Abstract
Rationale
Assays to quantify antiretrovirals in hair samples are increasingly used to monitor adherence and exposure in both HIV prevention and treatment studies. Atazanavir (ATV) is a protease inhibitor used in combination antiretroviral therapy (ART). We developed and validated a liquid chromatography/tandem mass spectrometry (LC/MS/MS)-based method to quantify ATV in human hair, per the NIH Division of AIDS Clinical Pharmacology Quality Assurance (CPQA) program and the FDA bioanalytical method validation guidelines.
Methods
ATV was extracted from hair using optimized methods and the extracts were injected onto a BDS C-18 column (5 μm, 4.6 × 100 mm), followed by isocratic elution via a mobile phase composed of 55% acetonitrile, 45% water, 0.15% acetic acid, and 4 mM ammonium acetate, at a flow rate of 0.8 mL/min prior to analysis by MS/MS. Levels were quantified using positive electrospray ionization by multiple reaction monitoring (MRM) for the transitions MH+ m/z 705.3 to m/z 168.0 and MH+ m/z 710.2 to m/z 168.0 for ATV and ATV-d5 (internal standard), respectively.
Results
Our assay demonstrated a linear standard curve (r = 0.99) over the concentration range of 0.0500 ng ATV/mg hair to 20.0 ng/mg hair. The inter- and intraday accuracy of ATV quality control (QC) samples was −1.33 to 4.00% and precision (% coefficient of variation (%CV)) was 1.75 to 6.31%. The %CV for ATV levels in hair samples from highly adherent patients (incurred samples) was less than 10%. No significant endogenous peaks or crosstalk were observed in the specificity test with other HIV drugs. The overall extraction efficiency of ATV from incurred hair samples was greater than 95%.
Conclusions
This highly sensitive, highly specific and validated assay can be considered for therapeutic drug monitoring for HIV-infected patients on ATV-based ART.
1 | INTRODUCTION
Morbidity and mortality has declined largely from human immunodeficiency virus (HIV) -1 infection as the result of access to antiretroviral therapy (ART).1 There are, however, major challenges with adhering to ART over a lifetime. Moreover, there is no gold standard for measuring adherence in either HIV treatment or pre-exposure prophylaxis (PrEP). One way to objectively monitor adherence is to analyze antiretroviral (ARV) drug concentrations in different biomatrices. Our group has developed expertise in the measurement of ARV levels in hair to assess adherence and its exposure in the context of both HIV prevention and treatment.2–34
Atazanavir (ATV) is an azapeptide HIV protease inhibitor (PI) that targets the viral protease enzyme during the late stages of HIV replication. ATV is metabolized primarily by cytochrome P450 3A (CYP3A) isoenzymes and is typically given at a dose of 300 mg once-daily with a pharmacoenhancer (e.g. CYP3A4 inhibitor, ritonavir (RTV) or cobicistat).35 The maximum plasma concentration (Cmax) of RTV-boosted ATV is 5.23 μg/mL and the minimum plasma concentration (Cmin) is 0.86 μg/mL.36 The elimination half-live of RTV-boosted ATV is 8.6 h, time to Cmax is approximately 2 h,37 and steady-state plasma concentrations are achieved by 6 days.37 The Cmax and area under the plasma ATV concentration vs time curve (AUC) values increase in a dose-proportional manner, suggesting that clearance is constant. Patients prescribed an ATV-based regimen may experience adverse events which are dependent on ATV levels,38–41 including nausea and indirect hyperbilirubinemia.35,37,42
The most commonly used technique to analyze ARVs in plasma is high-performance liquid chromatography (HPLC) coupled with ultraviolet (UV), fluorescence or mass spectrometry (MS) detection systems. Several analytical methods have also been reported for quantifying ATV in dried blood spots (DBS)43 and plasma43–45 using HPLC coupled with UV,44 fluorescence (FL)46 and tandem mass spectrometry (MS/MS) techniques.43,45,47 The HPLC-UV-based method detects plasma concentrations of ATV between 0.09 and 4.38 μg/mL (retention time: 8.3 min, flow rate: 1.8 mL/min),44 and the HPLC-FL-based method detects plasma concentrations of ATV between 0.025 and 10 μg/mL (retention time: 11.8 min, flow rate: 1 mL/min).43 By comparison, MS detection, especially MS/MS, is highly sensitive, selective, and specific.48 HPLC-coupled with MS/MS (LC/MS/MS) can detect ATV in DBS between 0.025 and 20 μg/mL (retention time: 3.0 min, flow rate: 0.6 mL/min),43 and in plasma between 1 and 1000 ng/mL (retention time: 4.96 min, flow rate: 0.35 mL/min)45 and between 19.53 and 5000 ng/mL (retention time: 2.9 min, flow rate: 0.6 mL/min).47 In LC/MS/MS, the separation allows for differentiation between co-eluting analytes, particularly deuterated internal standards, which eliminates overlapping analyte peak signals and miscalculation of drug levels. Furthermore, LC/MS/MS allows for shorter analysis times because the drug can be eluted faster without interference based on selective analyte-specific multiple reaction monitoring.48,49 One of the disadvantages of LC/MS/MS is the potential influence of the matrix effect on the sensitivities of an analyte via ionization suppression or enhancement, which can be eliminated through sample clean-up and extraction steps.48
Our Hair Analytical Laboratory (HAL) at the University of California, San Francisco, has previously reported on the development of ARV hair assays utilizing LC/MS/MS, including efavirenz, lopinavir and ritonavir,3 nevirapine,4 tenofovir and emtricitabine.23 In this paper, we describe a validated effective extraction method and a validated sensitive and specific LC/MS/MS-based method for the analysis of ATV in human hair following NIH Division of AIDS Clinical Pharmacology Quality Assurance (CPQA) program50 and FDA bioanalytical method validation guidelines.51
2 | EXPERIMENTAL
2.1 | Materials
Atazanavir (ATV) sulfate reference compound and deuterated atazanavir-d5 (ATV-d5) internal standard (IS) were purchased from U.S. Pharmacopeia (USP, Rockville, MD, USA) and Toronto Research Chemicals, Inc. (TRC, Toronto, Canada), respectively. Acetonitrile (ACN), acetic acid, ethyl acetate (EA), methanol (MeOH), methyl tert- butyl ether (MTBE), sodium hydroxide (NaOH), and sodium phosphate dibasic anhydrous were all purchased from Thermo Fisher (Waltham, MA, USA). Trifluoracetic acid (TFA) was purchased from Acros Organics (Thermo Fisher). Ammonium acetate was obtained from Spectrum Chemical (New Brunswick, NJ, USA). All reagents and solvents used for LC/MS/MS analysis were of HPLC or analytical grade. Water was deionized and filtered using a NANOpure II water purification system (Barnstead, Boston, MA, USA).
Control human hair samples were acquired from healthy HIV- negative volunteers who had not received ATV and used as ARV-free blank hair samples. Hair samples for positive controls were obtained from HIV-positive patients recruited under the “Shaved Heads Study” from the UCSF San Francisco General Hospital (SFGH) -based HIV clinic (“Ward 86”). Patients enrolled into the “Shaved Heads Study” had to be on ATV-containing ART for at least 6 months, maintained good adherence as reported by both provider and patient, and have documented sustained virologic suppression (HIV RNA < 40 copies/mL) on ART. After the participants had been deemed eligible for the study, their hair was cut down to 2 centimeters (cm) and the remainder shaved into bags to serve as “incurred” (drug dosed) QC human hair samples for the laboratory. UCSF Institutional Review Board approved study procedures for the “Shaved Heads Study” (IRB#14-13687) and all participants provided written informed consent prior to participation.
