Abstract
Objective
The aim of this study was to determine the association between chronic placental inflammation and amniotic fluid (AF) markers of maternal anti-fetal rejection as well as the presence of microorganisms in the AF fluid of patients with fetal death.
Study Design
This cohort study included 40 patients with fetal death whose placentas were examined for chronic inflammatory lesions and whose AF chemokine ligand (CXCL)10 and interleukin (IL)-6 concentrations were determined by immunoassays. AF was processed for bacteria, mycoplasmas and viruses using cultivation and molecular microbiologic techniques (i.e. PCR-ESI/MS).
Results
(1) The most prevalent placental findings were maternal vascular underperfusion (63.2%, 24/38), followed by chronic inflammatory lesions (57.9%, 22/38); (2) chronic chorioamnionitis (18/38) was three times more frequent than villitis of unknown etiology (6/38); (3) an elevated AF CXCL10 concentration (above the 95th centile) was present in 60% of the cases, and a receiver operating characteristics (ROC)-derived cut-off of 2.9 ng/mL had a sensitivity of 73% and a specificity of 75% in the identification of chronic placental inflammatory lesions; (4) only five cases had microbial invasion of the amniotic cavity, and the presence of microorganisms did not correlate with chronic placental inflammation.
Conclusion
In women with unexplained fetal death, there is an association between elevated AF CXCL10 and chronic placental inflammatory lesions. Therefore, we conclude that a subset of patients with fetal death may have endured a breakdown of maternal-fetal tolerance, which cannot be attributed to microorganisms in the amniotic cavity.
Keywords: amniotic fluid, bacteria, chorioamnionitis, chronic deciduitis, chronic inflammation, CXCL10/IP-10 interleukin-6 (IL-6), placenta, villitis of unknown etiology (VUE), viruses
Introduction
Fetal death, characterized by the cessation of heart activity after 20 weeks of gestation [1], is considered one of the “great obstetrical syndromes” [2–4] caused by multiple etiologies, including failure of the placenta to provide oxygen/nutrients to support fetal growth [5–14]; infections such as syphilis [15–19], malaria [16, 20–22], Zika virus [23–25] and Listeria monocytogenes [26–30]; fetal cardiac arrhythmias [31, 32]; congenital anomalies [6, 33–36]; umbilical cord accidents (i.e. “true” knots of the umbilical cord) [10, 35–39] and complications of monochorial placentation in multiple gestation pregnancies [40–42].
Affecting more than three million pregnancies per year, fetal death is a clinical challenge; at present, there are no good methods to assess the magnitude of risk or to prevent this devastating complication of pregnancy [43–45]. Although several classification systems have been proposed and are used to attempt to identify co-morbid events associated with fetal death [36, 46–63], a firm causal link between the events labeled in these classifications as “causes” of fetal death and the actual cause of death is extremely difficult to prove [39, 49, 58, 64–66]. Therefore, these classification systems provide a general idea of the context in which fetal death occurred, rather than providing unequivocal proof of the cause of death (e.g. total placental abruption [67, 68] or maternal trauma [69–71]).
The fetus and placenta are semi-allografts to the maternal host, as 50% of their genomes are paternally derived [72–76]. The placenta is widely considered to be the most successful transplant in nature, a biological adaptation required for viviparity, and accomplished by immune tolerance [72, 76–86]. Sir Peter Medawar, the founder of the field of Reproductive Immunology, crafted the fundamental question of the field [87, 88]: “The immunological problem of pregnancy may be formulated thus: how does the pregnant mother contrive to nourish within itself, for many weeks or months, a foetus that is an antigenically foreign body?” In other words, why are the fetus and placenta not rejected?
Tolerance, “the active state of antigen-specific nonresponsiveness,” leading to diminished reactivity to paternal antigens expressed by the placenta and/or fetus, is considered key for a successful pregnancy [75–78, 83–86, 89–104]. The mechanisms responsible for tolerance in pregnancy have been reviewed by Elizabeth Bonney [90, 91, 93, 104–106], Sing-Sing Way [107, 108], and Adrian Erlebacher [72, 86, 109–112].
A fundamental question, largely overlooked, is whether semi-allograft rejection mediated by a breakdown of tolerance is a mechanism of disease responsible for adverse pregnancy outcome. Transplant rejection is characterized by the infiltration of donor CD8 + (cytotoxic) T cells into the graft and by organ dysfunction or failure [113–115]. Renal transplant rejection is diagnosed in patients who have a drop in the glomerular filtration rate (i.e. creatinine clearance) and an infiltration of lymphocytes from the donor into the transplanted organ [116, 117]. The presence of maternal lymphocytes at the maternofetal interfaces, such as the chorioamniotic membranes and villous tree, as seen in chronic chorioamnionitis and villitis of unknown etiology (VUE), respectively, demonstrates a breakdown in maternal (host) tolerance of the fetus (graft) [118–123]. In parallel fashion, chemokine markers of chronic inflammation, such as chemokine ligand 10 (CXCL10) were elevated in the amniotic fluid (AF) when signs of rejection were identified by placental histology [118, 121, 123, 124].
We have provided evidence that maternal anti-fetal rejection is operative in a subset of patients with spontaneous preterm labor [118–120, 122, 124–126], preterm prelabor rupture of the membranes (PPROM) [118], maternal floor infarction [127], and other obstetrical syndromes [121, 122, 126]. Three placental pathology lesions have been considered manifestations of maternal anti-fetal rejection: (1) chronic chorioamnionitis [118–122, 124, 126, 128, 129]; (2) VUE [122, 123, 126, 130, 131]; and (3) chronic deciduitis [122, 126, 129]. In these lesions, maternal lymphocytes infiltrate the fetal tissue (the chorioamniotic membranes in chronic chorioamnionitis, the villous tree in VUE, and the basal plate of the placenta in chronic deciduitis) [118, 123, 126, 130–134].
The ultimate form of transplant rejection is a total failure of the transplanted organ, which manifests itself as renal failure [117], cardiac failure [135], etc. Fetal death in the context of maternal anti-fetal rejection can be considered as the most severe expression of such a rejection. We previously reported that chronic chorioamnionitis is frequent in patients with fetal death [121, 126]; however, a pervasive question is whether some cases of chronic chorioamnionitis may be due to subclinical infection rather than to maternal anti-fetal rejection. Therefore, we conducted this study to determine whether fetal death associated with placental lesions consistent with maternal anti-fetal rejection is related to intra-amniotic infection. This clinical condition was diagnosed by the presence of bacteria and/or viruses using cultivation and molecular microbiologic techniques.
Materials and methods
Patient population and study materials
This cohort study was performed at Hutzel Women’s Hospital, Detroit, MI, USA. Subjects included patients who presented with unexplained fetal death (n = 40) defined as spontaneous death of a fetus at or after 20 weeks of gestation and not attributed to an identifiable cause. Patients with a multifetal gestation, pre-gestational diabetes, and prenatal diagnoses of fetal anomalies as well as chromosomal abnormalities were excluded from the study.
Patients diagnosed with fetal death by ultrasound were offered amniocentesis for karyotype determination and assessment for the presence of intra-amniotic infection. We selected cases in which AF samples were available from the Bank of Biological Materials of the Perinatology Research Branch, Wayne State University, and the Detroit Medical Center. Each participant provided written informed consent for the use of their information and biological specimens for research. The Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), approved the collection of clinical information and use of biological materials for research purposes.
Small for gestational age was defined as birthweight less than the 10th percentile for gestational age [136]. Chronic maternal condition was defined as a chronic medical illness affecting an organ system that may require medication (e.g. asthma, lupus). Prior preterm birth was defined as a pregnancy delivering at or after 20+0 and before 37+0 weeks of gestation. Preeclampsia was defined as the presence of new-onset hypertension and proteinuria at or beyond 20+0 weeks of gestation [137]. Mean arterial blood pressure [138] was defined as diastolic blood pressure + (1/3*pulse pressure). The use of illicit drugs was determined by urine drug screening. Microbial invasion of the amniotic cavity was defined as a positive culture or positive result from molecular microbiologic techniques [139]. Clinical chorioamnionitis and placental abruption were diagnosed by physicians in the clinical setting.
Histopathologic examination
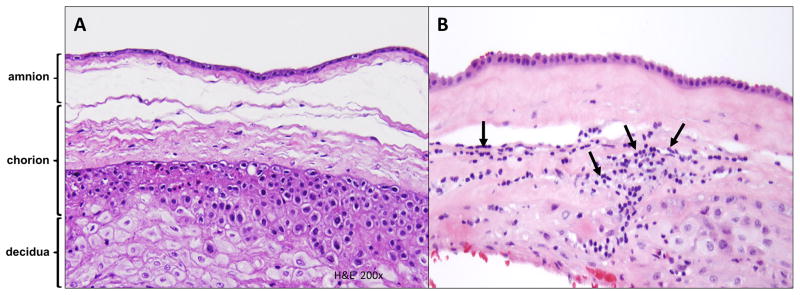
Histopathologic assessment of placental lesions was performed by examination of hematoxylin and eosin (H&E)-stained sections of the chorioamniotic membranes, placental disc and umbilical cord in each case. Diagnoses of findings were made by perinatal pathologists, according to previously published criteria [118, 126, 140–144]. The following categories of placental findings were characterized: findings consistent with amniotic fluid infection (AFI), fetal vascular thrombo-occlusive disease, maternal vascular underperfusion, and chronic inflammatory lesions. Sub-classes of chronic inflammatory lesions included in this study were chronic chorioamnionitis, VUE and chronic deciduitis. Chronic chorioamnionitis was diagnosed when lymphocytic infiltrates were observed in the chorionic trophoblast layer or chorioamniotic connective tissue [118, 126] (Figure 1). The severity of inflammation was scored on grade and stage. The extent of inflammation was graded as 0 (no inflammation), 1 (more than two foci or patchy inflammation), or 2 (diffuse inflammation). The stage of inflammation was scored as stage 1 if amniotropic lymphocytic infiltration was limited to the chorionic trophoblast layer sparing the chorioamniotic connective tissue, and stage 2 if lymphocytic infiltration into the chorioamniotic connective tissue was noted. Villitis of unknown etiology was defined as the presence of lymphohistiocytic infiltrates in the chorionic villi [126, 144, 145]. Grading of VUE is based on the number of affected villi and the extent of involvement. Low-grade lesions affect less than 10 villi; high-grade lesions affect more than 10 villi. VUE can be characterized by whether the pattern of involvement is distal (terminal and intermediate villi), proximal (stem villi), or basal (anchoring villi). In this study, the pattern of VUE was characterized as either proximal or basal, according to the protocol at our institution. Chronic deciduitis was diagnosed by the presence of both lymphocytes and plasma cells in the basal plate of the placenta [133].
