with a burn injury ≥ 20% TBSA and need for burn excision and grafting was anticipated. Patients were excluded if they were: <18 years of age; pregnant; unable or unwilling to receive blood products; chronically anemic (hemoglobin <9.0 g/dl one month prior to enrollment); on renal dialysis prior to injury; brain dead, imminent brain death, or a non-survivable burn; experiencing angina or acute myocardial infarction on admission; preexisting hematologic disease; or closed head injury with Glasgow coma scale <9. Informed consent was obtained by the one of the Investigators or research personnel. Eligible subjects were approached for informed consent within 72 hours of admission. If the patient lacked decision-making capacity, surrogate consent was obtained and formal patient consent obtained when the patient regained decision-making capacity.
Randomization
Consecutive burn patients admitted with the above criteria were assigned to one of two treatment groups (restrictive versus liberal transfusion strategy) using an adaptive random allocation procedure to balance groups with respect to the screening prognostic variables. The treatment groups were balanced across sites with respect to age category (18–39 versus 40–59 versus ≥ 60 years) and TBSA of the burn (20%–39% versus 40%–59% versus ≥60%), and within site with respect to overall restrictive and liberal totals. Each subject was randomized with a “biased coin” procedure, which used randomization probabilities, favoring the treatment with the deficit enrollment, to improve the balance on group assignment.16 We used an “intention-to-treat” analysis plan.
Interventions
Patients were block randomized across centers and within centers for burn size and age. Baseline patient demographic data were collected on the following parameters within 72 hours of enrollment: age, gender, TBSA burn (second and third degree), inhalation injury, Acute Physiology and Chronic Health Evaluation (APACHE II) score, multiple organ dysfunction score (MODS),17 tobacco use, recreational drug use, and associated illnesses.
ICU Transfusion Protocol
Patients assigned to the restrictive transfusion strategy received red blood cells (RBC) when hemoglobin was <7 g/dl. In the liberal transfusion group, blood was transfused when the hemoglobin was <10 g/dl. Patients received blood transfusions one unit at a time with hemoglobin measured after each unit was transfused. Compliance was assessed with monitoring of hemoglobin concentrations for each patient throughout hospitalization. Hemoglobin concentration was recorded daily and prior to each blood transfusion. The number and volume of red-cell transfusions, age of red blood cells, use of leukocyte-reduced blood, use of other blood products (albumin, fresh frozen plasma, platelets, cryoprecipitate) were recorded, including the number of units and the volume of the transfusion.
Operating Room Protocols
The operative period was defined as the time the patient entered the operating room and ended when the patient left the operating room. Hemoglobin levels were obtained within 8 hours prior to surgery. Hemoglobin was measured prior to and immediately after each blood transfusion, and the amount of blood transfused recorded. If the patient was hypotensive due to blood loss, blood was transfused as needed to maintain hemodynamic stability without waiting for the results of the hemoglobin level. The reason for transfusion was recorded, as was the hemoglobin at the time of transfusion. The operative procedure, estimated blood loss, number of units and volume of blood transfused in the operating room, and other fluid administered (crystalloid, colloid, other blood products) during the operation were recorded. A hemoglobin level was obtained postoperatively within 30 minutes of completion of the surgical procedure.
Daily Monitoring
Organ dysfunction was assessed with lab values obtained within the first 24 hours and daily. If routine clinical care presented multiple values on any given day, those indicating the highest level of dysfunction were recorded. Parameters recorded daily included: complete blood count, electrolytes, arterial blood gases, fluid intake and output, medications given, mechanical ventilation, dialysis, blood stream infections, and other infection (catheter, urine, pneumonia, wound) as defined by the Burn Consensus conference.17 (Appendix 1) Criteria for a burn wound infection included change in burn wound appearance or character (i.e. rapid eschar separation; violaceous discoloration of the eschar, or edema at the wound edges) and histologic examination of burn biopsy showing invasion of organisms in adjacent viable tissue.
