Concurrent KIAA1549-BRAF fusion and chromosome 1p deletion with or without concomitant chromosome 19q loss are recurrent molecular alterations in the diffuse leptomeningeal glioneuronal tumor (DLGNT) [6,7,10], in which widespread leptomeningeal dissemination is a diagnostic feature [1,6,10]. We have reviewed five spinal cord tumors (Table 1) that show the morphologic and molecular genetic characteristics of DLGNT, but in which radiographically apparent leptomeningeal dissemination was not identified at presentation. At initial review, we sought to understand the relationship between these tumors and DLGNT by undertaking immunohistochemical analyses with a panel of neural markers: GFAP, OLIG2, and synaptophysin, and with antibodies to the BRAF V600E, histone H3.1/H3.3 K27M, and IDH1 R132H mutant proteins. Duplication of chromosome 7q34 (a surrogate marker for the presence of KIAA1549-BRAF fusion) and deletion of chromosome arms 1p and 19q were examined by interphase fluorescence in situ hybridization (iFISH). The presence of KIAA1549-BRAF fusion transcripts was also tested by reverse transcription polymerase chain reaction (RT-PCR). We collected relevant clinical information and follow-up radiologic studies to characterize the tumors’ clinical behavior and tendency for leptomeningeal dissemination.
Table 1.
Patient Demographics, Tumor Locations and Pathologic Findings
| Case | Age (yrs) | Gender | Site (spinal cord) | Follow-up | OLCs | NIs | LM | GFAP | Olig2 | Syn |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | M | T10–12 | 5 m | + | + | + | − | + | + |
| 2 | 20 | F | Obex-Conus | 10 m | + | + | − | − | + | + |
| 3 | 5 | M | C4-T4 | 4 y | + | + | + | − | n/d | + |
| 4 | 14 | F | Obex-Conus | 2 m | + | + | + | − | + | + |
| 5 | 5 | M | C2-T9 | 7.5 y | + | + | + | − | + | + |
Follow-up; m: months; y: years
OLCs: oligodendrocyte-like cells
NIs: neuropil-like islands
LM: microscopic leptomeningeal involvement
Syn: synaptophysin
n/d: not done
DLGNT presents most commonly in children and young adults [6]. Similarly, among the five patients in our series, four were children (5-14 years old) and one was a young adult (20 years old). There was no apparent gender predilection, although female patients were older in our series. Pre-operative imaging was not available for one child. While diffuse leptomeningeal enhancement was not identified by MRI in any of the five cases, extensive intramedullary involvement across multiple segments was typical (Supplemental Figure). Except for one distal thoracic tumor spanning three segments, all tumors spanned ≥7 segments, with holospinal involvement from obex to conus in two cases. All four pre-operatively imaged tumors appeared central within the cord, filling and expanding the central canal (Fig. 1). All were multicystic, with peripheral variably solid or nodular enhancement. This mixed solid/cystic appearance, with T1 hypointensity and T2 hyperintensity, matches the imaging characteristics of DLGNT with a spinal cord mass [3,6,8–10]. None of the five patients had documented leptomeningeal metastasis during follow-up (2 months – 7.5 years).
Figure 1. Imaging Features of Spinal BRAF-fused and 1p-deleted Glioneuronal Tumors.
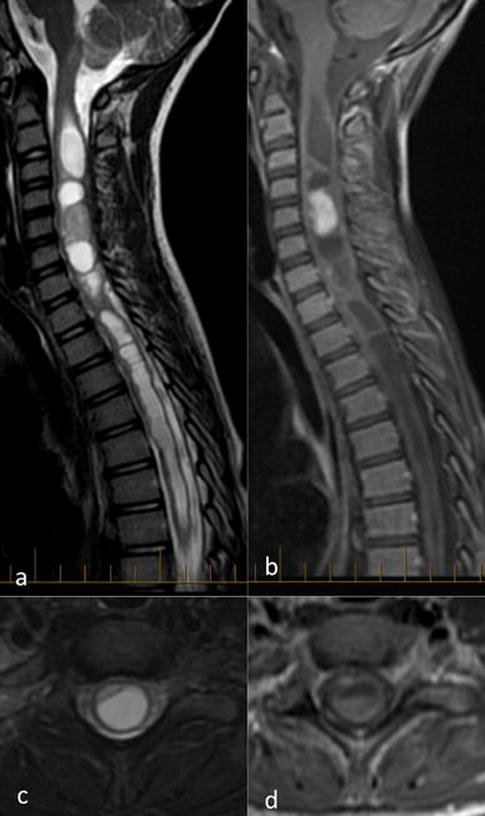
Sagittal (a, b) and axial (c, d) fat-saturated T2 (a, c) and contrast-enhanced T1-weighted images (b, d) show multi-cystic tumor with peripheral and nodular enhancement, filling and expanding the central canal of the spinal cord across multiple spinal segments.