2.2 | Instrumentation
2.2.1 | LC/MS/MS conditions
The LC/MS/MS analyses were performed using a Prominence UFLCXR system (Shimadzu, Kyoto, Japan) consisting of an LC pump (LC-20 ADXR), an autosampler (SIL-20 ACXR), a column oven (CTO-20 AC), a communications bus module (CBM-20A) coupled to an API 5000 triple quadrupole mass spectrometer (AB Sciex, Redwood City, CA, USA) and fitted with a Turbo V ion source. The analyses were performed using a reversed-phase column (BDS, C-18, 5.0 μm, 4.6 × 100 mm; Thermo Fisher). Detection of ATV and ATV-d5 was carried out by electrospray ionization (ESI) in positive ionization mode with an isocratic system that utilized a mobile phase composed of 55% ACN, 45% water, 0.15% acetic acid, and 4 mM ammonium acetate and a flow rate of 0.8 mL/min. Multiple reaction monitoring (MRM) was used to detect the analytes at the mass transitions MH+ m/z 705.3 to m/z 168.0 and MH+ m/z 710.2 to m/z 168.0 for ATV and ATV-d5, respectively. The standard curves were generated by plotting peak area ratios of ATV to ATV-d5 vs concentration of ATV with the weighted linear regression factor of 1/x. Data processing was performed with Analyst software (version 1.6.2; AB Sciex).
2.3 | Validation methods
2.3.1 | Standard and QC solutions
ATV was weighed and prepared independently in two sets of stock solutions with 50% MeOH: standard curve (SC) and quality control (QC) at a concentration of 1.00 mg/mL. Working solutions were diluted with 50% MeOH from the stock solutions at a concentration of 4.00 μg/mL for the high working and 0.100 μg/mL for the low working solutions. One set of working solutions is prepared for calibrating the assay (SC), while another set is made for QC. The stock and working solutions were stable for at least 6 months at 4°C. The internal standard (IS) of ATV-d5 was dissolved in 100% MeOH and prepared at a final concentration of 1.00 mg/mL. A working solution of 1.00 mg/mL of IS stock was diluted with 50% MeOH at a final concentration of 0.200 μg/mL and kept at 4°C.
2.3.2 | Sample preparation (with preparation of standard controls and QCs)
The standard curve (SC) was prepared by spiking ATV working solutions in accordance to the standard curve range (0 to 20.0 ng/mg hair) to cut blank human hair (2 mg) in borosilicate glass test tubes containing 9:1 MeOH/TFA (v/v) solution. Afterwards, IS working solution was added, the sample was vortexed, and the mixture incubated at 37°C overnight in a water shaking bath. The MeOH/TFA was evaporated to dryness by nitrogen (N2) gas at room temperature. The samples were mixed with 0.2 M sodium phosphate aqueous solution (pH adjusted to 9.4 with 1 M NaOH) followed by 3 mL of MTBE/EA (1:1, v/v). Following centrifugation at 3000 rpm for 10 min, the samples were frozen in a MeOH/dry ice bath. The organic layer was transferred to a glass test tube, evaporated to dryness by N2 gas, and reconstituted with 50% ACN (200 μL). Aliquots (2 μL) were injected for analysis by LC/MS/MS.
QC drug concentrations for high, medium, and low, 14.0, 1.00, and 0.150 ng/mg, respectively, were spiked into cut blank hair (2 mg) in borosilicate glass test tubes containing MeOH/TFA solution. Preparation of the QC samples was carried out employing the same method as described above.
For incurred hair QC sample analysis, approximately 2 mg of incurred hair was weighed and processed for the preparation of standard curve and QC samples as described above.
2.3.3 | Specificity
Six lots of blank human hair samples were obtained from healthy volunteers (HIV non-infected, not on medications). The blank hair samples were assayed in duplicate along with one lower limit of quantitation (LLOQ) sample following the SC and QC preparation steps described above. Interference from endogenous peaks was assessed at the retention times of the test analyte by evaluating the drug and IS peak areas of the LLOQ sample to any endogenous peaks observed in the blank hair samples.
2.3.4 | Linearity
Linearity was determined by generating the standard curve. The standard curve was made up of one double blank sample (blank hair without ATV and IS), one blank sample (blank hair with only IS), and standard curve samples spiked at nine ATV concentrations (0.0500, 0.100, 0.200, 0.500, 1.00, 2.00, 4.00, 10.0, and 20.0 ng/mg hair). The linearity of the standard curve was evaluated by the analyte to IS peak area ratio using a 1/x weighted linear regression equation and the correlation coefficient (r). Normalized concentration was defined by the calculated concentration obtained from the standard curve multiplied by 2 mg (the target weight) and divided by the actual weight of the hair sample. The accuracy and precision acceptance criteria were ≤ ±15% for %RE and ≤15% for %CV for the standard curve samples except for LLOQ, which was ≤ ±20% for %RE and ≤20% for %CV. Following the FDA Guidance for Bioanalytical Method Validation,52 up to two standard curve samples (not including the LLOQ and upper limit of quantitation) which do not meet acceptance criteria were excluded from the standard curve.
2.3.5 | Accuracy and precision
The inter- and intraday accuracy and precision of the method were evaluated on three separate days by analyzing QC samples containing human hair with known spiked concentrations of ATV, through 6 sets (n = 3) of control samples at three QC concentrations; high (14.0 ng/mg hair), medium (1.00 ng/mg hair), and low (0.150 ng/mg hair). We also tested the precision of the assay on incurred QC samples obtained from HIV-infected patients on ATV-containing ART. In addition, the accuracy and precision of the LLOQ samples were evaluated. Accuracy was defined as the percent relative error (%RE) between the mean and nominal concentrations. The acceptance criterion for the LLOQ samples was ≤ ±20% for %RE; the criterion for SC samples, and QC samples at the three QC concentrations was ≤ ±15% for %RE. Precision was defined as the percent coefficient of variation (%CV) between the mean and standard deviation (SD). The acceptance criterion for LLOQ samples was ≤20% for %CV; the criterion for incurred QC hair samples, SC samples, and QC samples at three QC concentrations was ≤15% for %CV.
2.3.6 | Recovery
The recovery of spiked ATV was obtained by comparing the peak area ratio of extracted to unextracted hair samples at three QC concentrations (high, medium, low) in triplicates. The IS was added after the extraction process. The recovery of IS (ATV-d5) is obtained by comparing the IS peak area ratio of extracted IS with spiked IS to unextracted IS in hair samples in triplicates. QC medium concentration was added after the extraction process. The acceptance criterion is that the percent difference between the averages of three QC concentration recoveries should be no more than 30%.
2.3.7 | Extraction efficiency of incurred samples
The extraction efficiency of incurred QC hair samples was determined by measuring the ATV concentration present in the hair residue after repeated extractions. ATV from incurred hair samples, in triplicate, was extracted following normal sample preparation conditions (for the first extraction). The hair was then separated from the first extracted solution, dried under N2 gas, combined with new extraction solution, including IS, and processed for further extraction. For the second and third extractions, the incurred hair samples were treated under the same conditions as in the first extraction.