Figure 1. Histology of the extraplacental chorioamniotic membranes.
(A) Normal chorioamniotic membranes with no evidence of chronic inflammatory cell infiltration in the amnion and choriodecidua.
(B) Chronic chorioamnionitis, stage 2: chorioamniotic membranes from FD, gestational age 31.4 weeks. Chronic inflammatory cells (arrows) infiltrate into the chorioamniotic connective tissues beyond the chorionic trophoblast layer. FD: fetal death.
Enzyme-linked immunosorbent assay for CXCL10 and IL-6
Amniotic fluid concentrations of CXCL10 and interleukin (IL)-6 were measured by specific ELISA assays (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. The sensitivity of the assay for AF CXCL10 and IL-6 was 6.82 pg/mL and 2.24 pg/mL, respectively. The inter-assay coefficient of variation was 2.75% for CXCL10 and 2.84% for IL-6; the intra-assay coefficient of variation was 3.87% and 3.68%, respectively. Gervasi et al. [146] reported reference ranges for AF CXCL10 sampled in the mid-trimester for pregnancies later delivered at term without complications; the 95th percentile for CXCL10 was defined as 2.2 ng/mL and this cut-off was used in our study [146]. IL-6 is an inflammation-related cytokine associated with systemic inflammation in the maternal [147], fetal [148, 149], and placental compartments [150] as well as with intra-amniotic infection and intra-amniotic inflammation [146, 151–167]. We used a cut-off of 2.6 ng/mL for the identification of intra-amniotic inflammation [157,168, 169].
Microbiologic evaluation
Amniotic fluid was transported in a capped sterile syringe to the clinical laboratory where it was cultured for aerobic and anaerobic bacteria, including genital Mycoplasmas and viruses. Determinations of the AF white blood cell count [170], glucose concentration [171] and Gram stain [172] were performed first. The AF was then centrifuged at 1300 × g for 10 min at 4°C and then stored at −80°C until analyzed by immunoassay. The analysis for the presence of bacteria, genital mycoplasmas and/or viruses was performed by cultivation and broad-range polymerase chain reaction (PCR) coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) (Ibis® Technology, Athogen, Carlsbad, CA, USA) [139, 173–181].
Statistical analysis
Distributions of continuous variables were examined for skewness and normality. The Shapiro-Wilk test was performed to assess deviations of arithmetic data from normality. Bivariate analysis was performed with the Mann-Whitney U and Kruskal-Wallis tests for the assessment of non-parametric continuous variables (e.g. CXCL10, IL-6). Spearman’s rho was used to assess correlations. Odds ratios and 95% confidence intervals (CI) were calculated to assess magnitudes of association. Statistical analysis was performed using SPSS Version 19 (SPSS Inc., Chicago, IL, USA). A two-tailed P value of <0.05 was considered significant.
Results
Study population
Forty patients with a fetal death met the inclusion criteria. Table 1 provides the clinical characteristics, obstetrical histories and laboratory results of the AF samples. Patients of African-American origin comprised 87.5% (35/40) of the study population. Only one patient had a prior fetal demise. The median gestational age at presentation was 30.9 weeks, indicating that most fetal deaths occurred in the third trimester, and 27.5% of fetal deaths occurred in small-for-gestational-age cases. The median interval from diagnosis to delivery was 0.1 weeks [range 0–0.5 weeks], one patient delivered spontaneously, 37 had induction of labor, and two were delivered by cesarean section. A Kleihauer-Betke test was performed in 33 patients and only one had a positive result.
Table 1.
Clinical, Obstetrical and laboratory results of patients with fetal death
| Stillbirths (n=40 ) | |
|---|---|
| Maternal age (years) | 25.0 (±4.6) |
| Race/Ethnicity | |
| African American | 87.5% (35/40) |
| Caucasian | 2.5% (1/40) |
| Hispanic | 5% (2/40) |
| Other | 5% (2/40) |
| Body mass index (kg/(m)2 ) | 30.2 (±8.7) |
| Mean arterial pressure (mm Hg) | 95 (±13) |
| Nulliparity | 37.5% (15/40) |
| Prior preterm birth | 17.5% (7/40) |
| Maternal medical comorbiditiesǂ | 37.5% (15/40) |
| Active tobacco smoker | 20% (8/40) |
| Drug use during index pregnancy (positive urine drug screen) | 28.2% (11/39) |
| Gestational age at diagnosis & amniocentesis (weeks) | 30.9 [25.2–35.7] |
| Interval from diagnosis to delivery (weeks) | 0.1 [0–0.5] |
| Induction of labor | 92.5% (37/40) |
| Stillborn birthweight (grams) | 1403 [625–2283] |
| Birthweight <10th percentile | 27.5% (11/40) |
| Male fetus | 57.5% (23/40) |
| Kleihauer-Betke positive | 3% (1/33) |
| Amniotic fluid | |
| White blood cell count (cells/ml 3) | 1 [0–13] |
| Glucose (mg/dL) | 18 [10–22] |
| CXCL-10 (ng/ml) | 2.9 [1.7–7. 6)] |
| IL-6 (ng/ml) | 2.2 [0.5–5.8] |
| Positive bacterial culture | 2.5% (1/40) |
| Positive viral culture | 2.9% (1/34) |
| Positive bacterial molecular study | 2.5% (1/40) |
| Positive viral molecular study | 10% (4/40) |
Data presented as median [IQR] or mean (± SD) and % (n/N).
Medical comorbidities included (n): asthma (7), cerebral palsy (1), chronic hypertension (3), Crohn’s disease (1), drug abuse (2), gastroesophageal reflux disease (1), liver disease (1), systemic lupus erythematosus (1), psychiatric mood disorder (1), Wiskott-Aldridge Carrier (1).
Placental lesions
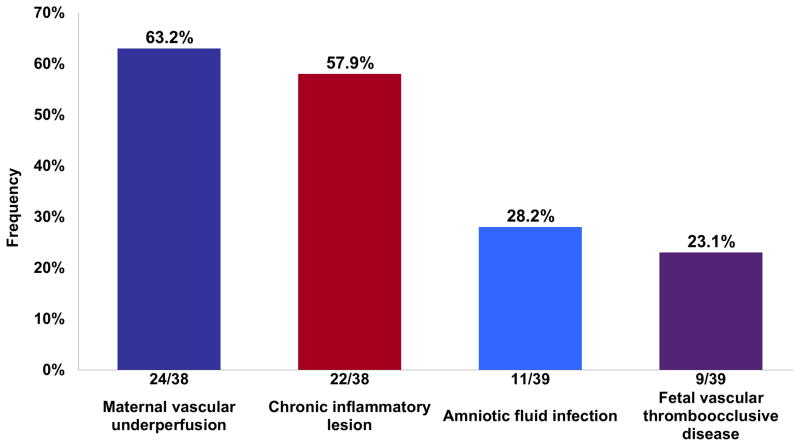
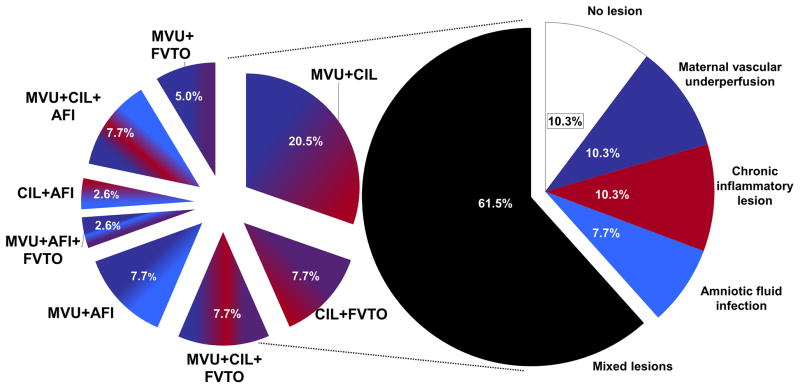
The most frequent lesions were those consistent with maternal vascular underperfusion (63.2%; 24/38), followed by chronic inflammatory lesions (57.9%; 22/38), acute inflammatory lesions consistent with AFI (28.2%; 11/39), and fetal vascular thrombo-occlusive disease (23.1%; 9/39) (Figure 2). More than one type of lesion was diagnosed in 61.5% (24/39) of the placentas. The most frequent combination of lesions (14/22) included those consistent with maternal vascular underperfusion and chronic placental inflammation (Figure 3). Chronic placental inflammatory lesions consisted of 18 cases with chronic chorioamnionitis (17/18 cases were preterm gestation), six cases with VUE (5/6 cases were preterm gestation) and seven cases with chronic deciduitis. In regard to the staging and grading of chronic chorioamnionitis, 61.1% (11/18) were stage 2 (involvement of the chorionic connective tissue) (Figure 1), 38.9% (7/18) were grade 2 (a diffuse, rather than localized, inflammatory process), and 33.3% (6/18) also involved the chorionic plate. In four of the cases, chronic deciduitis was also present.
Figure 2. Frequency of placental lesions in fetal death.
The classification of placental lesions was based on those categorized by the Society for Pediatric Pathology. Chronic inflammatory lesions encompassed chronic chorioamnionitis, villitis of unknown etiology and chronic deciduitis. Chronic inflammatory lesions were the second most predominant histopathological lesion observed in unexplained fetal death.
Figure 3. Frequency of isolated and mixed placental lesions in unexplained fetal death.