Outcomes
The primary outcome measure was number of blood stream infections as defined by the Burn Consensus Conference.18 Secondary outcomes included: mortality, number of infectious episodes (urinary tract infections, pneumonia, wound infection), burn ICU length of stay (LOS), hospital LOS, duration of mechanical ventilation, organ dysfunction (MODs score), and time to 90% burn wound healing (defined as 7 days after the last excision and grafting procedure). Patients who died were assigned a MODS score of 24.
Reporting of Adverse Events
All adverse events were reported according to institutional policy by the individual site coordinator using a standardized adverse events report form. Adverse events were categorized per institutional policy in terms of severity (severe, moderate, or mild), relationship to the study (definitely, probably, possibly, remote, or definitely not), action taken in response to the adverse event, and the outcome (recovered, on-going, treated, untreated, unknown). The lead site coordinator, Principle Investigator (tlp), and the chair of the data safety monitoring board were notified of all adverse events.
Statistical Considerations
Sample Size
The study was a multicenter randomized trial with two parallel treatment arms comparing two transfusion strategies. To estimate the sample size needed to detect differences in blood stream infection, retrospective pilot data from 666 patients with burns >20% TBSA were used to estimate the effect of blood transfusions on the likelihood of blood stream infection.15 Since some of the effect may represent a surrogate of disease severity, the effect of blood transfusion on the likelihood of blood stream infection was adjusted for disease severity using TBSA burn, gender and age. To be conservative, the total number of blood transfusions received during hospital stay was dichotomized at the median of 7. Using this threshold, the pilot demonstrated a significant association between occurrence of BSI and the dichotomized number of transfusions with an odds ratio (OR) of 2.3 (SE=0.3, p<0.001). In the pilot data, 18 of 217 patients with <7 total transfusions developed blood stream infection (8% chance).
Patients were randomized to receive blood transfusions when their hemoglobin was less <7g/dl in the restrictive group vs. <10 in the liberal group. This would result in the restrictive group receiving a fewer number of transfusions. Since the restrictive and liberal groups had a mixture of patients receiving less or more than 7 transfusions, the OR = 2.3 observed in the pilot data analysis would be attenuated towards zero. We estimated the magnitude of attenuation based on the law of total probability which resulted in the expected chance of blood stream infection (BSI) of
in the Restrictive group vs. 15% in the Liberal group, by a similar argument.
Therefore, the power of a test for binomial proportions (two-sided, α = 0.05) will exceed 90% with a total sample size of 100 patients per arm. Given an anticipated average drop-out rate of 15% and noncompliance of 5%, we estimated that 120 patients per arm were required.
The power calculation for equivalence in mortality, (secondary outcome indicator), indicated that a sample size of 295 patients was required (α = 0.05, power = 0.8, using a one-sided test). Given the drop-out/incomplete data rate of approximately 15%, a total of 345 patients were recruited for study participation to assure that the study was adequately powered for secondary endpoints.
Blinding
The study was a prospective unblinded open labelled randomized prospective trial. Investigators were informed of treatment group by calling the randomization center, which used the computer-generated randomization scheme described above to provide treatment assignments.
Statistical Analyses
Simple descriptive statistics were generated to summarize distributions and proportions on study variables. Bivariate analyses of continuous variables were compared across treatment groups using Wilcoxon rank sum tests as most variables deviated from normality; medians [25th percentile, 75th percentile] are reported. For dichotomous variables, χ2 tests were used to compare proportions between treatment groups. Analyses were conducted in R (version 3.2.3) and SAS (version 9.4). Unconditional logistic regression was used to test for differences in the occurrence BSI and other infections between treatment groups. Hospital LOS was included in the model as a covariate to adjust for varying time at risk for BSI and for other infections among patients. We conducted multiple logistic regressions to test for differences in the occurrence of BSI and other infections between treatment groups after adjusting for age, gender, TBSA, inhalation injury, and APACHE score in addition to LOS. All tests were two-sided with a significance level of 0.05. Kaplan-Meier survival curves were estimated for both treatment groups and compared with a log-rank test.