2.3.8 | Matrix effect
The matrix effect was determined by comparing the peak responses of ATV and IS in post-extracted blank samples (in the presence of hair) to those in pure solution samples (in the absence of hair). The matrix effect was evaluated at three QC concentrations (high, medium, low) in six different lots of blank human hair and processed under the sample preparation conditions as described above. The acceptance criterion was set at ≤15% for %CV.
2.3.9 | Stability
The stability of the extracted samples in the refrigerator at 4°C was evaluated at two concentrations (high and low QC) in triplicate for up to 1 week. The drug to IS area ratio was evaluated by using a freshly prepared standard curve and QC samples. The exclusion criterion of ATV nominal mean concentration deviation is defined as less than or equal to ±15% of the spiked nominal concentration at day 0.
The stability of spiked samples in incubation was evaluated at three QC concentrations, in triplicate. Incubated samples were analyzed against non-incubated samples at the same concentrations. The %RE of the stability test was defined as incubated peak area ratio minus the non-incubated peak area ratio and divided by non-incubated peak area ratio. The acceptance criterion of ATV concentration deviation is %RE being less than or equal to ±15% between incubated and non-incubated samples.
The stability of stored incurred QC samples was assessed at room temperature in triplicate and at selected time points. The acceptance criterion of ATV concentration deviation is %RE being less than or equal to ±15% between time points and initial test samples.
The light-sensitivity stability of incurred hair samples was evaluated by placing the incurred QC hair samples underneath a light source for 24 and 48 h. After each time interval, the concentrations of incurred QC hair samples underneath a light source were compared against the concentrations of incurred QC hair samples stored in the absence of light, which serve as 0 h. %RE between average normalized concentrations was defined as n-h concentration minus the 0-h concentration and then divided by the 0-h concentration. The acceptance criterion of ATV concentration deviation is %RE being less than or equal to ±15% between light-exposed hair samples and hair samples kept in the dark (0-h samples).
3 | RESULTS
3.1 | Specificity
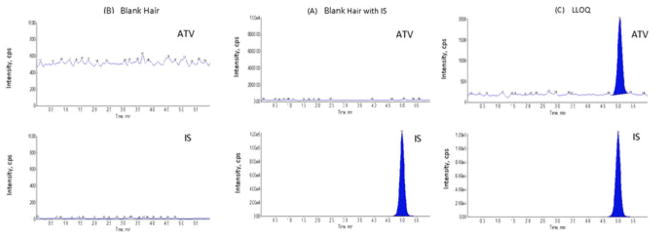
The specificity of the extraction and analytical method for ATV from human hair was tested using six lots of blank hair acquired from healthy HIV-non-infected volunteers. Chromatograms are shown in Figure 1. The chromatograms of blank hair samples showed no interference peaks at ATV or IS retention times in tandem with an ATV LLOQ sample. This indicated that the present method is specific for the analysis of ATV in hair samples.
FIGURE 1.
LC/MS/MS chromatograms obtained by applying the present method: (A) blank hair sample, (B) blank hair sample with internal standard, and (C) LLOQ sample [Color figure can be viewed at wileyonlinelibrary.com]
3.2 | Linearity
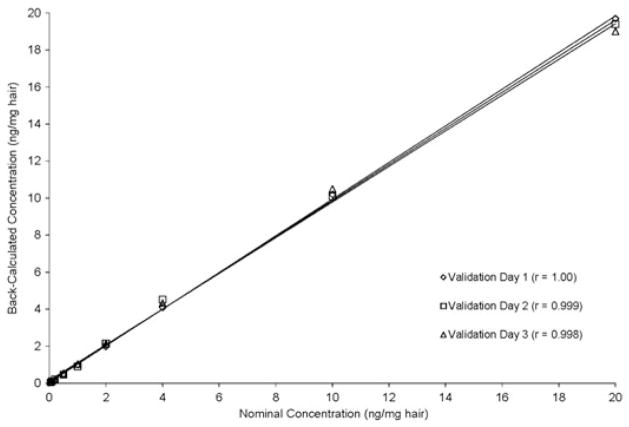
The linearity of the standard curve was back-calculated by plotting peak area ratios of ATV to IS against the nominal concentrations of the standard curve range (0.0500–20.0 ng/mg hair) and is shown in Figure 2. The three individual validation standard curves showed good correlation coefficients (r) of 1.00, 0.999, and 0.998, respectively.
FIGURE 2.
Plot of back-calculated concentrations of calibration standards of atazanavir
3.3 | Accuracy and precision
The inter- and intraday accuracy and precision for spiked ATV into blank hair samples at four concentrations (LLOQ, high QC, medium QC, and low QC) were evaluated over three sets of preparations and are summarized in Table 1. The interday precisions (%CVs) of the four concentrations were between 3.13 and 10.8%, and accuracies (%RE) were between -4.00 and 2.00%. The intraday precisions of four concentrations were between 1.75 and 6.31% with accuracies from -14.6 to 8.00%. The incurred QC hair sample analysis was assessed for three preparations, as summarized in Table 2. The intra- and interday precisions for incurred hair samples were 6.15% and between 5.22 and 7.35%, respectively. These results suggest that the present method is accurate, precise, and reproducible for extraction as well as quantitation of ATV from hair samples.
TABLE 1.