The placental examination demonstrated lesions from multiple histological classes (mixed) more frequently than those confined to one class (isolated). The most abundant findings were lesions of maternal vascular underperfusion and chronic inflammation. Some placentas even had lesions from 3 different classes. The frequency of isolated lesions is represented in solid colors and the frequency of mixed lesions in colors derived from the blend of the classes involved. AFI: amniotic fluid infection; CIL: chronic inflammatory lesion; FVTO: fetal vascular thrombo-occlusive disease; MVU: maternal vascular underperfusion.
All six cases of VUE had basal, low-grade lesions (five cases were preterm gestation). In all six cases, the AF was negative for bacteria and viruses. In one-half of the VUE cases (3/6), there was evidence of chronic chorioamnionitis, and equally in 3/6 cases, there was chronic deciduitis.
Amniotic fluid concentrations of CXCL10 and interleukin-6 in fetal death
The median AF concentration of CXCL10 (a chemokine elevated in cases of allograft rejection) was 2.9 ng/mL [IQR 1.7–7.6] – this is higher than the 95th centile of CXCL10 measured in the mid-trimester AF (2.2 ng/mL) of patients with a normal pregnancy outcome (see Supplemental Material Figure 1). An elevated AF CXCL10 concentration was present in 60% (24/40) of patients with a fetal death.
The AF CXCL10 concentration was elevated for 17 of 22 (77.2%) patients with chronic placental inflammatory lesions, including two cases with both chronic chorioamnionitis and VUE – this included 13 cases with chronic chorioamnionitis and five with VUE. There was a positive correlation between the concentration of CXCL10 and the occurrence of chronic placental inflammatory lesions (r = 0.37, P = 0.02). We found no correlation between gestational age and CXCL10 for the entire study population (r = −0.23; P = 0.16).
The median AF concentration of CXCL10 in patients with and without chronic placental inflammatory lesions was [5.2 (2.2–9.2) vs. 2.1 (1.6–3.3), P = 0.01], respectively. In the current study, a receiver operating characteristics (ROC) curve was plotted using sensitivity and 1-specificity; an AF CXCL10 concentration of 2.9 ng/mL had a sensitivity of 73%, a specificity of 75%, a positive likelihood ratio of 2.9, and a negative likelihood ratio of 0.4 with an area under the curve of 0.72 (P = 0.02) for the presence of chronic placental inflammatory lesions. Of note, using the ROC curve, the previously reported cut-off for the 95th percentile of CXCL10 (2.2 ng/mL) [146] would have had a sensitivity of 77%, a specificity of 56%, a positive likelihood ratio of 1.8, and a negative likelihood ratio of 0.4 for the presence of placental lesions consistent with chronic inflammation.
Forty-five percent (18/40) of patients had intra-amniotic inflammation (defined as an AF IL-6 concentration ≥2.6 ng/ml) [157, 168, 169]. The median concentration of AF IL-6 was 2.2 [0.5–5.8] ng/ml; neither acute inflammatory lesions of the placenta nor microbial invasion of the amniotic cavity was associated with AF IL-6 (P = 0.14, P = 0.26, respectively).
In 13 of 40 (32.5%) patients, both AF markers of acute (IL-6) and chronic (CXCL10) inflammation were greater than the 95th percentile. The odds of co-occurrence of elevated AF CXCL10 and IL-6 were nine times higher among patients who had mixed placental histologic lesions compared to those with an isolated lesion or no lesion (OR 9; 95% CI 1.2–62); adjustment for gestational age at diagnosis slightly increased the odds ratio (OR 11; 1.4–83).
Microbial invasion of the amniotic cavity
A positive AF result for microorganisms was present in 12.5% (5/40) of the study population (Table 1). In most cases, the organisms were viruses (i.e. parvovirus); in one case, it was Lactobacillus sakei (Table 2). The organisms, clinical characteristics, results of AF analysis, IL-6, and CXCL10, as well as the results of placental pathologic findings, are described in Table 2.
Table 2.
Microbial invasion of the amniotic cavity in fetal death: microorganisms, amniotic fluid analysis and placental findings.
| Organism | Culture | PCR | Genome/ well |
GA diagnosis/ amniocente sis (wks) |
AF WBC (cells/ml3 |
AF glucose (mg/dl) |
AF IL-6 (ng/ml) |
AF CXCL-10 (ng/ml) |
Placental histological classes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFI | CIL | FVTO | MVU | |||||||||
| Parvovirus B-19 | NS | + | 921 | 26 | --- | 11 | 13.8 | 1.7 | -- | +ǂ | -- | + |
| Parvovirus B-19 | NS | + | 931 | 39 | 260 | 22 | 0.3 | 1.2 | -- | +ǂ | -- | -- |
| Cytomegalovirus | + | + | 685 | 27 | 0 | 18 | 0.4 | 2.0 | -- | -- | -- | -- |
| Human enterovirus§ | -- | + | 23 | 33 | 4 | 10 | 1.2 | 1.9 | -- | -- | -- | + |
| Coagulase negative staphylococci§ | + | -- | NA | |||||||||
| Lactobacillus sakei | -- | + | 15 | 29 | 10 | 9 | 0.5 | 7. 7 | -- | +ǂ | -- | + |
NS, no specimen. NA, not applicable. PCR, polymerase chain reaction. GA, gestational age. AF, amniotic fluid. WBC, white blood cell. AFI, amniotic fluid infection. CIL, chronic inflammatory lesion. FVTO, fetal vascular thromboocclusive disease. MVU, maternal vascular underperfusion.
These microorganisms were recovered from the same AF specimen.
Chronic chorioamnionitis was the only chronic inflammatory lesion present—there were no chronic villitis cases.
Standard cultivation techniques identified one bacteria and one virus. Cytomegalovirus was identified by culture and confirmed with molecular microbiology. One culture of AF was positive for coagulase negative staphylococci – it is noteworthy that this patient had only four AF white blood cells per cubic milliliter, a glucose of 10 mg/dL, and a normal IL-6/CXCL10, and no evidence of acute inflammatory lesions of the placenta (acute chorioamnionitis, funisitis, or chorionic villitis). Therefore, it is possible that this represents contamination of the specimen, given that this organism is frequently present on the skin. Molecular microbiologic techniques identified two cases of parvovirus B-19, one of Lactobacillus sakei and one of human enterovirus.
None of the cases with microbial invasion of the amniotic cavity had acute inflammatory lesions of the placenta. Indeed, if pathological lesions were present, these cases had either chronic inflammatory lesions or maternal underperfusion or sometimes both. Three cases with chronic inflammatory lesions had microbial invasion: two with parvovirus B-19 and one case with Lactobacillus sakei.
Discussion
Principal findings of the study
(1) Chronic placental inflammatory lesions were present in 57.9% (22/38) of cases; (2) chronic chorioamnionitis was the most frequent chronic inflammatory lesion of the placenta, followed by chronic deciduitis with plasma cells and VUE; (3) one-half of the VUE cases (3/6) also demonstrated chronic chorioamnionitis; (4) most cases of chronic chorioamnionitis had no evidence of microbial invasion of the amniotic cavity, except for three (two with parvovirus B-19, one with Lactobacillus sakei). However, these cases did not have acute inflammatory lesions, indicating the absence of either a maternal inflammatory response (acute chorioamnionitis) or a fetal inflammatory response (acute chorionic vasculitis or funisitis); (5) an elevated AF concentration of CXCL10 (above the 95th centile) was present in 60% of cases with fetal death; (6) an elevated AF CXCL10 concentration of 2.9 ng/mL had a sensitivity of 73% and a specificity of 75% in the identification of chronic placental inflammatory lesions; (7) microbial invasion of the amniotic cavity, detected by a combination of cultivation and molecular microbiologic techniques, was present in only 12.5% of fetal death cases. Importantly, most of the organisms were viruses (parvovirus B-19, Cytomegalovirus and enterovirus); (8) acute placental inflammatory lesions were infrequent in cases of fetal death; and (9) the placentas of pregnancies complicated with fetal death often had more than one lesion – the most frequent combination included maternal vascular lesions of underperfusion and chronic inflammatory lesions; 10) the odds of both an elevation in acute (IL-6) and chronic (CXCL10) inflammation markers were nine times greater when more than one class of placental lesion was diagnosed compared to a single class or when no lesion was found. Collectively, this evidence suggests that maternal anti-fetal rejection plays a role in unexplained fetal death. This is supported in patients with a fetal death by a high frequency of chronic placental inflammatory lesions, a high concentration of CXCL10, and a low frequency of microbial invasion of the amniotic cavity.
Fetal death: a manifestation of allograft failure
Fetal death is syndromic in nature and can be caused by maternal insults (i.e. maternal trauma leading to placental abruption [182, 183], a metabolic disorder such as diabetic ketoacidosis [184–186], maternal sepsis [187, 188], etc.), placental disease (i.e. observed in patients who have absent or reversed end-diastolic velocities of the umbilical artery [189, 190], massive perivillous fibrin deposition [191–193], etc.), or fetal disorders (such as cardiac arrhythmias [31, 32], hydrops fetalis [194, 195], critical congenital anomalies [33, 34] and some chromosomal abnormalities [196, 197]).
Although the fetus and placenta are known to be semi-allografts [87] and immune disorders can cause fetal death (i.e. Rh alloimmunization [198, 199]), the role of maternal anti-fetal rejection as a mechanism of disease responsible for fetal death has been considered only recently [121, 126].
Allograft rejection is a dynamic process that occurs frequently in transplanted organs [200, 201]. Infiltration of lymphocytes from the recipient into the donor organ is not uncommon [113–115], as is the deposition of complement by antibody-mediated rejection [202, 203]. However, histopathologic evidence of an immune response in the graft is not sufficient to define rejection – the latter requires organ dysfunction, which is clinically manifested by a functional disorder of the transplanted organ, such as renal failure, congestive heart failure, pulmonary insufficiency, etc. [204–207].