RESULTS
Patient Enrollment
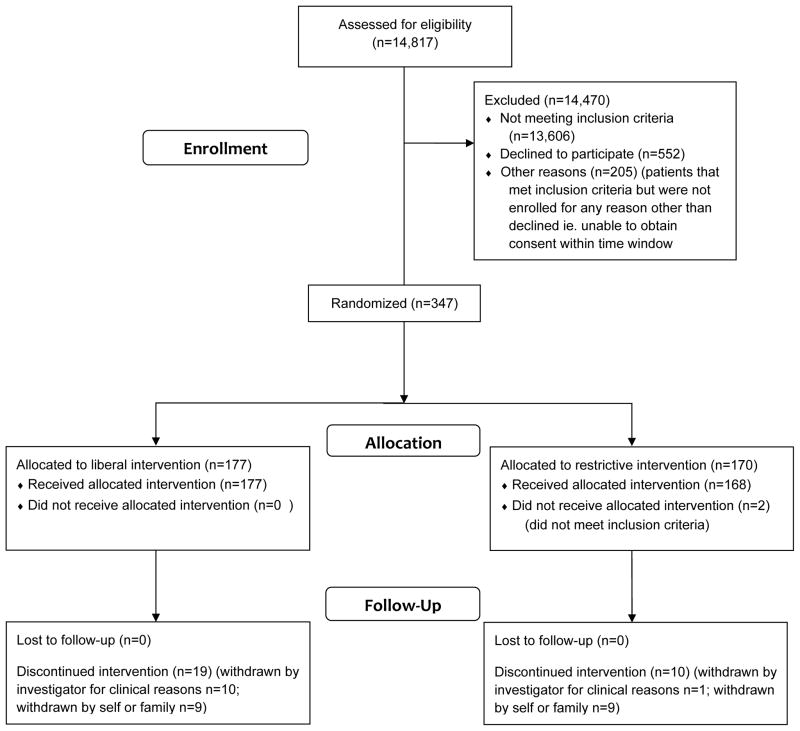
Between August 16, 2010 and August 28, 2015 a total of 347 patients were randomized to one of two treatment groups in 18 centers. (Figure 1) Two did not meet eligibility criteria and were excluded leaving 345 patients for analysis.
Figure 1.
CONSORT diagram illustrating patient allocation
Patient and transfusion data
The two treatment groups had comparable patient characteristics including age, gender, TBSA, % full thickness, % partial thickness burn, admit MOD and APACHE scores, and proportion of patients with inhalation injury (Table 1). Days on study were similar between treatment groups (p = 0.664). Overall compliance with the transfusion protocol (defined as transfusion within parameters of randomized group) was 90.6% in the liberal group and 88.0% in the restrictive group; ICU compliance was 98.5% and 97.5% for liberal and restrictive groups, respectively. Virtually all episodes of noncompliance were due to acute intraoperative bleeding or hypotension.
Table 1.
Summary statistics of patient characteristics. Data expressed as medians [25th, 75th quantiles] or percentage (n) of selected variables for each treatment group. APACHE=Acute Physiology and Chronic Health Evaluation; MOD=Multiple Organ Dysfunction score.
| Variable | Liberal(n = 177) | Restrictive(n = 168) | p-value |
|---|---|---|---|
| Age (years) | 41 [30, 55] | 41 [27, 55] | 0.40 |
| Gender (% male) | 78.5% (n = 139) | 79.8% (n = 134) | 0.88 |
| TBSA (%) | 33 [25, 47] | 31 [25, 44.25] | 0.43 |
| Full Thickness (%) | 20 [4, 33] | 20 [6, 32] | 0.75 |
| Partial Thickness (%) | 12 [1, 25] | 12 [0.5, 23.25] | 0.62 |
| Inhalation Injury (%) | 20.9% (n = 37) | 24.4% (n = 41) | 0.52 |
| APACHE Score | 19 [12, 24] | 17 [12.75, 23] | 0.46 |
| Admission MOD Score | 4 [1, 7] | 4 [1, 7] | 0.91 |
Strikingly, patients in the restrictive group received fewer total blood product transfusions overall (3,411 vs. 5,636 total, median 8 [3, 24.2] vs. 16 [7, 40] units/patient, p < 0.001) and RBC transfusions (2,574 vs. 4,480 total, mean 20.3±32.7 vs. 31.8±44.3 units/patient, median 7 [2, 19] vs. 15 [7, 31] units/patient, p < 0.001) (Table 2) and there was no significant difference in time from admission to first transfusion (4 [0, 16] days in liberal vs. 5 [0, 19] days in restrictive). The percentage of patients not receiving a transfusion was greater in the restrictive group compared to the liberal group (16.1% vs. 6.8%, p = 0.011). However, there was no significant difference between groups in number of transfusions received in the operating room (restrictive: 2 [0, 10] vs. liberal: 3 [1, 2], p =0.20). Although the majority of transfusions occurred in the ICU, 4,868 (89.0%) of the non-OR transfusions were given within 24 hours after operation. Median time to RBC transfusion post-operation were similar: liberal 7.70 [3.29, 14.89] vs. restrictive: 7.83 [3.14, 15.75] hours, with a median of 10 [6,9] units liberal vs. 5 [2,9] restrictive p<0.001 administered with 24 hours of operation.