Accuracy and precision of quality controls concentrations calculated by standard curve
| Run No. | Sample No. | LLOQ 0.0500 (ng/mg hair) | Low 0.150 (ng/mg hair) | Medium 1.00 (ng/mg hair) | High 14.0 (ng/mg hair) | Run No. | Intraday statistics | LLOQ 0.0500 (ng/mg hair) | Low 0.150 (ng/mg hair) | Medium 1.00 (ng/mg hair) | High 14.0 (ng/mg hair) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0470 | 0.153 | 1.07 | 14.3 | Mean | 0.0471 | 0.151 | 1.03 | 14.0 | ||
| 2 | 0.0483 | 0.149 | 1.08 | 14.3 | SD | 0.0013 | 0.003 | 0.04 | 0.4 | ||
| 1 | 3 | 0.0474 | 0.154 | 1.02 | 14.1 | 1 | %CV | 2.76 | 1.99 | 3.88 | 2.86 |
| 4 | 0.0452 | 0.150 | 1.03 | 14.1 | %RE | −5.80 | +0.667 | +3.00 | 0.00 | ||
| 5 | 0.0463 | 0.147 | 1.01 | 13.8 | n | 6 | 6 | 6 | 6 | ||
| 6 | 0.0486 | 0.152 | 0.981 | 13.3 | |||||||
| 1 | 0.0540 | 0.159 | 1.04 | 14.2 | Mean | 0.0540 | 0.153 | 0.990 | 13.9 | ||
| 2 | 0.0563 | 0.151 | 0.993 | 13.4 | SD | 0.0034 | 0.003 | 0.049 | 0.5 | ||
| 2 | 3 | 0.0524 | 0.149 | 1.05 | 14.7 | 2 | %CV | 6.30 | 1.96 | 4.95 | 3.60 |
| 4 | 0.0506 | 0.153 | 0.966 | 13.8 | %RE | +8.00 | +2.00 | −1.00 | −0.714 | ||
| 5 | 0.0596 | 0.153 | 0.972 | 13.3 | n | 6 | 6 | 6 | 6 | ||
| 6 | 0.0513 | 0.155 | 0.921 | 13.9 | |||||||
| 1 | 0.0432 | 0.140 | 0.945 | 13.0 | Mean | 0.0427 | 0.148 | 1.04 | 13.9 | ||
| 2 | 0.0415 | 0.142 | 1.04 | 13.5 | SD | 0.0008 | 0.006 | 0.06 | 0.7 | ||
| 3 | 3 | 0.0431 | 0.146 | 1.02 | 13.5 | 3 | %CV | 1.87 | 4.05 | 5.77 | 5.04 |
| 4 | 0.0437 | 0.152 | 1.11 | 14.8 | %RE | −14.6 | −1.33 | +4.00 | −0.714 | ||
| 5 | 0.0420 | 0.150 | 1.06 | 14.5 | n | 6 | 6 | 6 | 6 | ||
| 6 | 0.0428 | 0.156 | 1.06 | 14.3 | |||||||
| Interday statistics | Intraday ranges | ||||||||||
| Theoretical conc. | 0.0500 | 0.150 | 1.00 | 14.0 | |||||||
| Mean | 0.0480 | 0.151 | 1.02 | 13.9 | Mean | 0.0427–0.0540 0.148–0.153 | 0.990–1.04 | 13.9–14.0 | |||
| SD | 0.0052 | 0.005 | 0.05 | 0.5 | SD | 0.0008–0.0034 0.003–0.006 | 0.04–0.06 | 0.4–0.7 | |||
| %CV | 10.8 | 3.31 | 4.90 | 3.60 | %CV | 1.87–6.30 | 1.96–4.05 | 3.88–5.77 | 2.86–5.04 | ||
| %RE | −4.00 | +0.667 | +2.00 | −0.714 | %RE | −14.6 to +8.00 | −1.33 to +2.00 | −1.00 to +4.00 | −0.714 to 0.00 | ||
| n | 18 | 18 | 18 | 18 | n | 6 | 6 | 6 | 6 | ||
TABLE 2.
Precision of incurred sample concentrations calculated by standard curve
| Run No. | Sample No. | Weight of hair (mg) | Nominal conc. (ng/mg hair) | Normalized conc. (ng/mg hair) | Run No. | Intraday statistics | Normalized conc. (ng/mg hair) |
|---|---|---|---|---|---|---|---|
| 1 | 1.70 | 1.56 | 1.84 | ||||
| 2 | 1.76 | 1.49 | 1.69 | Mean | 1.70 | ||
| 1 | 3 | 1.72 | 1.47 | 1.71 | 1 | SD | 0.13 |
| 4 | 1.94 | 1.78 | 1.84 | %CV | 7.65 | ||
| 5 | 1.73 | 1.40 | 1.62 | n | 6 | ||
| 6 | 1.92 | 1.46 | 1.52 | ||||
| 1 | 1.81 | 1.59 | 1.76 | ||||
| 2 | 2.02 | 1.69 | 1.67 | Mean | 1.70 | ||
| 2 | 3 | 1.74 | 1.38 | 1.59 | 2 | SD | 0.11 |
| 4 | 2.02 | 1.57 | 1.55 | %CV | 6.47 | ||
| 5 | 1.92 | 1.77 | 1.84 | n | 6 | ||
| 6 | 1.85 | 1.63 | 1.76 | ||||
| 1 | 1.75 | 1.38 | 1.58 | ||||
| 2 | 1.98 | 1.59 | 1.61 | Mean | 1.66 | ||
| 3 | 3 | 1.86 | 1.63 | 1.75 | 3 | SD | 0.09 |
| 4 | 1.77 | 1.46 | 1.65 | %CV | 5.42 | ||
| 5 | 1.78 | 1.59 | 1.79 | n | 6 | ||
| 6 | 1.72 | 1.38 | 1.60 | ||||
| Interday statistics | Intraday ranges | ||||||
| Theoretical Conc. | |||||||
| Mean | 1.69 | Mean | 1.66–1.70 | ||||
| SD | 0.10 | SD | 0.09–0.13 | ||||
| CV | 5.92 | %CV | 5.42–7.65 | ||||
| n | 18 | n | 6 | ||||
3.4 | Recovery
The recovery of spiked samples of ATV was assessed at three QC concentrations (high, medium and low), along with the internal standard, and the results are summarized in Table 3. The percentage of recoveries for the three QC concentrations and internal standard were 76.6%, 88.4%, 93.7%, and 104%, respectively. The overall average recovery for the three QC ATV concentrations was 86.2%. The percentage difference between high QC and medium QC, high QC and low QC, and medium QC and low QC were 11.8%, 17.1%, and 5.30%, respectively.
TABLE 3.
Recovery of spiked samples of atazanavir
| Sample No. | Spiked conc. (ng/mg hair) | Peak area ratio | |
|---|---|---|---|
| Unprocessed sample | Processed sample | ||
| 1 | 14.0 | 8.29 | 6.83 |
| 2 | 9.11 | 6.67 | |
| 3 | 9.47 | 7.08 | |
| MEAN ± SD | 8.96 ± 0.61 | 6.86 ± 0.21 | |
| RECOVERY (6.86/8.96) X 100 = 76.6% | |||
| 1 | 1.00 | 0.578 | 0.526 |
| 2 | 0.639 | 0.615 | |
| 3 | 0.694 | 0.547 | |
| MEAN ± SD | 0.637 ± 0.058 | 0.563 ± 0.047 | |
| RECOVERY (0.563/0.637) X 100 = 88.4% | |||
| 1 | 0.150 | 0.0915 | 0.0801 |
| 2 | 0.0961 | 0.0839 | |
| 3 | 0.0898 | 0.0962 | |
| MEAN ± SD | 0.0925 ± 0.0033 | 0.0867 ± 0.0084 | |
| RECOVERY (0.0867/0.0925) × 100 = 93.7% | |||
| OVERALL AVERAGE RECOVERY = 86.2% | |||
| % DIFFENCE BETWEEN H QC AND M QC |76.6 –88.4%| = 11.8% | |||
| % DIFFENCE BETWEEN H QC AND L QC |76.6–93.7%| = 17.1% | |||
| % DIFFENCE BETWEEN M QC AND L QC |88.4–93.7%|= 5.30% | |||
| INTERNAL STANDARD (IS) | |||
| 1 | 1.49 | 1.55 | |
| 2 | 1.35 | 1.42 | |
| 3 | 1.55 | 1.60 | |
| MEAN ± SD | 1.46 ± 0.10 | 1.52 ± 0.09 | |
| IS RECOVERY (1.52/1.46) × 100 = 104% | |||
3.5 | Extraction efficiency
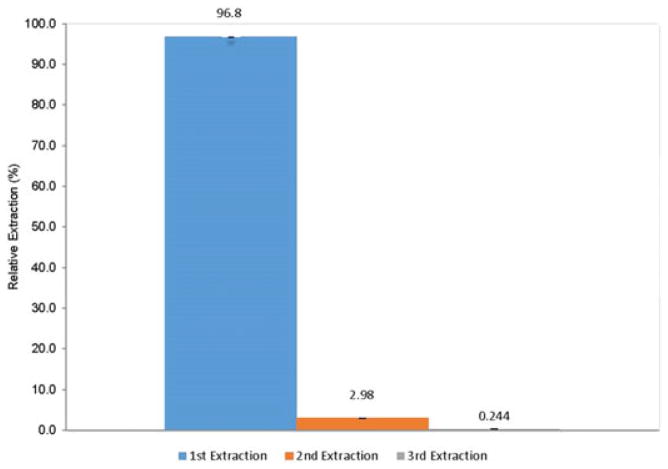
The extraction efficiency of ATV from incurred hair samples was evaluated in repeated extractions (three extraction processes) under the current condition to measure the amounts of ATV extracted from the hair residue. The percentage extracted at each of the three steps is presented in Figure 3. The sum of the total extracted ATV in these three extraction steps was used as the total amount of ATV in the hair. More than 95% of ATV was extracted during the first extraction, and the remaining hair residue contained an insignificant amount of ATV, as demonstrated by the second and third extractions. Since more than 95% of ATV was extracted at the first extraction, we decided to proceed with only one extraction process when finalizing our method.