The placenta is a multi-functional organ that has respiratory, nutritive, excretory, endocrine and immune functions, among others [208–210]. Therefore, severe placental dysfunction could manifest in a number of ways, such as inadequate transport of oxygen, leading to fetal myocardial failure and subsequent death [5]. Nutritive failure of the placenta could lead to fetal growth restriction, a risk factor of fetal death; however, this in and of itself would not be sufficient to cause death unless placental respiratory failure also occurs. Other forms of placental dysfunction may be more subtle, e.g. placental sulfatase deficiency can lead to an enzymatic inability to hydrolyze the sulfate group from DHA sulfate (DHA-S) and 16a-hydroxy-DHA sulfate. This condition is associated with low levels of estrogens and is clinically manifested with post-term pregnancy [211].
The immune functions of the placenta are complex and involve host defense against microorganisms that may gain access to the uterus via an ascending pathway or through a hematogenous route [73, 212–220]. Some infections are known to be a cause of fetal death (i.e. syphilis [15–19]). Another major immunological function is the successful establishment and maintenance of maternal-fetal tolerance [72, 75, 83, 84, 92, 94, 99, 104, 111], the breakdown of which may lead to maternal antifetal rejection. The clinical spectrum of maternal antifetal rejection may include subclinical semi-allograft rejection detected only at the time of examination of the placenta (by identification of chronic chorioamnionitis [118–122, 124, 126, 128, 129], VUE [122, 123, 126, 130, 131], or chronic deciduitis [122, 126, 129]), preterm labor with intact membranes [118–120, 122, 124–126], PPROM [118], maternal perivillous fibrin deposition [127], and other obstetrical syndromes [121, 122, 126]. However, the extreme form of rejection would include a functional failure of the placenta of such magnitude that it would lead to fetal death [121, 126].
The precise mechanisms whereby rejection may lead to fetal death are not yet known; however, we have described a new entity, the fetal inflammatory response syndrome type 2 (FIRS type 2), which occurs in fetuses whose placentas have lesions consistent with maternal anti-fetal rejection [122, 126]. FIRS type 2 is characterized by a unique change in the profile of cytokines in the peripheral blood, with an elevation of CXCL10, and by a stereotypic transcriptome [122]. Gene ontology analysis of differentially expressed genes in fetal white blood cells with FIRS type 2 indicates enrichment for genes that participate in immune-related processes [122]. In contrast, in FIRS type 1, the systemic inflammatory response associated with microorganisms or “danger signals” can lead to a situation that resembles septic shock in the fetus and newborn, and some reports of fetal death [221–225]. However, the natural history of FIRS type 2 has not been elucidated; thus, it is unclear how maternal antifetal rejection and FIRS type 2 can progress toward fetal death. However, the data presented herein show a clear association between fetal death, placental lesions of maternal anti-fetal rejection, and an elevation of CXCL10 in the AF, as well as the absence of microbial invasion of the amniotic cavity in most cases. Therefore, the immune disorder detected in the placenta and AF cannot be attributed to a viral or bacterial infection.
Placental pathology in fetal death
The most common lesion associated with fetal death was maternal vascular lesions of underperfusion [6, 7, 61, 144, 226–233]. These lesions fall into two categories: villous changes and vascular lesions. At the most recent meeting of the Amsterdam Placental Workshop, experts recommended to re-label this category of lesions into “maternal vascular malperfusion” [234]. It is believed that an inadequate supply of nutrients and oxygen would occur in patients who have lesions such as massive perivillous fibrin deposition and massive placental infarction [235].
An important finding of this study is that chronic placental inflammatory lesions were observed in 57.9% (22/38) of cases. Chronic inflammatory lesions included chronic chorioamnionitis, VUE, and chronic deciduitis with plasma cells. Chronic placental inflammatory lesions were associated in 63.6% (14/22) of cases with lesions of maternal vascular underperfusion. The precise mechanism responsible for the association between chronic placental inflammatory lesions and maternal vascular lesions of underperfusion is unknown. It is noteworthy that in one condition, massive perivillous fibrin deposition, there is evidence of antibody-mediated maternal anti-fetal rejection (documented by a mismatch of HLA antigens between the mother and the fetus, maternal sensitization demonstrated by the presence of anti-HLA antibodies, and complement fixation of the umbilical vein shown as C4a deposit) [127]. This condition has also been found to be associated with an abnormal, severe angiogenic/antiangiogenic profile which begins in early pregnancy [236] and has been successfully treated with pravastatin [237]. Further studies are required to determine the extent of the association between chronic placental inflammation and maternal vascular lesions of underperfusion in other obstetrical syndromes.
Acute placental inflammatory lesions were present in 28.2% of cases. Although placental pathologists have considered that acute chorioamnionitis, funisitis, and chorionic vasculitis are manifestations of an amniotic fluid infection syndrome, it is now known that these lesions can occur in the absence of bacteria or viruses in the amniotic cavity and that these processes have been attributed to danger signals that can initiate an inflammatory response in the absence of microbial products [139, 173–176]. These danger signals are considered to be alarmins [238] (e.g. high-mobility group box-1 (HMGB1) [169, 239] and IL-1α [240]), which are present in the AF. It is noteworthy that only 12.5% of patients in our study had bacteria or viruses in the AF. Therefore, an infection-related etiology appears to be rare in high-income countries. In low-income countries, infection appears to play a more important role [21]. The frequency of the acute placental inflammatory lesions (28.2%) is at odds with the frequency of microbial invasion of the amniotic cavity, which was 12.5%. A possible explanation is that some patients may have developed acute inflammatory lesions and, specifically, acute chorioamnionitis during the induction of labor because of fetal death. The induction process may result in the administration of agents such as prostaglandins [241–252], insertion of a Foley catheter [253], or any other method of induction of labor that may predispose to inflammation. Additionally, labor per se is an inflammatory state associated with the infiltration of inflammatory cells into the cervix [254–264], myometrium [256, 265–271] and chorioamniotic membranes [272–277]. It should also be considered that amniocenteses were performed at the time of diagnosis and placentas were examined after delivery. While there is a temporal dissociation between the assessment of the microbial state of the amniotic cavity and placental examination, the overwhelming majority (92.5%) of patients in our study were induced and the median interval from diagnosis to delivery was short [0.1 (0–0.5) weeks]. Therefore, a smaller fraction of those with acute inflammation may not be fully explained by the process of labor induction.
The least common of the four major categories of lesions recognized by the Society of Pediatric Pathology was fetal vascular thrombo-occlusive disease – this was present in 23.1% of cases and associated with maternal vascular lesions of underperfusion in two-thirds of cases. Whether this represents simultaneous activation of the coagulation system in the mother and fetus, due to acquired or congenital thrombophilic states, remains to be determined. Some investigators have suggested that thrombophilia is a risk factor for fetal death; however, a systematic review and meta-analysis have not confirmed the initial reports [278, 279]. This may represent a lack of association or an incomplete knowledge of thrombophilic mutations at the present time.
In only 10.3% of cases did placental pathology not reveal a lesion belonging to the four major categories recognized by the Society of Pediatric Pathology. The circumstances of these fetal deaths were examined and there were no explanations for these occurrences (i.e. cord accident, maternal fetal hemorrhage, etc.).
Amniotic fluid cytokines in fetal death
The current study focuses on the examination of the concentration of two cytokines: IL-6, a marker of acute inflammatory lesions of the placenta [150], and CXCL10, a marker of chronic placental inflammatory lesions [126]. The widely used cut-off for intra-amniotic inflammation is an AF IL-6 concentration of 2.6 ng/mL [157, 168, 169]. Only one patient with microbial invasion of the amniotic cavity had an elevated AF IL-6 concentration – parvovirus B19 was detected and the IL-6 concentration was 13.7 ng/mL. This patient did not have evidence of acute chorioamnionitis but rather of chronic placental inflammatory lesions and, specifically, chronic chorioamnionitis, as well as maternal vascular lesions of underperfusion. Parvovirus B19 is typically transmitted transplacentally [280] and this may be the reason for the absence of acute chorioamnionitis.
Mixed histologic lesions were the most frequently observed placental finding, occurring in 61.5% (24/39) of placentas. The odds of a woman having a combined elevation of AF IL-6 and CXCL10 were nine times greater when mixed placental lesions were present than when no lesion or only a solitary lesion class was diagnosed. The most common classes of placental lesions found when mixed lesions were present were those of maternal vascular underperfusion and chronic inflammation. Microbial invasion of the amniotic cavity does not fully explain the elevation of IL-6 seen in unexplained fetal death. This finding raises the question of whether dysregulation of inflammatory pathways (both acute and chronic) is underway as mixed lesions are developing in the placenta. More investigation into the overlap of acute and chronic inflammation may help to answer this question.
The association between chronic placental inflammatory lesions, especially chronic chorioamnionitis, and fetal death has been previously reported by our group [121, 126]. The novel aspect of this report was the use of PCR techniques to evaluate for the presence of microorganisms in the AF. Using these advanced microbiological techniques, we determined that chronic inflammatory lesions, particularly VUE, could not be attributed to intraamniotic infection by bacteria or viruses.
The diagnostic performance of CXCL10 in the identification of patients with chronic inflammatory lesions indicted a sensitivity of 73%, a specificity of 75%, a positive likelihood ratio of 2.9, and a negative likelihood ratio of 0.4. Further studies are required to determine whether examination of the behavior of the inflammatory/cytokine network can enhance the diagnostic performance of CXCL10. CXCL10, as well as CXCL9 and CXCL11, are T-cell chemokines, which may play a role in the rejection of the semi-allograft fetus [118, 121, 124]. Similarly, the receptor for these T-cell chemokines is CXCR3 and its soluble form is increased in the AF of women who underwent spontaneous preterm labor and whose placentas presented lesions of maternal anti-fetal rejection [281]. Thus, it is possible that a combination of chemokines and their receptors may be of help in identifying the patients with maternal antifetal rejection. The same can be said for the determination of these factors in maternal and fetal blood.
Strengths and limitations
A strength of this study is that we investigated placental pathology, AF cytokines, and microbial invasion of the amniotic cavity in a cohort of patients with fetal death. One of the limitations of the study is the time interval between amniocentesis and delivery, even though the majority of patients had labor induced following the diagnosis of fetal death at admission. Another limitation is the uncertainty about the relationship between the frequency of acute inflammatory lesions of the placenta, microbial invasion of the amniotic cavity, and the interval to delivery. This relationship questions whether acute inflammatory lesions may be due to the process of induction of labor and labor per se rather than a pre-existing pathologic process related to fetal death.