Table 2.
Transfusions received during the study. Data expressed as medians [25th, 75th quantiles] by blood component and treatment group. RBC=Red Blood Cell units; FFP=Fresh Frozen Plasma units.
| Variable | Liberal (n = 177) | Restrictive (n = 168) | P-value |
|---|---|---|---|
| Operating Room + Non-Operating Room Transfusions | |||
| Total Transfusions | 16 [7, 40] | 8 [3, 24.2] | < 0.001 |
| RBC | 15 [7, 31] | 7 [2, 19] | < 0.001 |
| FFP | 0 [0, 6] | 0 [0, 4] | 0.56 |
| Platelet | 0 [0, 1] | 0 [0, 0] | 0.20 |
| Cryoprecipitate | 0 [0, 0] | 0 [0, 0] | 0.21 |
| No Transfusions (%) | 6.8% (n = 12) | 16.1% (n = 27) | 0.011 |
| Operating Room Transfusions | |||
| Total Transfusions | 3 [1, 12] | 2 [0, 10] | 0.21 |
| RBC | 2 [0, 8] | 2 [0, 7] | 0.18 |
| FFP | 0 [0, 3] | 0 [0, 3] | 0.86 |
| Platelet | 0 [0, 0] | 0 [0, 0] | 0.18 |
| Cryoprecipitate | 0 [0, 0] | 0 [0, 0] | 0.53 |
| No Transfusions (%) | 24.8% (n = 44) | 33.3% (n = 56) | 0.106 |
| Non-Operating Room Transfusions | |||
| Total Transfusions | 12 [6, 27] | 5 [1, 14] | < 0.001 |
| RBC | 11 [5, 22] | 4.5 [1, 10] | < 0.001 |
| FFP | 0 [0, 2] | 0 [0, 1] | 0.44 |
| Platelet | 0 [0, 0 ] | 0 [0, 0] | 0.19 |
| Cryoprecipitate | 0 [0, 0] | 0 [0, 0] | 0.18 |
| No Transfusions (%) | 7.3% (n = 13) | 17.3% (n= 29) | 0.008 |
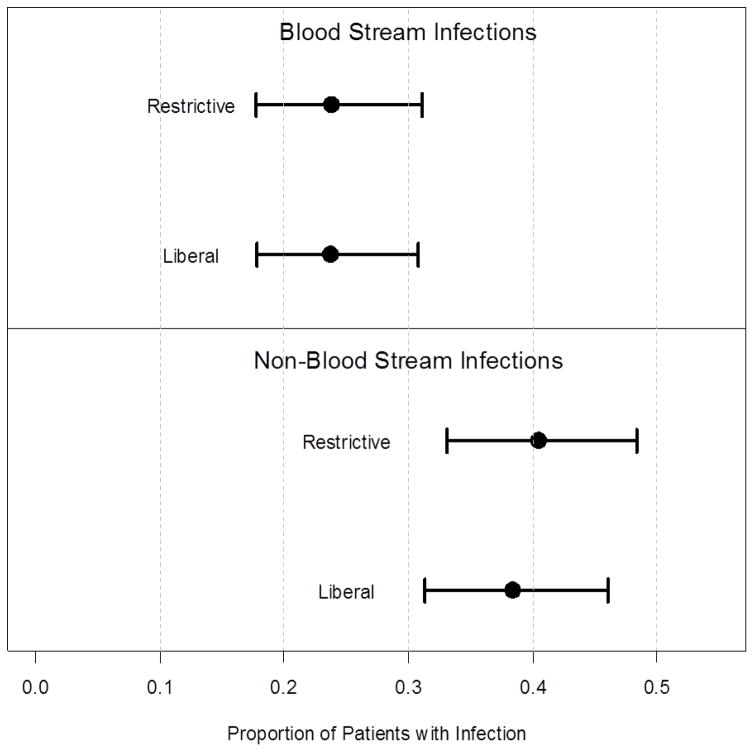
Primary Outcome: Blood Stream Infection (BSI)
BSI occurred in approximately 24% of patients in both the liberal and restrictive groups (Table 3, Figure 2). The risk of developing a BSI did not differ significantly between treatment groups (23.7% vs. 23.8%, p = 0.904), the OR (Odds Ratio) comparing the restrictive group relative to the liberal group was 1.034 [95% CI: 0.604, 1.767]. Multivariate adjustment for age, gender, TBSA, inhalation injury and APACHE score did not significantly change this result, ORadjusted = 1.130 [95% CI: 0.641, 1.993], similar to the unadjusted analysis.