FIGURE 3.
Extraction efficiency of atazanavir from incurred samples by present method (n = 3) [Color figure can be viewed at wileyonlinelibrary.com]
3.6 | Matrix effect
The matrix effect (ion suppression or enhancement) of the hair extract was evaluated at three QC concentrations (high, medium, and low) by determining the matrix factor (MF) using six lots of blank human hair samples in triplicate. The IS-normalized MF mean of three QC concentrations was 1.01, 0.986, and 1.00, and the %CVs were 2.11, 1.34, and 2.53, respectively. Based on these results, we demonstrated that there was minimum matrix effect for our method.
3.7 | Stability
ATV levels in extracted human hair were shown to be stable for 1 week in the refrigerator at 4°C; the percentage deviation was between 2.50 and 5.07% for processed sample stability. Furthermore, in an incubation shaker at 37°C overnight, the percentage change in ATV levels between the non-incubated and incubated samples at the three QC concentrations was -2.25 to 0.863%. ATV levels in hair when stored at room temperature in the absence of light (as represented by work with the incurred QC hair samples) were also stable for at least 10 months; the percentage deviation in ATV levels from the initial test (day 0) to levels determined at 10 months was -11.8 to 2.94%. The levels of ATV extracted from incurred QC hair were also stable when exposed to light for 24 h and 48 h; the percentage differences between the average normalized concentrations were 2.94% and 11.8%, respectively, demonstrating no significant changes in concentration after exposure to light. Since all the stability experiments met our acceptance criteria, we concluded extracted ATV was stable at 4°C for 1 week, 37°C overnight, and after being exposed to light. ATV levels in hair were not significantly changed after prolonged storage of the hair at room temperature and in the absence of light.
4 | DISCUSSION
4.1 | Why develop a method to analyze ARVs in hair samples?
Although ART has changed the course of HIV infection from a highly morbid process to a chronic condition, challenges in adhering to daily regimens remain. Moreover, there is no gold standard for assessing adherence in the context of HIV treatment or prevention. Self- reported adherence is subject to recall bias, social desirability bias, or failure to remember dosing patterns over time.53,54 Pill counts are subject to patients discarding pills before clinic or study visits and do not provide information on dose timing lapses, both of which can make this measure unreliable in predicting outcomes.55–57 Medication electronic monitoring devices on bottle caps (e.g. MEMS caps) to record openings are subject to patients not taking the pill despite bottle opening,58 expense,59 stigma due to bulk of the monitoring device,60,61 and incompatibility with pillboxes (a commonly employed adherence tool).62 There is therefore increasing interest in therapeutic drug monitoring (TDM) to analyze ARV levels in a specific biomatrix as an objective biomarker of adherence.
Plasma and serum are the most commonly used matrices in drug analysis and TDM, but have limitations including the requirement for a phlebotomist and sterile equipment and their infectious potential, requiring biohazardous precautions or viral deactivation. The use of anticoagulants in plasma collection tubes may interfere with analyses by formation of fibrinogen.63 Serum collection tubes do not use anticoagulant but clot formation can be incomplete or delayed, which may result in analyte instability.63 Moreover, single plasma levels of ARVs, like single glucose measurements, are limited in their ability to predict long-term treatment outcomes, because they reflect only a short duration of exposure,64–66 demonstrate significant day-to-day variation,64 and are subject to “white-coat” effects, where adherence improves transiently prior to study or clinic visits.67,68
Assessing drug levels in DBS for TDM has some advantages in that blood collection can be less invasive if fingerprick testing is available, the sample required is small, and DBS can be stored and transported for short periods of time at ambient temperature before being put in cold storage.69 Furthermore, pharmacogenomics testing (genotyping)52 can be performed using DBS. Caveats to DBS monitoring include the need to ensure that the blood spot (and therefore drug levels) on the filter paper is uniformly distributed,49 the requirement for a dry time of 90 min to 4 h, and the need to avoid exposure to sunlight. Moreover, biological factors (e.g. blood viscosity and hematocrit) may contribute to variability in sample quality.69 Analytes in DBS must remain stable during the drying process and during the process of selectively eluting for the analyte of interest.52,69 Since DBS contain blood, the same biohazardous safety precautions during sample collection, storage and shipment are required.
Hair can provide a retroactive timeline of a person's drug exposure over prolonged periods, from weeks to months (even years).2,70,71 Drug is incorporated into the hair through the systemic circulation. Since the rate of hair growth is approximately 1 cm per month, a rate that remains steady in different populations and age groups,72–74 the length of the hair serves as a marker of time and can provide drug exposure over different time points through the use of segmental analysis.2,70,71 Furthermore, hair has certain advantages as a clinical sample, such as ease of collection (no phlebotomy required so noninvasive), stability at room temperature (obviating the need of a cold chain in storage and shipping), and the fact that it is non-infectious (so it can be stored and shipped without biohazardous precautions).73
There are also disadvantages to analyzing drug levels in hair due to both external and internal factors, such as cosmetic treatments or cleaning products (e.g. hair shampoo, conditioner and hair-dye), weather (such as sun exposure or humidity), hair color, and structure (e.g. straight, curly). The pigmentation in hair is from melanin, which is associated with the drug binding rate in hair only if the drug is primarily basic (although not acidic).72 Despite these limitations, our group (the UCSF Hair Analytical Laboratory) has found that hair ARV measurements are stronger predictors of HIV outcomes than self-reported adherence,6,7,9,10,20,21 or single plasma ARV concentrations,8,11 in HIV treatment, and are useful for adherence and toxicity monitoring in the context of pre-exposure prophylaxis (PrEP).22–34 Moreover, we have not found significant differences in median and range hair concentrations of ARVs by race/ethnicity.
4.2 | Overcoming challenges to develop a TDM method using hair
The biggest challenge of drug analysis using hair as a biological matrix is in the sample extraction process. The extraction recovery of ATV in human hair in our assay was assessed in two ways: (1) spiked standard curve and QC concentrations into blank hair samples, and (2) analyzing hair from shaved head specimens from HIV-infected patients on ATV- based therapy with documented, sustained virologic suppression and good adherence. These large hair samples (from shaving the head of highly adherent patients on ART) represent a strength in our method development process, allowing for repeated experiments (e.g. different solvents, different lengths of time for extraction) to ultimately optimize the method.