Conclusions
This cohort study of patients with unexplained fetal death suggests that placental lesions associated with chronic placental inflammatory lesions are second only to the prevalence of lesions consistent with maternal underperfusion; and there is a strong correlation with the AF concentration of CXCL10, a chemokine involved in allograft rejection and fetal death. The mechanisms whereby maternal anti-fetal rejection may lead to fetal death require further investigation and may be related to the deployment of FIRS type 2.
Supplementary Material
Acknowledgments
Funding: This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Blencowe H, Cousens S, Jassir FB, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2016;4:e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- 2.Romero R. Prenatal medicine: the child is the father of the man. Prenat Neonat Med. 1996;1:8–11. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 3.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 4.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282:1646–1651. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- 6.Kidron D, Bernheim J, Aviram R. Placental findings contributing to fetal death, a study of 120 stillbirths between 23 and 40 weeks gestation. Placenta. 2009;30:700–704. doi: 10.1016/j.placenta.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Korteweg FJ, Erwich JJ, Holm JP, et al. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol. 2009;114:809–817. doi: 10.1097/AOG.0b013e3181b72ebe. [DOI] [PubMed] [Google Scholar]
- 8.Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA. 2011;306:2459–2468. doi: 10.1001/jama.2011.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananth CV, Lavery JA, Vintzileos AM, et al. Severe placental abruption: clinical definition and associations with maternal complications. Am J Obstet Gynecol. 2016;214:272e1–272.e9. doi: 10.1016/j.ajog.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 10.Bendon RW. Review of some causes of stillbirth. Pediatr Dev Pathol. 2001;4:517–531. doi: 10.1007/s10024001-0084-4. [DOI] [PubMed] [Google Scholar]
- 11.Hoyert DL, Gregory EC. Cause of fetal death: data from the Fetal Death Report, 2014. Natl Vital Stat Rep. 2016;65:1–25. [PubMed] [Google Scholar]
- 12.Korzeniewski SJ, Romero R, Chaiworapongsa T, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. 2016;214:629e1–629.e17. doi: 10.1016/j.ajog.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YM, Chaemsaithong P, Romero R, et al. Placental lesions associated with acute atherosis. J Matern Fetal Neonatal Med. 2015;28:1554–1562. doi: 10.3109/14767058.2014.960835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ptacek I, Sebire NJ, Man JA, Brownbill P, Heazell AE. Systematic review of placental pathology reported in association with stillbirth. Placenta. 2014;5:552–562. doi: 10.1016/j.placenta.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Sheffield JS, Sanchez PJ, Wendel GD, Jr, et al. Placental histopathology of congenital syphilis. Obstet Gynecol. 2002;100:126–133. doi: 10.1016/s0029-7844(02)02010-0. [DOI] [PubMed] [Google Scholar]
- 16.Yakoob MY, Lawn JE, Darmstadt GL, Bhutta ZA. Stillbirths: epidemiology, evidence, and priorities for action. Semin Perinatol. 2010;34:387–394. doi: 10.1053/j.semperi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:684–691. doi: 10.1016/S1473-3099(11)70104-9. [DOI] [PubMed] [Google Scholar]
- 18.Qin J, Yang T, Xiao S, Tan H, Feng T, Fu H. Reported estimates of adverse pregnancy outcomes among women with and without syphilis: a systematic review and meta-analysis. PLoS One. 2014;9:e102203. doi: 10.1371/journal.pone.0102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rac MW, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;216:352–363. doi: 10.1016/j.ajog.2016.11.1052. [DOI] [PubMed] [Google Scholar]
- 20.Nosten F, McGready R, Simpson JA, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375:1482–1490. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhutta ZA, Yakoob MY, Lawn JE, et al. Stillbirths: what difference can we make and at what cost? Lancet. 2011;377:1523–1538. doi: 10.1016/S0140-6736(10)62269-6. [DOI] [PubMed] [Google Scholar]
- 23.Sarno M, Sacramento GA, Khouri R, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis. 2016;10:e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasil P, Pereira JP, Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Citil Dogan A, Wayne S, Bauer S, et al. The Zika virus and pregnancy: evidence, management, and prevention. J Matern Fetal Neonatal Med. 2017;30:386–396. doi: 10.3109/14767058.2016.1174210. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 27.Lamont RF, Sobel J, Mazaki-Tovi S, et al. Listeriosis in human pregnancy: a systematic review. J Perinat Med. 2011;39:227–236. doi: 10.1515/JPM.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Committee on Obstetric Practice American College of Obstetricians Gynecologists. Committee Opinion No. 614: Management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241–1244. doi: 10.1097/01.AOG.0000457501.73326.6c. [DOI] [PubMed] [Google Scholar]
- 29.McLauchlin J. Human listeriosis in Britain, 1967–85, a summary of 722 cases. 1. Listeriosis during pregnancy and in the newborn. Epidemiol Infect. 1990;104:181–189. doi: 10.1017/s0950268800059343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plaza MC, Gilbert-Barness E. Fetal death in utero secondary to Listeria monocytogenes placental infection. Pediatr Pathol Mol Med. 2001;20:433–437. [PubMed] [Google Scholar]
- 31.Crotti L, Tester DJ, White WM, et al. Long QT syndrome-associated mutations in intrauterine fetal death. JAMA. 2013;309:1473–1482. doi: 10.1001/jama.2013.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusiol T, Roncati L, Lavezzi AM, Taddei F, Piscioli F, Ottaviani G. Sudden fetal death due to dualism of the sino-atrial node. Cardiovasc Pathol. 2016;25:325–328. doi: 10.1016/j.carpath.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Boyle B, McConkey R, Garne E, et al. Trends in the prevalence, risk and pregnancy outcome of multiple births with congenital anomaly: a registry-based study in 14 European countries 1984–2007. BJOG. 2013;120:707–716. doi: 10.1111/1471-0528.12146. [DOI] [PubMed] [Google Scholar]
- 34.Frey HA, Odibo AO, Dicke JM, Shanks AL, Macones GA, Cahill AG. Stillbirth risk among fetuses with ultrasound-detected isolated congenital anomalies. Obstet Gynecol. 2014;124:91–98. doi: 10.1097/AOG.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getahun D, Ananth CV, Kinzler WL. Risk factors for antepartum and intrapartum stillbirth: a population-based study. Am J Obstet Gynecol. 2007;196:499–507. doi: 10.1016/j.ajog.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Vergani P, Cozzolino S, Pozzi E, et al. Identifying the causes of stillbirth: a comparison of four classification systems. Am J Obstet Gynecol. 2008;199:319.e1–319.e4. doi: 10.1016/j.ajog.2008.06.098. [DOI] [PubMed] [Google Scholar]
- 37.Collins JH. Umbilical cord accidents: human studies. Semin Perinatol. 2002;26:79–82. doi: 10.1053/sper.2002.29860. [DOI] [PubMed] [Google Scholar]
- 38.Sherer DM, Dalloul M, Ward K, Nakagawa J, Joseph I, Grube S. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk for adverse perinatal outcome. Ultrasound Obstet Gynecol. 2017;50:404–405. doi: 10.1002/uog.17389. [DOI] [PubMed] [Google Scholar]
- 39.Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196:433–444. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victoria A, Mora G, Arias F. Perinatal outcome, placental pathology, and severity of discordance in monochorionic and dichorionic twins. Obstet Gynecol. 2001;97:310–315. doi: 10.1016/s0029-7844(00)01111-x. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman HK, Hume RF, Jr, Calhoun BC, et al. Natural history of twin gestation complicated by in utero fetal demise: associations of chorionicity, prematurity, and maternal morbidity. Fetal Diagn Ther. 2003;18:442–446. doi: 10.1159/000073140. [DOI] [PubMed] [Google Scholar]
- 42.Lewi L, Gucciardo L, Van Mieghem T, et al. Monochorionic diamniotic twin pregnancies: natural history and risk stratification. Fetal Diagn Ther. 2010;27:121–133. doi: 10.1159/000313300. [DOI] [PubMed] [Google Scholar]
- 43.Fretts R. Stillbirth epidemiology, risk factors, and opportunities for stillbirth prevention. Clin Obstet Gynecol. 2010;53:588–596. doi: 10.1097/GRF.0b013e3181eb63fc. [DOI] [PubMed] [Google Scholar]
- 44.Smith GC. Screening and prevention of stillbirth. Best Pract Res Clin Obstet Gynaecol. 2017;38:71–82. doi: 10.1016/j.bpobgyn.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Reddy UM. Prediction and prevention of recurrent stillbirth. Obstet Gynecol. 2007;110:1151–1164. doi: 10.1097/01.AOG.0000287616.71602.d0. [DOI] [PubMed] [Google Scholar]
- 46.de Galan-Roosen AE, Kuijpers JC, van der Straaten PJ, Merkus JM. Fundamental classification of perinatal death. Validation of a new classification system of perinatal death. Eur J Obstet Gynecol Reprod Biol. 2002;103:30–36. doi: 10.1016/s0301-2115(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 47.Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. Br Med J. 2005;331:1113–1117. doi: 10.1136/bmj.38629.587639.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korteweg FJ, Gordijn SJ, Timmer A, et al. The Tulip classification of perinatal mortality: introduction and multidisciplinary inter-rater agreement. BJOG. 2006;113:393–401. doi: 10.1111/j.1471-0528.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 49.Flenady V, Froen JF, Pinar H, et al. An evaluation of classification systems for stillbirth. BMC Pregnancy Childbirth. 2009;9:24. doi: 10.1186/1471-2393-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordijn SJ, Korteweg FJ, Erwich JJ, et al. A multilayered approach for the analysis of perinatal mortality using different classification systems. Eur J Obstet Gynecol Reprod Biol. 2009;144:99–104. doi: 10.1016/j.ejogrb.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Reddy UM, Goldenberg R, Silver R, et al. Stillbirth classification--developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;114:901–914. doi: 10.1097/AOG.0b013e3181b8f6e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyd TK, Wright CA, Odendaal H, et al. The Stillbirth Classification System for the Safe Passage Study: incorporating mechanism, etiology, and recurrence. Pediatr Dev Pathol. 2017;20:120–132. doi: 10.1177/1093526616686251. [DOI] [PubMed] [Google Scholar]
- 53.Hey EN, Lloyd DJ, Wigglesworth JS. Classifying perinatal death: fetal and neonatal factors. BJOG. 1986;93:1213–1223. doi: 10.1111/j.1471-0528.1986.tb07854.x. [DOI] [PubMed] [Google Scholar]
- 54.Wigglesworth JS. Monitoring perinatal mortality. A pathophysiological approach. Lancet. 1980;2:684–686. doi: 10.1016/s0140-6736(80)92717-8. [DOI] [PubMed] [Google Scholar]