Table 3.
Summaries of infections for each treatment group
| Variable | Liberal (n = 177) | Restrictive (n = 168) |
|---|---|---|
| BSI (Y/N) | 23.7% (n = 42) | 23.8% (n = 40) |
| Number of BSI | 0.5 ± 1.4 | 0.4 ± 0.9 |
| BSI (#/1,000 patient-days)c | 9.87 | 7.89 |
| Wound Infections (Y/N) | 11.9% (n = 21) | 11.9% (n = 20) |
| Number of Wound Infections | 0.2 ± 0.6 | 0.2 ± 0.6 |
| UTI (Y/N) | 13.6% (n= 24) | 14.3% (n= 24) |
| Number of UTI | 0.2 ± 0.6 | 0.2 ± 0.6 |
| UTI (#/1,000 patient-days)c | 3.80 | 3.88 |
| Pneumonia (Y/N) | 27.7% (n = 49) | 29.2% (n = 49) |
| Number of Pneumonia | 0.5 ± 1.1 | 0.5 ± 1.1 |
| Pneumonia (#/1,000 patient-days)c | 10.23 | 9.35 |
| Wound Infections (Y/N) | 11.9% (n = 21) | 11.9% (n = 20) |
| Number of Wound Infections | 0.2 ± 0.6 | 0.2 ± 0.6 |
| Wound (#/1,000 patient-days)c | 3.80 | 3.76 |
Hospital length of stay was used to determine patient-days. P>0.05 in all groups
Figure 2.
Comparison of proportion of patients developing a blood stream infection or non-blood stream infection by treatment group.
Secondary Outcomes
There were 31 deaths within 30 days and 11 deaths after 30 days (Table 4). Fifteen subjects (8.5%) in the liberal group died within 30 days compared with 16 deaths (9.5%) in the restrictive group, not statistically significantly different (χ2 = 0.018, p = 0.89) between groups. There was no difference in hospital mortality between groups. Kaplan-Meier survival curves did not differ significantly between treatment groups (χ2 = 0.666, p = 0.414).
Table 4.
Secondary outcome measures for each treatment group. Data expressed as medians [25th, 75th quantiles) or percentage (n) of outcomes for each treatment group.