We report our results in the form of weight of drug/weight of hair as opposed to concentration of weight of drug/volume of hair because hair is a solid and not a liquid like plasma. During sample processing, the exact weight of the hair sample is recorded, which should be within 20% of the target weight (2 mg). For the reported results, the final drug concentration in hair are normalized by the actual hair weight (e.g. Normalized concentration = [(Calculated concentration) × (Target hair weight)]/(Actual hair weight)). Normalizing the calculated concentration to 2 mg allows for the accurate reporting of drug concentration at measured weight ±20% of the target weight. The results are still consistent in regard to the relationship between weights to concentration. Huang et al examined if different hair weights alter the accuracy of the assay by weighing and analyzing 0.1–4.0 mg of shaved hair sample under the assay's conditions;3,4 the results showed linearity for the drug to IS peak area ratio and consistency for the peak area ratio to hair weight. This work led us to conclude that hair weights have no influence on the assay once the weighed weights are normalized.
We chose the target weight of 2 mg because this mass can be weighed accurately on a laboratory balance, is sufficient for drug detection, and lower weights can be accommodated for dilutions if a sample's concentration is over the highest calibrator (e.g. 1 mg vs 2 mg). Participant hair samples received from research study sites can be scant (<10 strands of hair), despite our request for 20–30 strands to analyze ATV. This becomes a challenge, as a result of insufficient amounts of hair, when we need to reanalyze the sample due to unacceptable chromatograms or dilutions because the concentration is over the highest calibrator on our standard curve.
Crosstalk between other ARVs as well as other concomitant medications is a possibility because ARVs are prescribed in combination; hence, it is important that we do not see any crosstalk between the drugs in our method. We have tested effects of ritonavir, which is a booster and often co-administered with ATV, and lopinavir, which is another PI, and did not see crosstalk between these drugs and ATV (data not shown). Therefore, the current analytical method is specific and suitable for ATV quantitation in hair.
Even though matrix effects were tested with six lots of blank hair, these are not representative of all types of hair; they represent hair that is clean, devoid of chemical products, and not chemically processed. We did an experiment with six lots of blank hair weighing approximately to 2, 5, and 10 mg, following the same processing procedure as described above, testing the relationship between increase in the hair matrix and matrix effect. The results of the experiment indicated that more matrix can affect the assay. However, due to our use of the deuterated IS, we can compensate for the matrix effect. Finally, our analytical method does not take into account potential variables caused by hair dyes, shampoos, conditioners, bleach, hair products (e.g. gel, baby powder, hair spray, etc.), and other treatments (e.g. straighteners, permanent wave chemicals) that may be present in research samples. All of these can potentially cause matrix effects but, from our experiments (relationship between hair weights and matrix effect), these variables can be compensated for by using a deuterated IS, which helps in interpreting the results due to its behavioral similarities to the drug. We are currently working on experiments to elucidate the effects of hair dyes and other treatments on ARV levels in hair.
4.3 | Reproducibility of our assay and validation standards
Studies have shown strong relationships between ATV levels in hair and virologic outcomes among HIV-infected patients on ATV-based therapy.7,20 The most recently published study was conducted among adolescents in Zimbabwe failing second-line ART20 and our laboratory analyzed the ATV concentrations in hair samples collected at baseline and 3 months. The median ATV hair concentrations among those experiencing virologic failure (defined by HIV RNA >400 copies/mL) was 0.99 ng/mg and the median ATV hair levels among those with virologic success was 3.33 ng/mg (p = 0.0042). To confirm our assay's reproducibility we conducted further experiments, where we randomly selected 20 patient hair samples from that study20 and reanalyzed them. Table 4 shows the original ATV concentrations of the assay runs for these 20 samples, the re-run concentrations, and the percentage differences between the two values. The percentage difference between the original and reanalyzed results met the acceptance criterion by the Food and Drug Administration (FDA) guidelines for Bioanalytical Method Validation,51 thus demonstrating our method's reproducibility for participant samples.
TABLE 4.
Reproducibility of clinical sample results for our LC/MS/MS-based method to analyze ATV in small hair samples*
| Participant sample no | Normalized original conc. (ng/mg) | Normalized repeat conc. (ng/mg) | Average normalized conc. (ng/mg) | Difference (%)** |
|---|---|---|---|---|
| 1 | 2.75 | 2.99 | 2.87 | 8.36 |
| 2 | 6.09 | 5.49 | 5.79 | −10.4 |
| 3 | 2.25 | 2.85 | 2.55 | 23.5 |
| 4 | 3.20 | 3.40 | 3.30 | 6.06 |
| 5 | 17.3 | 15.7 | 16.5 | −9.70 |
| 6 | 1.92 | 1.61 | 1.77 | −17.5 |
| 7 | 2.97 | 2.80 | 2.89 | −5.88 |
| 8 | 1.27 | 1.50 | 1.39 | 16.5 |
| 9 | 1.87 | 2.28 | 2.08 | 19.7 |
| 10 | NA | NA | NA | ND |
| 11 | 3.30 | 3.62 | 3.46 | 9.25 |
| 12 | 3.07 | 3.60 | 3.34 | 15.9 |
| 13 | 2.46 | 3.06 | 2.76 | 21.7 |
| 14 | 2.09 | 2.11 | 2.10 | 0.952 |
| 15 | 3.72 | 3.84 | 3.78 | 3.17 |
| 16 | 2.39 | 2.61 | 2.50 | 8.80 |
| 17 | 2.20 | 2.18 | 2.19 | −0.913 |
| 18 | 3.25 | 3.45 | 3.35 | 5.97 |
| 19 | 2.20 | 2.37 | 2.29 | 7.42 |
| 20 | 5.15 | 5.59 | 5.37 | 8.19 |
FDA incorporated Incurred Sample Reanalysis Table.
Percentage (%) Difference = ((Normalized repeat conc. − Normalized original conc.)/(Average normalized conc.) × 100.
NA – Not Applicable; ND – No Difference
Validation of our method was conducted based on FDA guidelines for analytical procedures51 and methods validation for drugs and biologics; Clinical Pharmacology Quality Assurance (CPQA) guidelines for chromatographic method development and validation50 from the National Institutes of Health' Division of AIDS (DAIDS); and European Medicines Agency guidelines on bio-analytical method validation.75 In 2017, our assay was peer-reviewed and approved by the DAIDS' supported CPQA program.
5 | CONCLUSIONS
The aim of this study was to develop and validate a sensitive and specific LC/MS/MS analytical method for ATV in human hair. Our method was accurate and precise, displayed good linearity, had minimal matrix effect, and extracted samples were stable under a variety of conditions. The proposed method can be utilized to monitor long-term adherence and exposure to ATV in HIV-infected patients.