- 55.Rayburn W, Sander C, Barr M, Jr, Rygiel R. The stillborn fetus: placental histologic examination in determining a cause. Obstet Gynecol. 1985;65:637–641. [PubMed] [Google Scholar]
- 56.VanderWielen B, Zaleski C, Cold C, McPherson E. Wisconsin stillbirth services program: a multifocal approach to stillbirth analysis. Am J Med Genet A. 2011;155A:1073–1080. doi: 10.1002/ajmg.a.34016. [DOI] [PubMed] [Google Scholar]
- 57.Leisher SH, Teoh Z, Reinebrant H, et al. Seeking order amidst chaos: a systematic review of classification systems for causes of stillbirth and neonatal death, 2009–2014. BMC Pregnancy Childbirth. 2016;16:295. doi: 10.1186/s12884-016-1071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horn LC, Langner A, Stiehl P, Wittekind C, Faber R. Identification of the causes of intrauterine death during 310 consecutive autopsies. Eur J Obstet Gynecol Reprod Biol. 2004;113:134–138. doi: 10.1016/S0301-2115(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 59.Dudley DJ, Goldenberg R, Conway D, et al. A new system for determining the causes of stillbirth. Obstet Gynecol. 2010;116(2 Pt 1):254–260. doi: 10.1097/AOG.0b013e3181e7d975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baird D, Walker J, Thomson AM. The causes and prevention of stillbirths and first week deaths. III. A classification of deaths by clinical cause; the effect of age, parity and length of gestation on death rates by cause. J Obstet Gynaecol Br Emp. 1954;61:433–448. doi: 10.1111/j.1471-0528.1954.tb07507.x. [DOI] [PubMed] [Google Scholar]
- 61.Hovatta O, Lipasti A, Rapola J, Karjalainen O. Causes of stillbirth: a clinicopathological study of 243 patients. Br J Obstet Gynaecol. 1983;90:691–696. doi: 10.1111/j.1471-0528.1983.tb09296.x. [DOI] [PubMed] [Google Scholar]
- 62.Cole SK, Hey EN, Thomson AM. Classifying perinatal death: an obstetric approach. BJOG. 1986;93:1204–1212. doi: 10.1111/j.1471-0528.1986.tb07853.x. [DOI] [PubMed] [Google Scholar]
- 63.Chan A, King JF, Flenady V, Haslam RH, Tudehope DI. Classification of perinatal deaths: development of the Australian and New Zealand classifications. J Paediatr Child Health. 2004;40:340–347. doi: 10.1111/j.1440-1754.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 64.Nappi L, Trezza F, Bufo P, et al. Classification of stillbirths is an ongoing dilemma. J Perinat Med. 2016;44:837–843. doi: 10.1515/jpm-2015-0318. [DOI] [PubMed] [Google Scholar]
- 65.Laury A, Sanchez-Lara PA, Pepkowitz S, Graham JM., Jr A study of 534 fetal pathology cases from prenatal diagnosis referrals analyzed from 1989 through 2000. Am J Med Genet A. 2007;143A:3107–3120. doi: 10.1002/ajmg.a.32094. [DOI] [PubMed] [Google Scholar]
- 66.Korteweg FJ, Erwich JJ, Timmer A, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206:53.e1–53.e12. doi: 10.1016/j.ajog.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 67.Kasai M, Aoki S, Ogawa M, Kurasawa K, Takahashi T, Hirahara F. Prediction of perinatal outcomes based on primary symptoms in women with placental abruption. J Obstet Gynaecol Res. 2015;41:850–856. doi: 10.1111/jog.12637. [DOI] [PubMed] [Google Scholar]
- 68.Nkwabong E, Tiomela Goula G. Placenta abruption surface and perinatal outcome. J Matern Fetal Neonatal Med. 2017;30:1456–1459. doi: 10.1080/14767058.2016.1219988. [DOI] [PubMed] [Google Scholar]
- 69.Kvarnstrand L, Milsom I, Lekander T, Druid H, Jacobsson B. Maternal fatalities, fetal and neonatal deaths related to motor vehicle crashes during pregnancy: a national population-based study. Acta Obstet Gynecol Scand. 2008;87:946–952. doi: 10.1080/00016340802302184. [DOI] [PubMed] [Google Scholar]
- 70.Klinich KD, Flannagan CA, Rupp JD, Sochor M, Schneider LW, Pearlman MD. Fetal outcome in motor-vehicle crashes: effects of crash characteristics and maternal restraint. Am J Obstet Gynecol. 2008;198:450.e1–450.e9. doi: 10.1016/j.ajog.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 71.McClure EM, Garces A, Saleem S, et al. Global Network for Women’s and Children’s Health Research: probable causes of stillbirth in low- and middle-income countries using a prospectively defined classification system. BJOG. 2018;125:131–138. doi: 10.1111/1471-0528.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erlebacher A. Why isn’t the fetus rejected? Curr Opin Immunol. 2001;13:590–593. doi: 10.1016/s0952-7915(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 73.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 75.PrabhuDas M, Bonney E, Caron K, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 77.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 78.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leslie M. Immunology. Fetal immune system hushes attacks on maternal cells. Science. 2008;322:1450–1451. doi: 10.1126/science.322.5907.1450b. [DOI] [PubMed] [Google Scholar]
- 80.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burlingham WJ. A lesson in tolerance--maternal instruction to fetal cells. N Engl J Med. 2009;360:1355–1357. doi: 10.1056/NEJMcibr0810752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bluestone JA. Mechanisms of tolerance. Immunol Rev. 2011;241:5–19. doi: 10.1111/j.1600-065X.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 83.Betz AG. Immunology: Tolerating pregnancy. Nature. 2012;490:47–48. doi: 10.1038/490047a. [DOI] [PubMed] [Google Scholar]
- 84.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams Z. Inducing tolerance to pregnancy. N Engl J Med. 2012;367:1159–1161. doi: 10.1056/NEJMcibr1207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 87.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Soc Exp Biol. 1953:320–338. [Google Scholar]
- 88.Billington WD. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol. 2003;60:1–11. doi: 10.1016/s0165-0378(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 89.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 90.Bonney EA, Matzinger P. Much IDO about pregnancy. Nat Med. 1998;4:1128–1129. doi: 10.1038/2624. [DOI] [PubMed] [Google Scholar]
- 91.Bonney EA. Maternal tolerance is not critically dependent on interleukin-4. Immunology. 2001;103:382–389. doi: 10.1046/j.1365-2567.2001.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szekeres-Bartho J. Immunological relationship between the mother and the fetus. Int Rev Immunol. 2002;21:471–495. doi: 10.1080/08830180215017. [DOI] [PubMed] [Google Scholar]
- 93.Bonney EA, Onyekwuluje J. Maternal tolerance to H-Y is independent of IL-10. Immunol Invest. 2004;33:385–395. doi: 10.1081/imm-200032732. [DOI] [PubMed] [Google Scholar]
- 94.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 95.Moffett A, Loke YW. The immunological paradox of pregnancy: a reappraisal. Placenta. 2004;25:1–8. doi: 10.1016/S0143-4004(03)00167-X. [DOI] [PubMed] [Google Scholar]
- 96.Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85:71–75. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shepard MT, Bonney EA. PD-1 regulates T cell proliferation in a tissue and subset-specific manner during normal mouse pregnancy. Immunol Invest. 2013;42:385–408. doi: 10.3109/08820139.2013.782317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hemberger M. Immune balance at the foeto-maternal interface as the fulcrum of reproductive success. J Reprod Immunol. 2013;97:36–42. doi: 10.1016/j.jri.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 100.Xin L, Ertelt JM, Rowe JH, et al. Cutting edge: committed Th1 CD4+ T cell differentiation blocks pregnancy-induced Foxp3 expression with antigen-specific fetal loss. J Immunol. 2014;192:2970–2974. doi: 10.4049/jimmunol.1302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72:107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinder JM, Jiang TT, Ertelt JM, et al. Cross-Generational Reproductive Fitness Enforced by Microchimeric Maternal Cells. Cell. 2015;162:505–515. doi: 10.1016/j.cell.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaturvedi V, Ertelt JM, Jiang TT, et al. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest. 2015;125:1713–1725. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonney EA. Immune regulation in pregnancy: a matter of perspective? Obstet Gynecol Clin North Am. 2016;43:679–698. doi: 10.1016/j.ogc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonney EA, Onyekwuluje J. The H-Y response in mid-gestation and long after delivery in mice primed before pregnancy. Immunol Invest. 2003;32:71–81. doi: 10.1081/imm-120019209. [DOI] [PubMed] [Google Scholar]
- 106.Norton MT, Fortner KA, Oppenheimer KH, Bonney EA. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology. 2010;131:426–437. doi: 10.1111/j.1365-2567.2010.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol. 2016;16:90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang TT, Chaturvedi V, Ertelt JM, et al. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol. 2014;192:4949–4956. doi: 10.4049/jimmunol.1400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Erlebacher A. Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol Metab. 2010;21:428–434. doi: 10.1016/j.tem.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tagliani E, Erlebacher A. Dendritic cell function at the maternal-fetal interface. Expert Rev Clin Immunol. 2011;7:593–602. doi: 10.1586/eci.11.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 112.Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. 2014;58:189–198. doi: 10.1387/ijdb.140054ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strom TB, Tilney NL, Paradysz JM, Bancewicz J, Carpenter CB. Cellular components of allograft rejection: identity, specificity, and cytotoxic function of cells infiltrating acutely rejecting allografts. J Immunol. 1977;118:2020–2026. [PubMed] [Google Scholar]
- 114.Chan SY, DeBruyne LA, Goodman RE, Eichwald EJ, Bishop DK. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59:1155–1161. [PubMed] [Google Scholar]
- 115.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3:844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 116.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 117.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 118.Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee J, Romero R, Dong Z, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J, Romero R, Chaiworapongsa T, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70:265–284. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oggé G, Romero R, Lee DC, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223:553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee J, Romero R, Xu Y, et al. Detection of anti-HLA antibodies in maternal blood in the second trimester to identify patients at risk of antibody-mediated maternal anti-fetal rejection and spontaneous preterm delivery. Am J Reprod Immunol. 2013;70:162–175. doi: 10.1111/aji.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53–69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Romero R, Whitten A, Korzeniewski SJ, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70:285–298. doi: 10.1111/aji.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee J, Kim JS, Park JW, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013;34:681–689. doi: 10.1016/j.placenta.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 129.Bang H, Bae GE, Park HY, et al. Chronic Placental Inflammation in Twin Pregnancies. J Pathol Transl Med. 2015;49:489–496. doi: 10.4132/jptm.2015.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993;143:473–479. [PMC free article] [PubMed] [Google Scholar]