Tumor cells in DLGNT have a characteristic oligodendrocyte-like morphology and express OLIG2. Expression of GFAP or synaptophysin is more variable. Signs of neurocytic or ganglion cell differentiation are present in 10-20% of cases [6,10]. Neuropil-like hypocellular zones and glial foci that resemble a pilocytic astrocytoma are sometimes seen [10]. Mimicking DLGNT, the five spinal cord tumors in our series contained many oligodendrocyte-like cells (OLCs), which either infiltrated spinal cord parenchyma or grew in small nests (Table 1, Fig. 2). By immunohistochemistry, they expressed OLIG2 and synaptophysin, but not GFAP. All contained rare neuropil-like islands, which were often surrounded by tumor cells with neurocytic differentiation and had a rosette-like configuration, a feature distinct from conventional low-grade ganglion cell tumors. The rosetted neuropil islands were much larger than similar structures in the rosette-forming glioneuronal tumor. Glial foci resembling pilocytic astrocytoma (PA) were also evident. Mitotic counts (<1/10hpfs) and Ki-67 immunolabeling (<5%) were low, in line with our experience of DLGNTs. While leptomeningeal spread was not found radiographically, microscopic leptomeningeal involvement was identified at the site of biopsy in four cases.
Figure 2. Histologic Features of Spinal BRAF-fused and 1p-deleted Glioneuronal Tumors.
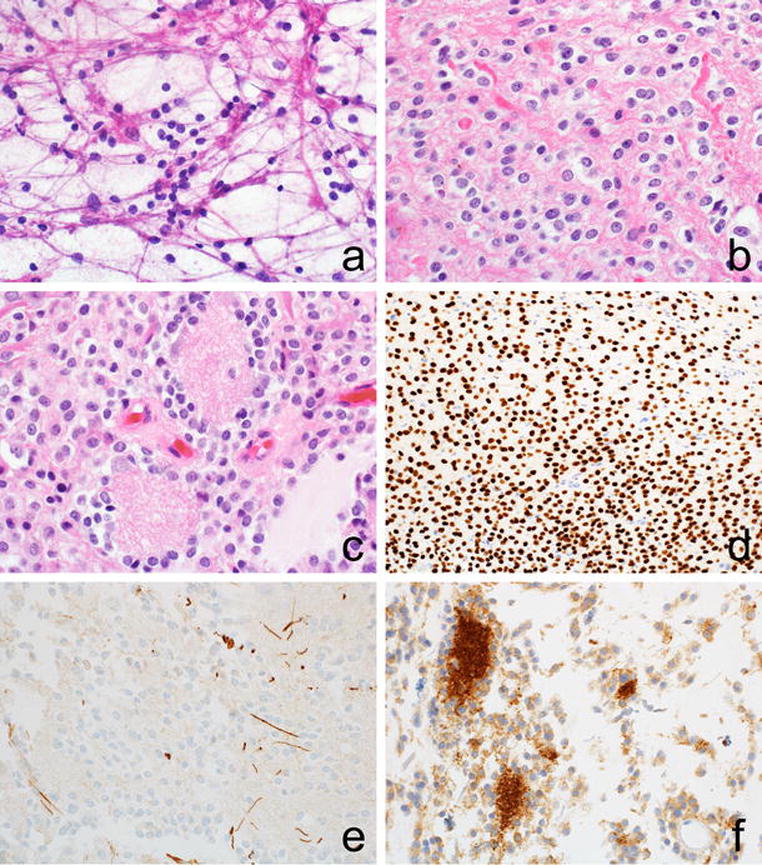
The oligodendrocyte-like cytology of the tumor is evident on both smear preparations (a) and fixed histologic sections (b). Axons are seen among oligodendrocyte-like cells (OLCs), giving away their infiltrative nature. Neuropil-like islands are often surrounded by tumor cells and have a rosette-like configuration (c). OLCs are immunopositive for OLIG2 (d) and immunonegative for GFAP (e). Synaptophysin immunoreactivity marks tumor cells and highlights neuropil islands (f).
Chromosome 7q34 duplication, a surrogate marker for the presence of KIAA1549-BRAF fusions, was identified in all five cases by iFISH (Table 2). The presence of fusion transcripts was further confirmed in four cases tested by RT-PCR. In two cases, exon 15 of KIAA1549 was fused to exon 9 of BRAF (15-9), which is also commonly seen in extra-cerebellar midline PAs [2]. The 15-11 fusion seen in case #3 is occasionally found in supratentorial PAs [5]. Both 15-9 and 15-11 fusions have been detected in DLGNTs at our institution. The 13-11 fusion in case #4 occurs rarely in PAs [11]. Chromosome 1p loss was present in all five cases. Chromosome 19q loss was not identified in the four cases tested. Immunostains for BRAF V600E, histone H3.1/H3.3 K27M and IDH1 R132H mutant proteins were negative in tumors with sufficient tissue for testing.
Table 2.