Acknowledgments
Funding information National Institutes of Health, Grant/Award Numbers: 2R01AI098472, R21AI112362 and RO1MH109310
Support for this work came from the National Institutes of Allergy and Infectious Diseases (NIAID)/NIH R21AI112362 (P.I. Gandhi) and 2R01AI098472 (P.I. Gandhi). We thank the program office – Hao Zhang PhD – for both NIH grants for his support of and contribution to our work. Further support came from the National Institutes of Mental Health (NIMH)/NIH RO1MH109310. The authors would like to thank Shirley Yee from the Hair Analytical Laboratory (HAL) for work on this assay. We thank the AIDS Clinical Trials Group leadership and participants for collaboration on a treatment study (A5257) that examined atazanavir-based regimens. Finally, we give our heartfelt thanks to shaved heads participants who contributed hair for assay development.
References
- 1.Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137:696–697. doi: 10.7326/0003-4819-137-8-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Yang Q, Yoon K, et al. Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:1923–1933. doi: 10.1007/s00216-011-5278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M, Greenblatt RM, Bacchetti P, et al. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis. 2012;206:1453–1461. doi: 10.1093/infdis/jis508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23:471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52:1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Zyl GU, van Mens TE, McIlleron H, et al. Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56:333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koss C, Natureeba P, Mwesigwa J, et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding ugandan women. AIDS. 2015;29:825–830. doi: 10.1097/QAD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxi SM, Greenblatt RM, Bacchetti P, et al. Nevirapine concentration in hair samples is a strong predictor of virologic suppression in a prospective cohort of HIV-infected patients. PLoS One. 2015;10(6):e0129100. doi: 10.1371/journal.pone.0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasitsuebsai W, Kerr SJ, Truong KH, et al. Using lopinavir concentrations in hair samples to assess treatment outcomes on second-line regimens among Asian children. AIDS Res Hum Retroviruses. 2015;31:1009–1014. doi: 10.1089/aid.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi M, Mwesigwa J, Aweeka F, et al. Hair and plasma data show that lopinavir, ritonavir, and efavirenz all transfer from mother to infant in utero, but only efavirenz transfers via breastfeeding. J Acquir Immune Defic Syndr. 2013;63:578–584. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey MD, Salmen CR, Tessler RA, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66:311–315. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olds PK, Kiwanuka JP, Nansera D, et al. Assessment of HIV antiretrovi-ral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care. 2015;27:327–332. doi: 10.1080/09540121.2014.983452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartelink IH, Savic RM, Mwesigwa J, et al. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. J Clin Pharmacol. 2014;54:121–132. doi: 10.1002/jcph.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohan D, Natureeba P, Koss CA, et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29:183–191. doi: 10.1097/QAD.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Yang Q, Bacchetti P, Huang Y. A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource- limited settings. AIDS Res Hum Retroviruses. 2014;30:25–28. doi: 10.1089/aid.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwadz M, Cleland CM, Applegate E, et al. Behavioral intervention improves treatment outcomes among HIV-infected individuals who have delayed, declined, or discontinued antiretroviral therapy: A randomized controlled trial of a novel intervention. AIDS Behav. 2015;19:1801–1817. doi: 10.1007/s10461-015-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickey MD, Salmen CR, Omollo D, et al. Pulling the network together: quasi-experimental trial of a patient-defined support network intervention for promoting engagement in HIV care and medication adherence on Mfangano Island, Kenya. J Acquir Immune Defic Syndr. 2015;66:311–315. doi: 10.1097/QAI.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawana TD, Gandhi M, Nathoo K, et al. Defining a cut-off for atazanavir in hair samples associated with virological failure among adolescents failing second-line antiretroviral treatment. J Acquir Immune Defic Syndr. 2017;76:55–59. doi: 10.1097/QAI.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pintye J, Bacchetti P, Teeraananchai S, et al. Brief report: Lopinavir hair concentrations are the strongest predictor of viremia in HIV-infected Asian children and adolescents on second-line antiretroviral therapy. J Acquir Immune Defic Syndr. 2017;76:367–371. doi: 10.1097/QAI.0000000000001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: Hair as a potential adherence measure for pre-exposure prophylaxis (PrEP) PLoS One. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. https://doi.org/10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxi SM, Liu A, Bacchetti P, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68:13–20. doi: 10.1097/QAI.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi M, Glidden DV, Liu A, et al. Strong correlation between concentrations of tenofovir (TFV) and emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx Open Label Extension: Implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis. 2015;212:1402–1406. doi: 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheth AN, Rolle CP, Gandhi M. HIV pre-exposure prophylaxis for women. J Virus Erad. 2016;2:149–155. doi: 10.1016/S2055-6640(20)30458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi M, Glidden DV, Mayer K, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3(11):e521–e528. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koss CA, Bacchetti P, Hillier SL, et al. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses. 2017;33:778–783. doi: 10.1089/aid.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saberi P, Neilands TB, Ming K, et al. Strong correlation between concentrations of antiretrovirals in home-collected and study-collected hair samples: Implications for adherence monitoring. J Acquir Immune Defic Syndr. 2017;76(4):e101–e103. doi: 10.1097/QAI.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi M, Murnane PM, Bacchetti P, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS. 2017;31:2245–2251. doi: 10.1097/QAD.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koss CA, Hosek SG, Bacchetti P, et al. Comparison of measures of adherence to HIV preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis. 2018;66:213–219. doi: 10.1093/cid/cix755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abaasa A, Hendrix C, Gandhi M, et al. Utility of different adherence measures for PrEP: Patterns and incremental value. AIDS Behav. 2017 doi: 10.1007/s10461-017-1951-y. https://doi.org/10.1007/s10461-017-1951-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 32.Seifert SM, Castillo-Mancilla JR, Erlandson K, et al. Adherence bio-marker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001596. https://doi.org/10.1097/QAI.0000000000001596. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 33.Baxi SM, Vittinghoff E, Bacchetti P, et al. Comparing pharmacologic measures of tenofovir exposure in a U.S. pre-exposure prophylaxis randomized trial. PLOS One. 2018;13(1):e0190118. doi: 10.1371/journal.pone.0190118. https://doi.org/10.1371/journal.pone.0190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markowitz M, Grossman H, Anderson PL, et al. Newly acquired infection with multidrug-resistant HIV-1 in a patient adherent to preexposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:e104–e106. doi: 10.1097/QAI.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertz RJ, Persson A, Chung E, et al. Pharmacokinetics and pharmacody-namics of atazanavir-containing antiretroviral regimens, with or without ritonavir, in patients who are HIV-positive and treatment- naive. Pharmacotherapy. 2013;33:284–294. doi: 10.1002/phar.1205. [DOI] [PubMed] [Google Scholar]
- 36.McCabe SM, Ma Q, Slish JC, et al. Antiretroviral therapy: pharmacoki-netic considerations in patients with renal or hepatic impairment. Clin Pharmacokinet. 2008;47:153–172. doi: 10.2165/00003088-200847030-00002. [DOI] [PubMed] [Google Scholar]