- 131.Kim JS, Romero R, Kim MR, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 133.Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000;31:292–295. doi: 10.1016/s0046-8177(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 134.Myerson D, Parkin RK, Benirschke K, Tschetter CN, Hyde SR. The pathogenesis of villitis of unknown etiology: analysis with a new conjoint immunohistochemistry-in situ hybridization procedure to identify specific maternal and fetal cells. Pediatr Dev Pathol. 2006;9:257–265. doi: 10.2350/08-05-0103.1. [DOI] [PubMed] [Google Scholar]
- 135.Duquesnoy RJ, Demetris AJ. Immunopathology of cardiac transplant rejection. Curr Opin Cardiol. 1995;10:193–206. doi: 10.1097/00001573-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 136.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 137.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 138.Smulyan H, Safar ME. Blood pressure measurement: retrospective and prospective views. Am J Hypertens. 2011;24:628–634. doi: 10.1038/ajh.2011.22. [DOI] [PubMed] [Google Scholar]
- 139.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–358. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 141.Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:443–452. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 142.Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 143.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 144.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21–28. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 145.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 146.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–343. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smulian JC, Bhandari V, Vintzileos AM, et al. Intrapartum fever at term: serum and histologic markers of inflammation. Am J Obstet Gynecol. 2003;188:269–274. doi: 10.1067/mob.2003.11. [DOI] [PubMed] [Google Scholar]
- 148.Salafia CM, Sherer DM, Spong CY, et al. Fetal but not maternal serum cytokine levels correlate with histologic acute placental inflammation. Am J Perinatol. 1997;14:419–422. doi: 10.1055/s-2007-994172. [DOI] [PubMed] [Google Scholar]
- 149.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 150.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 153.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 154.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 155.Halgunset J, Johnsen H, Kjollesdal AM, Qvigstad E, Espevik T, Austgulen R. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur J Obstet Gynecol Reprod Biol. 1994;56:153–160. doi: 10.1016/0028-2243(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 156.Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171:901–911. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 157.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 158.Hsu CD, Meaddough E, Hong SF, Aversa K, Lu LC, Copel JA. Elevated amniotic fluid nitric oxide metabolites and interleukin-6 in intra-amniotic infection. J Soc Gynecol Investig. 1998;5:21–24. doi: 10.1016/s1071-5576(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 159.Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol. 1998;179:1267–1270. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 160.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78:379–382. [PubMed] [Google Scholar]
- 161.Baud O, Emilie D, Pelletier E, et al. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. BJOG. 1999;106:72–77. doi: 10.1111/j.1471-0528.1999.tb08088.x. [DOI] [PubMed] [Google Scholar]
- 162.Hsu CD, Aversa K, Meaddough E. The role of amniotic fluid interleukin-6, and cell adhesion molecules, intercellular adhesion molecule-1 and leukocyte adhesion molecule-1, in intra-amniotic infection. Am J Reprod Immunol. 2000;43:251–254. doi: 10.1111/j.8755-8920.2000.430501.x. [DOI] [PubMed] [Google Scholar]
- 163.Harirah H, Donia SE, Hsu CD. Amniotic fluid matrix metalloproteinase-9 and interleukin-6 in predicting intra-amniotic infection. Obstet Gynecol. 2002;99:80–84. doi: 10.1016/s0029-7844(01)01632-5. [DOI] [PubMed] [Google Scholar]
- 164.Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011;32:732–736. doi: 10.1016/j.placenta.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 165.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1–e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 166.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med. 2015;28:1510–1519. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29:360–367. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 169.Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–567. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165(4 Pt 1):821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 171.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 172.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 173.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2015;28:1343–1359. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–1409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016;44:23–32. doi: 10.1515/jpm-2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44:77–98. doi: 10.1515/jpm-2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016;44:53–76. doi: 10.1515/jpm-2015-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44:33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weiss HB, Songer TJ, Fabio A. Fetal deaths related to maternal injury. JAMA. 2001;286:1863–1868. doi: 10.1001/jama.286.15.1863. [DOI] [PubMed] [Google Scholar]
- 183.Chibber R, Al-Harmi J, Fouda M, El-Saleh E. Motor-vehicle injury in pregnancy and subsequent feto-maternal outcomes: of grave concern. J Matern Fetal Neonatal Med. 2015;28:399–402. doi: 10.3109/14767058.2014.918094. [DOI] [PubMed] [Google Scholar]
- 184.Lufkin EG, Nelson RL, Hill LM, Melton LJ, 3rd, O’Fallon WM, Evans AT., 3rd An analysis of diabetic pregnancies at Mayo Clinic, 1950–79. Diabetes Care. 1984;7:539–547. doi: 10.2337/diacare.7.6.539. [DOI] [PubMed] [Google Scholar]
- 185.Montoro MN, Myers VP, Mestman JH, Xu Y, Anderson BG, Golde SH. Outcome of pregnancy in diabetic ketoacidosis. Am J Perinatol. 1993;10:17–20. doi: 10.1055/s-2007-994692. [DOI] [PubMed] [Google Scholar]
- 186.Pinto ME, Villena JE. Diabetic ketoacidosis during gestational diabetes. A case report. Diabetes Res Clin Pract. 2011;93:e92–e94. doi: 10.1016/j.diabres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 187.White BA, Labhsetwar SA, Mian AN. Streptococcus bovis bacteremia and fetal death. Obstet Gynecol. 2002;100(5 Pt 2):1126–1129. doi: 10.1016/s0029-7844(02)02206-8. [DOI] [PubMed] [Google Scholar]
- 188.Barth T, Broscheit J, Bussen S, Dietl J. Maternal sepsis and intrauterine fetal death resulting from Candida tropicalis chorioamnionitis in a woman with a retained intrauterine contraceptive device. Acta Obstet Gynecol Scand. 2002;81:981–982. doi: 10.1034/j.1600-0412.2002.811014.x. [DOI] [PubMed] [Google Scholar]
- 189.Malcolm G, Ellwood D, Devonald K, Beilby R, Henderson-Smart D. Absent or reversed end diastolic flow velocity in the umbilical artery and necrotising enterocolitis. Arch Dis Child. 1991;66(7 Spec):805–807. doi: 10.1136/adc.66.7_spec_no.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Valcamonico A, Danti L, Frusca T, et al. Absent end-diastolic velocity in umbilical artery: risk of neonatal morbidity and brain damage. Am J Obstet Gynecol. 1994;170:796–801. doi: 10.1016/s0002-9378(94)70285-3. [DOI] [PubMed] [Google Scholar]
- 191.Naeye RL. Maternal floor infarction. Hum Pathol. 1985;16:823–828. doi: 10.1016/s0046-8177(85)80254-9. [DOI] [PubMed] [Google Scholar]
- 192.Andres RL, Kuyper W, Resnik R, Piacquadio KM, Benirschke K. The association of maternal floor infarction of the placenta with adverse perinatal outcome. Am J Obstet Gynecol. 1990;163:935–938. doi: 10.1016/0002-9378(90)91100-q. [DOI] [PubMed] [Google Scholar]
- 193.Mandsager NT, Bendon R, Mostello D, Rosenn B, Miodovnik M, Siddiqi TA. Maternal floor infarction of the placenta: prenatal diagnosis and clinical significance. Obstet Gynecol. 1994;83(5 Pt 1):750–754. [PubMed] [Google Scholar]
- 194.Yeom W, Paik ES, An JJ, et al. Clinical characteristics and perinatal outcome of fetal hydrops. Obstet Gynecol Sci. 2015;58:90–97. doi: 10.5468/ogs.2015.58.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim SA, Lee SM, Hong JS, et al. Ultrasonographic severity scoring of non-immune hydrops: a predictor of perinatal mortality. J Perinat Med. 2015;43:53–59. doi: 10.1515/jpm-2013-0208. [DOI] [PubMed] [Google Scholar]
- 196.Warburton D. Chromosomal causes of fetal death. Clin Obstet Gynecol. 1987;30:268–277. doi: 10.1097/00003081-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 197.Wapner RJ. Genetics of stillbirth. Clin Obstet Gynecol. 2010;53:628–634. doi: 10.1097/GRF.0b013e3181ee2793. [DOI] [PubMed] [Google Scholar]
- 198.Bowman JM. Alloimmunization in twin pregnancies. Am J Obstet Gynecol. 1985;153:7–13. doi: 10.1016/0002-9378(85)90581-2. [DOI] [PubMed] [Google Scholar]
- 199.Branch DW. Immunologic disease and fetal death. Clin Obstet Gynecol. 1987;30:295–311. doi: 10.1097/00003081-198706000-00009. [DOI] [PubMed] [Google Scholar]
- 200.Hara S. Current pathological perspectives on chronic rejection in renal allografts. Clin Exp Nephrol. 2017;21:943–951. doi: 10.1007/s10157-016-1361-x. [DOI] [PubMed] [Google Scholar]
- 201.Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl Surg. 1999;5(4 Suppl 1):S30–S36. doi: 10.1053/JTLS005s00030. [DOI] [PubMed] [Google Scholar]
- 202.Stites E, Le Quintrec M, Thurman JM. The Complement System and Antibody-Mediated Transplant Rejection. J Immunol. 2015;195:5525–5531. doi: 10.4049/jimmunol.1501686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8:670–678. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 204.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 205.Nankivell BJ, Chapman JR. Chronic allograft nephropathy: current concepts and future directions. Transplantation. 2006;81:643–654. doi: 10.1097/01.tp.0000190423.82154.01. [DOI] [PubMed] [Google Scholar]
- 206.Chang DH, Kobashigawa JA. Current diagnostic and treatment strategies for cardiac allograft vasculopathy. Expert Rev Cardiovasc Ther. 2015;13:1147–1154. doi: 10.1586/14779072.2015.1087312. [DOI] [PubMed] [Google Scholar]