| Variable | Liberal (n = 177) | Restrictive (n = 168) | P-value |
|---|---|---|---|
| 30-day Mortality | 8.5% (n = 15) | 9.5% (n = 16) | 0.89 |
| Overall Mortality (%) | 11.3% (n = 20) | 13.7% (n = 23) | 0.26 |
| LOS (days) | 31 [20, 59.2] | 31 [21, 58.2] | 0.84 |
| Ventilator Days | 6 [0, 20] | 6 [1, 27.5] | 0.64 |
| ICU Days | 20 [9, 40] | 22.5 [11, 42.2] | 0.61 |
| Days to Wound Healing | 24 [14, 43] | 23 [15, 41] | 0.70 |
| Days on Study | 26 [16, 51] | 27.5 [17, 56] | 0.66 |
| Maximum MOD Score | 7 [4, 10] | 8 [4, 11] | 0.22 |
| Surgery (Y/N) | 93.8% (n = 166) | 94.0% (n = 158) | 1.00 |
| Number of Operations | 3 [1, 5] | 2 [1, 5] | 0.18 |
Pneumonia (29% restrictive, 27% liberal), urinary tract infection (14.3% restrictive, 13.6% liberal), and wound infections (11.9% both groups) occurred with similar frequency in both transfusion groups (Table 3). The risk of developing an infection other than BSI did not differ significantly between treatment groups (p = 0.592), OR 1.136 [95% CI: 0.712, 1.812]. Adjusting for age, gender, TBSA, inhalation injury and APACHE score did not change this result (p = 0.516); ORadjusted = 1.172 [95% CI: 0.726, 1.892].
None of the remaining secondary outcomes differed significantly between treatment groups (Table 4). The maximum MOD score (restrictive: 8 [4, 11] vs. liberal: 7 [4, 10], p = 0.224) and days to wound healing were nearly identical between groups (restrictive: 23.0 [15.0, 41.0] vs. liberal 24.0 [14.0, 43.0], p = 0.700). LOS (restrictive: 31.0 [21.0, 58.2] vs liberal: 31.0 [20.0, 59.2], p = 0.840), ICU days (restrictive: 22.5 [11.0, 42.2] vs liberal: 20.0 [9.0, 40.0], p = 0.606) and ventilator days (restrictive: 6.0 [1, 27.5] vs liberal: 6.0 [0, 20.0], p = 0.638) also were very similar.
CONCLUSIONS
This multicenter randomized prospective trial comparing two transfusion strategies (transfusing for hemoglobin <10 g/dl compared to <7 g/dl) in patients with burn injury >20% TBSA found that a restrictive strategy markedly reduced transfusion volume, yet found no statistically significant differences in the primary outcome of BSI or in the secondary outcome measures, including mortality, pneumonia, urinary tract infection, wound infection, hospital length of stay, ICU length of stay, organ dysfunction, or wound healing. Treatment groups were highly comparable and compliance with study protocols was very high with hemoglobin levels maintained in the target range.
The findings of TRIBE further confirm and extend the results of the TRICC trial, in which a restrictive transfusion strategy was equally effective as a liberal strategy, as well as studies in hip fracture patients, cardiac surgery, and other ICU patients.5–8 A restrictive strategy significantly reduced blood utilization compared to the liberal strategy. However, a restrictive transfusion strategy did not decrease the incidence of blood stream infection or any other infectious complication, findings contrary to previous burn and critical care studies.19,20 To date, prospective randomized trials have not confirmed the notion that blood transfusion alone increases infection rates, despite the immunomodulatory effects of blood.8 Patients with major burn injury receive significant volumes of blood and are immunosuppressed after injury; hence, risk of infection should be magnified in this population. Our study suggests that the previous retrospective studies may suffer from association bias; i.e., sicker patients who are more prone to infection also receive more blood because they are sick.
A restrictive strategy in burn patients also did not result in a difference in mortality, organ dysfunction, hospital length of stay, or duration of mechanical ventilation, further confirming the findings of previous ICU trials but differing from studies in other populations. 5–8, 21–22 Prospective randomized trials evaluating transfusion strategies in symptomatic coronary artery disease and surgical oncology patients, respectively, described fewer cardiac events and fewer postoperative complications with the liberal strategy.21,22 Burn patients, due to the hypermetabolic state and multiple operations, experience significant cardiac stress, yet we found no difference in mortality or organ dysfunction between transfusion strategies in major burn injury.23 Likewise, burn patients undergo major operations with a need to heal surgical wounds, yet no significant difference in the time to wound healing was observed between the liberal and restrictive transfusion strategies.
Application of a restrictive transfusion strategy in major burn injury also has the potential to markedly decrease transfusion-associated costs. For example, on average 1714 patients with burns >20% TBSA are admitted to US burn centers every year.24 Given the estimated per patient transfusion cost of $1600–2400,25 use of a restrictive strategy could save between $31,543,220 and $47,314,680 a year for major burn injury alone.