- 37.Le Tiec C, Barrail A, Goujard C, Taburet AM. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin Pharmacokinet. 2005;44:1035–1050. doi: 10.2165/00003088-200544100-00003. [DOI] [PubMed] [Google Scholar]
- 38.Cattaneo D, Riva A, Clementi E, Milazzo L, Gervasoni C. Severe hyperbilirubinemia in an HIV-HCV-coinfected patient starting the 3D regimen that resolved after TDM-Guided atazanavir dose reduction. Ther Drug Monit. 2016;38:285–287. doi: 10.1097/FTD.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 39.Gervasoni C, Meraviglia P, Minisci D, et al. Metabolic and kidney disorders correlate with high atazanavir concentrations in HIV-infected patients: is it time to revise atazanavir dosages? PLoS One. 2015;10(4):e0123670. doi: 10.1371/journal.pone.0123670. https://doi.org/10.1371/journal.pone.0123670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nso Roca AP, Larru B, Bellon JM, et al. HIV-infected adolescents: relationship between atazanavir plasma levels and bilirubin concentrations. J Adolesc Health. 2011;48:100–102. doi: 10.1016/j.jadohealth.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Uglietti A, Novati S, Gulminetti R, Maserati R. Correlations between atazanavir C (trough)and hyperbilirubinemia: a case report. J Med Case Reports. 2009;3:9307. doi: 10.1186/1752-1947-3-9307. https://doi.org/10.1186/1752-1947-3-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lennox JL, Landovitz RJ, Ribaudo HJ, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med. 2014;161:461–471. doi: 10.7326/M14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe K, Varesio E, Hopfgartner G. Parallel ultra high pressure liquid chromatography-mass spectrometry for the quantification of HIV protease inhibitors using dried spot sample collection format. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;965:244–253. doi: 10.1016/j.jchromb.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Hirano A, Takahashi M, Kinoshita E, et al. High performance liquid chromatography using UV detection for the simultaneous quantification of the new non-nucleoside reverse transcriptase inhibitor etravirine (TMC-125), and 4 protease inhibitors in human plasma. Biol Pharm Bull. 2010;33:1426–1429. doi: 10.1248/bpb.33.1426. [DOI] [PubMed] [Google Scholar]
- 45.Koehn J, Ding Y, Freeling J, Duan J, Ho RJ. A simple, efficient, and sensitive method for simultaneous detection of anti-HIV drugs atazanavir, ritonavir, and tenofovir by use of liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother. 2015;59:6682–6688. doi: 10.1128/AAC.00869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbesselt R, Van Wijngaerden E, de Hoon J. Simultaneous determination of 8 HIV protease inhibitors in human plasma by isocratic high- performance liquid chromatography with combined use of UV and fluorescence detection: amprenavir, indinavir, atazanavir, ritonavir, lopinavir, saquinavir, nelfinavir and M8-nelfinavir metabolite. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;845:51–60. doi: 10.1016/j.jchromb.2006.07.068. [DOI] [PubMed] [Google Scholar]
- 47.Djerada Z, Feliu C, Tournois C, et al. Validation of a fast method for quantitative analysis of elvitegravir, raltegravir, maraviroc, etravirine, tenofovir, boceprevir and 10 other antiretroviral agents in human plasma samples with a new UPLC-MS/MS technology. J Pharm Biomed Anal. 2013;86:100–111. doi: 10.1016/j.jpba.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Leung KS, Fong BM. LC-MS/MS in the routine clinical laboratory: has its time come? Anal Bioanal Chem. 2014;406:2289–2301. doi: 10.1007/s00216-013-7542-5. [DOI] [PubMed] [Google Scholar]
- 49.Koal T, Burhenne H, Romling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2995–3001. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
- 50.DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93:479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.U.S. Department of Health and Human Services Food and Drug Administration (FDA) [accessed September 30, 2017];Guidance for Industry-Bioanalytical Method Validation. 2001 Available: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- 52.Lakshmy R. Analysis of the use of dried blood spot measurements in disease screening. J Diabetes Sci Technol. 2008;2:242–243. doi: 10.1177/193229680800200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osterberg L, Blaschke T. Adherence to medication. New Eng J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 54.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- 55.Rudd P, Byyny RL, Zachary V, et al. Pill count measures of compliance in a drug trial: variability and suitability. Am J Hypertens. 1988;1:309–312. doi: 10.1093/ajh/1.3.309. [DOI] [PubMed] [Google Scholar]
- 56.Pullar T, Kumar S, Tindall H, Feely M. Time to stop counting the tablets? Clin Pharmacol Ther. 1989;46:163–168. doi: 10.1038/clpt.1989.121. [DOI] [PubMed] [Google Scholar]
- 57.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed?A novel assessment technique. J Am Med Assn. 1989;261:3273–3277. [PubMed] [Google Scholar]
- 58.Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: practical considerations. AIDS Behav. 2005;9:103–110. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- 59.Martin S, Elliott-DeSorbo DK, Calabrese S, et al. A comparison of adherence assessment methods utilized in the United States: perspectives of researchers, HIV-infected children, and their caregivers. AIDS Patient Care STDS. 2009;23:593–601. doi: 10.1089/apc.2009.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Bruin M, Hospers HJ, van den Borne HW, Kok G, Prins JM. Theory- and evidence-based intervention to improve adherence to antiretrovi-ral therapy among HIV-infected patients in The Netherlands: a pilot study. AIDS Patient Care STDS. 2005;19:384–394. doi: 10.1089/apc.2005.19.384. [DOI] [PubMed] [Google Scholar]
- 61.Koss CA, Hosek SG, Bacchetti P, et al. Comparison of measures of adherence to HIV preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis. 2018;66(2):213–219. doi: 10.1093/cid/cix755. https://doi.org/10.1093/cid/cix755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45:908–915. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uges DR. Plasma or serum in therapeutic drug monitoring and clinical toxicology. Pharm Weekbl Sci. 1988;10:185–188. doi: 10.1007/BF01956868. [DOI] [PubMed] [Google Scholar]
- 64.Nettles RE, Kieffer TL, Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42:1189–1196. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- 65.Clevenbergh P, Garaffo R, Durant J, Dellamonica P. PharmAdapt: a randomized prospective study to evaluate the benefit of therapeutic monitoring of protease inhibitors: 12 week results. AIDS. 2002;16:2311–2315. doi: 10.1097/00002030-200211220-00011. [DOI] [PubMed] [Google Scholar]
- 66.Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, Losina E. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006;7:59–69. doi: 10.1310/hct.2006.7.2.004. [DOI] [PubMed] [Google Scholar]
- 67.Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med. 1990;150:1509–1510. [PubMed] [Google Scholar]
- 68.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. "White coat compliance" limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9:238–246. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 69.Zakaria R, Allen KJ, Koplin JJ, Roche P, Greaves RF. Advantages and challenges of dried blood spot analysis by mass spectrometry across the total testing process. EJIFCC. 2016;27:288–317. [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper GA, Kronstrand R, Kintz P. Society of Hair T. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218:20–24. doi: 10.1016/j.forsciint.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 71.Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Practice. 2001;55:353–357. [PubMed] [Google Scholar]
- 72.Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 2006;370:17–49. doi: 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 73.Xiang P, Shen M, Drummer OH. Review: Drug concentrations in hair and their relevance in drug facilitated crimes. J Forensic Leg Med. 2015;36:126–135. doi: 10.1016/j.jflm.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Kintz P. Bioanalytical procedures for detection of chemical agents in hair in the case of drug-facilitated crimes. Anal Bioanal Chem. 2007;388:1467–1474. doi: 10.1007/s00216-007-1209-z. [DOI] [PubMed] [Google Scholar]
- 75.Boterman M, Doig M, Breda M, et al. Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC) Bioanalysis. 2012;4:651–660. doi: 10.4155/bio.12.18. [DOI] [PubMed] [Google Scholar]