Summary of Molecular Findings
| Case | 7q34 dup | Fusion | 1p− | 19q− | V600E | K27M | IDH1 |
|---|---|---|---|---|---|---|---|
| 1 | + | 15–9 | + | − | − | − | − |
| 2 | + | 15–9 | + | − | − | − | − |
| 3 | + | 15–11 | + | − | n/d | n/d | n/d |
| 4 | + | 13–11 | + | − | − | − | − |
| 5 | + | n/d | + | n/d | − | − | n/d |
dup: duplication
Fusion: KIAA1549-BRAF fusion; 15-9: KIAA1549 exon 15 fused to BRAF exon 9; 15-11: KIAA1549 exon 15 fused to BRAF exon 11; 13-11: KIAA1549 exon 13 fused to BRAF exon 11
1p− chromosome arm 1p deletion; 19q− chromosome 19q deletion
V600E: immunostain for BRAF p.V600E mutant protein
K27M: immunostain for histone H3.1/H3.3 p.K27M mutant proteins
IDH1: immunostain for IDH1 p.R132H mutant protein
n/d: not done
The spinal tumors in our series share the patient demographics, radiologic characteristics, morphologic features, and genetic alterations of DLGNTs, but lack overt leptomeningeal dissemination at presentation and during follow-up. When a parenchymal mass is present in a DLGNT it is often in the spinal cord. Our findings suggest that a low-grade glioneuronal tumor with the same features can also present as a solitary spinal cord mass. Since DLGNTs demonstrate slow, but nonetheless relentless and debilitating clinical progression [1,4,6,8], it would be prudent to follow such intramedullary tumors closely to understand their long term clinical outcome. However, our findings suggest that DLGNTs form a spectrum with tumors that have no obvious leptomeningeal dissemination at presentation, which has implications for the DLGNT’s provisional status in the latest WHO classification.
Supplementary Material
Statement of Human Rights.
The study was approved by the appropriate institutional research ethics committees. All procedures were in accordance with their ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
The authors thank Drs. Susana Raimondi and Elizabeth Azzato for their assistance with iFISH and RT-PCR assays, respectively.
References
- 1.Cho HJ, Myung JK, Kim H, Park CK, Kim SK, Chung CK, et al. Primary diffuse leptomeningeal glioneuronal tumors. Brain Tumor Pathol. 2015;32:49–55. doi: 10.1007/s10014-014-0187-z. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner C, Ellis HP, Shaw A, Penman C, Palmer A, Wragg C, et al. BRAF Fusion Analysis in Pilocytic Astrocytomas: KIAA1549-BRAF 15-9 Fusions Are More Frequent in the Midline Than Within the Cerebellum. J Neuropathol Exp Neurol. 2015;74:867–872. doi: 10.1097/NEN.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardiman MP, Fassan M, Orvieto E, D’Avella D, Denaro L, Calderone M, et al. Diffuse leptomeningeal glioneuronal tumors: a new entity? Brain Pathol. 2010;20:361–366. doi: 10.1111/j.1750-3639.2009.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler BA, Bookhout C, Jaikumar S, Hipps J, Lee YZ. Disseminated oligodendroglial-like leptomeningeal tumor with anaplastic progression and presumed extraneural disease: case report. Clin Imaging. 2015;39:300–304. doi: 10.1016/j.clinimag.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Lin A, Rodriguez FJ, Karajannis MA, Williams SC, Legault G, Zagzag D, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez FJ, Perry A, Rosenblum MK, Krawitz S, Cohen KJ, Lin D, et al. Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol. 2012;124:627–641. doi: 10.1007/s00401-012-1037-x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez FJ, Schniederjan MJ, Nicolaides T, Tihan T, Burger PC, Perry A. High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN) Acta Neuropathol. 2015;129:609–610. doi: 10.1007/s00401-015-1400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi S, Rodriguez FJ, Mota RA, Dei Tos AP, Di Paola F, Bendini M, et al. Primary leptomeningeal oligodendroglioma with documented progression to anaplasia and t(1;19)(q10;p10) in a child. Acta Neuropathol. 2009;118:575–577. doi: 10.1007/s00401-009-0565-5. [DOI] [PubMed] [Google Scholar]
- 9.Ruppert B, Welsh CT, Hannah J, Giglio P, Rumboldt Z, Johnson I, et al. Glioneuronal tumor with neuropil-like islands of the spinal cord with diffuse leptomeningeal neuraxis dissemination. J Neurooncol. 2011;104:529–533. doi: 10.1007/s11060-010-0505-1. [DOI] [PubMed] [Google Scholar]
- 10.Schniederjan MJ, Alghamdi S, Castellano-Sanchez A, Mazewski C, Brahma B, Brat DJ, et al. Diffuse leptomeningeal neuroepithelial tumor: 9 pediatric cases with chromosome 1p/19q deletion status and IDH1 (R132H) immunohistochemistry. Am J Surg Pathol. 2013;37:763–771. doi: 10.1097/PAS.0b013e31827bf4cc. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