The strength of TRIBE is its randomized prospective nature applied to a defined patient population in a diverse selection of burn centers. TRIBE was not powered to detect differences in subsets of burn patients, such as those with inhalation injury. The composite for wound healing, namely 7 days after the last grafting procedure, is a surrogate marker for wound healing. Although it does not directly measure open wounds, it is a consistently documented endpoint not subject to investigator bias or interpretation, unlike many wound healing markers.
This randomized multicenter prospective transfusion trial, the first such study in burns, successfully united 18 centers from the Multicenter Trials Group to compare the efficacy of a restrictive vs. a liberal transfusion policy throughout hospitalization, including periods of intraoperative blood loss. As such, it is among the most comprehensive to date for the evaluation of both transfusion and infection in burn injury. The volume of blood transfused per patient (in general >1 blood volume) far exceeded that used in any other randomized prospective transfusion trial. A restrictive transfusion policy in major burn injury dramatically decreased the number of blood transfusions, but, similar to studies in other populations, did not decrease the incidence of BSI, mortality, organ dysfunction, other infections, wound healing, or length of stay.
Supplementary Material
Acknowledgments
This study was supported by the American Burn Association and funded by USAMRMC Award #W81XWH-08-1-0760 with support from the National Center for Research Resources, National Institutes of Health, through grant #UL1 RR024146, the National Center for Advancing Translational Sciences, National Institutes of Health, through grant #TR 000002
We would like to acknowledge the coordinators and participating centers:
University of California, Davis, CA: Katrina Falwell RN, BSN (lead study coordinator); Angela Mix RN, BSN, Cassandra Conover RN
Arizona Burn Center in Phoenix, AZ: Marlene Albrecht RN, CRC; Karen RicheyRN, BSN
University of Utah, Salt Lake City, UT: Iris Faraklas RN, BSN
University of Florida, Gainesville, FL: Tera Thigpin CRC
The Oregon Burn Center, Portland, OR: Marsha Ryan CRC
Middlemore Hospital, Auckland, New Zealand: Sue Olliff RN
US Army Institute of Surgical Research, Ft. Sam Houston, TX: Elsa Coates MS, RN, CCRN
University of North Carolina, Chapel Hill, NC: Carrie Nielsen MA
University of Kansas, Kansas City, KA: Annemarie Dalton RN, BSN; Doug Ross RN
University of Texas Southwestern, Dallas, TX: Agnes Burris RN
Sunnybrook Health Science Centre, Toronto, Canada: Kathy Popovski RN, CNS
University of Alberta, Edmonton, Canada: JiaJie Wu M.Sc., B.Eng.
Wake Forest Baptist Medical Center, Winston-Salem, NC: Courtney Gruver RN; Carmen Wells RN, Bill Martin
JMS Burn Center, Augusta, GA: Yvonne Daniel CRC
Community Regional Medical Center, Fresno, CA: Veronica Vidal CRC, Kim Nguyen RN
University of California San Diego, CA: Terry Curry RN, Emmer Trinidad CRC
Burn Center at Washington Hospital, Washington DC: Anna Pavlovich, RN
Arrowhead Regional Burn Center, Colton, CA: Jennifer Hardy, MSPA, PA-C
UC Davis Data Coordinating Center
• Director of Research Operations: Mary Beth Lawless RN, MSN
• Data auditor: Terese Curri, BS
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through grant # UL1 TR001860.
Footnotes
Presented at the American Surgical Association Meeting, Philadelphia, PA, April 2017
The views expressed are those of the authors and do not reflect the official policy or position of the Department of Defense or the U.S. Government
Conflicts of Interest and Sources of Funding
Dr. Palmieri: USAMRMC Award #W81XWH-08-1-0760 funded this study
Dr. Holmes: Equity positions in Abbott Labs, AbbVie, and Permeaderm Inc.