- 207.Verleden GM, Vos R, Vanaudenaerde B, et al. Current views on chronic rejection after lung transplantation. Transpl Int. 2015;28:1131–1139. doi: 10.1111/tri.12579. [DOI] [PubMed] [Google Scholar]
- 208.Burton GJ, Jauniaux E. What is the placenta? Am J Obstet Gynecol. 2015;213(4 Suppl):S6.e1–S6.e4. doi: 10.1016/j.ajog.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 209.Nelson DM. How the placenta affects your life, from womb to tomb. Am J Obstet Gynecol. 2015;213(4 Suppl):S12–S13. doi: 10.1016/j.ajog.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 210.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. 2015;213(4 Suppl):S14–S20. doi: 10.1016/j.ajog.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bedin M, Alsat E, Tanguy G, Cedard L. Placental sulfatase deficiency: clinical and biochemical study of 16 cases. Eur J Obstet Gynecol Reprod Biol. 1980;10:21–34. doi: 10.1016/0028-2243(80)90033-7. [DOI] [PubMed] [Google Scholar]
- 212.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 213.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 214.Abrahams VM, Aldo PB, Murphy SP, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu J, Feng Y, Wang J, et al. An “immune barrier” is formed in the placenta by hepatitis B immunoglobulin to protect the fetus from hepatitis B virus infection from the mother. Hum Vaccin Immunother. 2015;11:2068–2076. doi: 10.1080/21645515.2015.1010890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol. 2015;213(4 Suppl):S131–S137. doi: 10.1016/j.ajog.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Racicot K, Kwon JY, Aldo P, et al. Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth. Am J Reprod Immunol. 2016;75:451–460. doi: 10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mor G. Placental inflammatory response to Zika virus may affect fetal brain development. Am J Reprod Immunol. 2016;75:421–422. doi: 10.1111/aji.12505. [DOI] [PubMed] [Google Scholar]
- 221.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 222.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 223.Romero R, Maymon E, Pacora P, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–1077. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 224.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 225.Romero R, Savasan ZA, Chaiworapongsa T, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40:19–32. doi: 10.1515/JPM.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Heazell AE, Martindale EA. Can post-mortem examination of the placenta help determine the cause of stillbirth? J Obstet Gynaecol. 2009;29:225–228. doi: 10.1080/01443610802716042. [DOI] [PubMed] [Google Scholar]
- 227.Amir H, Weintraub A, Aricha-Tamir B, Apel-Sarid L, Holcberg G, Sheiner E. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intrauterine fetal death pregnancies. J Matern Fetal Neonatal Med. 2009;22:759–764. doi: 10.3109/14767050902929396. [DOI] [PubMed] [Google Scholar]
- 228.Tellefsen CH, Vogt C. How important is placental examination in cases of perinatal deaths? Pediatr Dev Pathol. 2011;14:99–104. doi: 10.2350/10-07-0870-OA.1. [DOI] [PubMed] [Google Scholar]
- 229.Bonetti LR, Ferrari P, Trani N, et al. The role of fetal autopsy and placental examination in the causes of fetal death: a retrospective study of 132 cases of stillbirths. Arch Gynecol Obstet. 2011;283:231–241. doi: 10.1007/s00404-009-1317-4. [DOI] [PubMed] [Google Scholar]
- 230.Flenady V, Middleton P, Smith GC, et al. Stillbirths: the way forward in high-income countries. Lancet. 2011;377:1703–1717. doi: 10.1016/S0140-6736(11)60064-0. [DOI] [PubMed] [Google Scholar]
- 231.Kim YM, Chaemsaithong P, Romero R, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28:2001–2009. doi: 10.3109/14767058.2014.976198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pinar H, Goldenberg RL, Koch MA, et al. Placental findings in singleton stillbirths. Obstet Gynecol. 2014;123(2 Pt 1):325–336. doi: 10.1097/AOG.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Roescher AM, Timmer A, Erwich JJ, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS One. 2014;9:e89419. doi: 10.1371/journal.pone.0089419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 235.Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol. 2002;5:159–164. doi: 10.1007/s10024001-0195-y. [DOI] [PubMed] [Google Scholar]
- 236.Whitten AE, Romero R, Korzeniewski SJ, et al. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. Am J Obstet Gynecol. 2013;208:310.e1–310.e11. doi: 10.1016/j.ajog.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Pravastatin to prevent recurrent fetal death in massive perivillous fibrin deposition of the placenta (MPFD) J Matern Fetal Neonatal Med. 2016;29:855–862. doi: 10.3109/14767058.2015.1022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 239.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 241.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet. 1986;1:1380. doi: 10.1016/s0140-6736(86)91685-5. [DOI] [PubMed] [Google Scholar]
- 242.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–1467. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 243.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988;34:141–145. doi: 10.1016/0952-3278(88)90137-8. [DOI] [PubMed] [Google Scholar]
- 244.Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins. 1989;37:149–161. doi: 10.1016/0090-6980(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 245.Romero R, Wu YK, Mazor M, Oyarzun E, Hobbins JC, Mitchell MD. Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1989;36:69–75. doi: 10.1016/0952-3278(89)90020-3. [DOI] [PubMed] [Google Scholar]
- 246.Bry K, Hallman M. Prostaglandins, inflammation, and preterm labor. J Perinatol. 1989;9:60–65. [PubMed] [Google Scholar]
- 247.Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci. 1990;26:425–428. [PubMed] [Google Scholar]
- 248.Hsu CD, Meaddough E, Aversa K, et al. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol. 1998;15:683–687. doi: 10.1055/s-2007-999302. [DOI] [PubMed] [Google Scholar]
- 249.Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2009;22:880–886. doi: 10.1080/14767050902994648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Maddipati KR, Romero R, Chaiworapongsa T, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 2014;28:4835–4846. doi: 10.1096/fj.14-254383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N, Yoon BH. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2016;29:2563–2572. doi: 10.3109/14767058.2015.1094794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Maddipati KR, Romero R, Chaiworapongsa T, et al. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. FASEB J. 2016;30:3296–3307. doi: 10.1096/fj.201600583R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lim SY, Kim YH, Kim CH, et al. The effect of a Foley catheter balloon on cervical ripening. J Obstet Gynaecol. 2013;33:830–838. doi: 10.3109/01443615.2013.831043. [DOI] [PubMed] [Google Scholar]
- 254.Liggins G. Cervical ripening as an inflammatory reaction. In: Ellwood E, Anderson A, editors. The cervix in pregnancy and labor: clinical and biochemical investigations. Edinburgh: Churchill Livingstone; 1981. pp. 1–9. [Google Scholar]
- 255.Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod. 1997 Mar;12:586–590. doi: 10.1093/humrep/12.3.586. [DOI] [PubMed] [Google Scholar]
- 256.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61:879–883. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 257.Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57:217–224. doi: 10.1016/s0165-0378(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 258.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 259.Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66:161–173. doi: 10.1016/j.jri.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 260.Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod. 2008;78:438–444. doi: 10.1095/biolreprod.107.063404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yellon SM, Oshiro BT, Chhaya TY, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011;85:498–502. doi: 10.1095/biolreprod.111.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod. 2011;84:587–594. doi: 10.1095/biolreprod.110.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87:106. doi: 10.1095/biolreprod.112.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Myers DA. The recruitment and activation of leukocytes into the immune cervix: further support that cervical remodeling involves an immune and inflammatory mechanism. Biol Reprod. 2012;87:107. doi: 10.1095/biolreprod.112.105049. [DOI] [PubMed] [Google Scholar]
- 265.Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 266.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 267.Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S2–S10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 268.Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 269.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, Lye SJ. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med. 2013;17:311–324. doi: 10.1111/jcmm.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20:154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 271.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol. 2016;13:462–473. doi: 10.1038/cmi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta. 1997;18:717–723. doi: 10.1016/s0143-4004(97)90014-x. [DOI] [PubMed] [Google Scholar]
- 273.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006;13:97–103. doi: 10.1016/j.jsgi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 274.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009 Jun;80:122–131. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 275.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205:235.e15–235.e24. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 276.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204:364.e9–364.e6. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 277.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–230. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Alonso A, Soto I, Urgelles MF, Corte JR, Rodriguez MJ, Pinto CR. Acquired and inherited thrombophilia in women with unexplained fetal losses. Am J Obstet Gynecol. 2002;187:1337–1342. doi: 10.1067/mob.2002.126849. [DOI] [PubMed] [Google Scholar]
- 279.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 2003;361:901–908. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 280.Lamont RF, Sobel JD, Vaisbuch E, et al. Parvovirus B19 infection in human pregnancy. BJOG. 2011;118:175–186. doi: 10.1111/j.1471-0528.2010.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Maymon E, Romero R, Bhatti G, Chaemsaithong P, Gomez-Lopez N, Panaitescu B, Chaiyasit N, Pacora P, Dong Z, Hassan SS, Erez O. Chronic inflammatory lesions of the placenta are associated with an up-regulation of amniotic fluid CXCR3: A marker of allograft rejection. J Perinat Med. 2018;46:123–137. doi: 10.1515/jpm-2017-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.