Dr. Arnoldo: No conflicts to declare
Dr. Peck: No conflicts to declare
Dr. Potenza: No conflicts to declare
Dr. Cochran: No conflicts to declare
Dr. King: No conflicts to declare
Dr. Dominick: No conflicts to declare
Dr. Cartotto: No conflicts to declare
Dr. Havsar: No conflicts to declare
Dr. Kemalyan: No conflicts to declare
Dr. Tredget: Contract research, Scar X™, KLOX Therapeutics, and Exciton (ExSALT™), collaborative research British Canadian BioSciences Corp (novel antifibrotic agent)
Dr. Stapelberg: No conflicts to declare
Dr. Mozingo: No conflicts to declare
Dr. Friedman: No conflicts to declare
Dr. Greenhalgh: No conflicts to declare
Dr. Taylor: National Center for Research Resources, National Institutes of Health, through grant #UL1 RR024146, the National Center for Advancing Translational Sciences, National Institutes of Health, through grant #TR 000002
Dr. Pollock: No conflicts to declare
References
- 1.Wallace EL, Churchill WH, Surgenor DM, et al. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion. 1998;38:625–636. doi: 10.1046/j.1537-2995.1998.38798346630.x. [DOI] [PubMed] [Google Scholar]
- 2.Hebert PC, Wells G, Martin C, et al. A Canadian survey of transfusion practices in critically ill patients. Crit Care Med. 1998;26:482–487. doi: 10.1097/00003246-199803000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Marini JJ. Transfusion triggers and Occam’s rusty razor. Crit Care Med. 1998;26:1775–1776. doi: 10.1097/00003246-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez G, Hebert PC, Szick S. Debate: transfusing to normal haemoglobin levels will not improve oucome. Crit Care. 2001;5(2):56–63. doi: 10.1186/cc987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;29365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajjar LA, Vincent J, Galas FRBG, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304:1550–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 8.Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews. 2016:10. doi: 10.1002/14651858.CD002042.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snell JA, Loh NW, Mahambrey T, Shokrollahi K. The critical care management of the burn patient. Crit Care. 2013;17(5):241. doi: 10.1186/cc12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curinga G, Jain A, Feldman M, et al. Red blood cell transfusion following burn. Burns. 2011;37:742–752. doi: 10.1016/j.burns.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Zhuang M, Fan X, et al. Blood transfusions in severe burn patients: Epidemiology and predictive factors. Burns. 2016;42:1721–1727. doi: 10.1016/j.burns.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Lu RP1, Lin FC, Ortiz-Pujols SM, et al. Blood utilization in patients with burn injury and association with clinical outcomes (CME) Transfusion. 2013;53(10):2212–2221. doi: 10.1111/trf.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sittig KM, Deitch EA. Blood transfusions: for the thermally injured or for the doctor? J Trauma. 1994;36:369–372. [PubMed] [Google Scholar]
- 14.Palmieri TL, Greenhalgh DG. Blood Transfusion in Burns: What Do We Do? J Burn Care Rehab. 2004;25:71–75. doi: 10.1097/01.BCR.0000105094.25999.0D. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri TL, Caruso DM, Foster KN, et al. Effect of Blood Transfusion on Outcome After Major Burn Injury: A Multicenter Study. Crit Care Med. 2006;34:1602–1607. doi: 10.1097/01.CCM.0000217472.97524.0E. [DOI] [PubMed] [Google Scholar]
- 16.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 17.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh DG, Saffle JR, Holmes JH, 4th, et al. American Burn Association consensus conference to define sepsis and infection. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302–8. doi: 10.1097/01.CCM.0000234034.51040.7F. [DOI] [PubMed] [Google Scholar]
- 20.Graves TA, Cioffi WG, Mason AD, et al. Relationship of transfusion and infection in a burn population. J Trauma. 1989;29:948–952. doi: 10.1097/00005373-198907000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion threshold for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–971. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Almeida JP, Vincent JL, Galas FR, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology. 2015;122:29–38. doi: 10.1097/ALN.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 23.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Burn Association. National Burn Repository Report of Data from 2006–2015. Version 12.0. Chicago, IL: 2016. p. 10. [Google Scholar]
- 25.Hannon Timothy. The Contemporary Economics of Transfusions. In: Spiess BD, Soence RK, Shander A, editors. Perioperative Transfusion Medicine. 2. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.