Abstract
Anaplasmosis is a tick-borne infectious disease that affects both human and animal health. This study was performed to characterize and investigate the prevalence of infection with Anaplasma bovis in Holstein cattle originating from two regions in the Republic of Korea (ROK). Blood samples (n = 151; 80 from Namwon and 71 from Jeju Island) were analyzed by polymerase chain reaction, and the prevalence of A. bovis infection was compared before and after grazing. In Namwon, A. bovis infection was not detected, while in the Jeju Island, A. bovis infection was detected in three of 13 animals after grazing. Phylogenetic analysis revealed that the A. bovis isolates had homology (97.1–99.7%) with a Korean spotted deer (Cervus nippon) isolate and Haemaphysalis longicornis tick isolates identified in the ROK. A. bovis infection has not previously been diagnosed in cattle in the ROK. This study shows that A. bovis infection in the Jeju Island is closely related to grazing.
Keywords: Anaplasma bovis, Grazing, Holstein cattle, Ticks
Findings
The climate of the Korean Peninsula is rapidly becoming subtropical, and warmer temperatures have already resulted in accelerated parasitic development and an extreme rise in vector populations [1]. These climatic changes have a widespread impact on the ecosystems. Temporal and spatial changes in temperature, precipitation, and humidity that occur under different climatic conditions affect the biology and ecology of vectors and intermediate hosts, and may increase the risk of infection transmission [2]. Tick distribution is also closely linked with climate, and there is growing concern that the prevalence of tick-borne diseases, such as theileriosis and anaplasmosis, may be increasing in the Republic of Korea (ROK) [3–6].
Anaplasmosis is a tick-transmitted disease that affects dogs, cats, horses, cattle, sheep, goats, and wild ruminants. The Anaplasma genus comprises six species showing differences in host cell tropism. A. centrale, A. marginale, and A. ovis are erythrocytic, while A. bovis, A. phagocytophilum, and A. platys infect monocytes, neutrophils, and platelets, respectively [7]. Bovine anaplasmosis is caused by A. bovis, A. centrale, A. marginale, and A. phagocytophilum. A. marginale is widely distributed in tropical and subtropical regions throughout the world. It causes a mild to severe hemolytic disease in cattle and wild ruminants, and is particularly highly pathogenic in cattle up to 2 years old [8]. The infection is characterized by persistent fever, lethargy, icterus, weight loss, abortion, reduced milk production, and death in more than 50% of untreated animals [8]. A. centrale is a less pathogenic species compared to A. marginale and causes mild symptoms in cattle and is considered a naturally attenuated subspecies [9]. A. phagocytophilum is known to infect humans and animals, and causes tick-borne fever being characterized by fever, respiratory signs, leukopenia, abortion, and sudden decrease in milk production [10, 11]. A. bovis causes fever, anemia, drowsiness, convulsions, weight loss, and enlargement of lymph nodes in cattle [12]. This infection has been found in China and Japan [13–15], but recently, A. bovis was also detected in Korean spotted deer (Cervus nippon) [16], Korean water deer (Hydropotes inermis argyropus) [17], and Haemaphysalis longicornis ticks in the ROK [18, 19]. However, information regarding A. bovis infection in cattle is not available in the ROK. Therefore, the aim of the present study was to investigate A. bovis infection in cattle before and after grazing and to characterize the evolutionary relationships of obtained A. bovis isolates.
Jugular vein EDTA stabilized blood samples (Vacutainer® tubes, Beckton Dickinson, Franklin Lakes, NJ, USA) were taken from 151 Holstein cattle in the ROK, consisting of 80 samples from one herd in the Namwon region and 71 samples from a herd on the Jeju Island (Fig. 1). The samples were taken twice from April to August 2016. Cattle raised at both farms were grazed on grass from the middle of May to the end of November. The samples were analyzed for erythrocyte numbers, hemoglobin, hematocrit, and white blood cell counts using the VetScan HM5 Hematology System (Abaxis, Union, CA, USA).
Fig. 1.
Map of the Republic of Korea. Dots indicate the location of the region of Namwon and the Jeju Island where blood samples were collected
Genomic DNA was extracted from blood samples using the DNeasy Blood kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A first round of polymerase chain reaction (PCR) was performed to amplify the 16S rRNA gene shared by all Anaplasma spp. (F, 5ʹ-TACCTCTGTGTTGTAGCTAACGC-3ʹ; R, 5ʹ-CTTGCGACATTGCAACCTATTGT-3ʹ). In a second round of PCR to identify individual Anaplasma spp., the following primers were used: AB1f/AB1r for A. bovis (F, 5′-CTCGTAGCTTGC TATGAGAAC-3′; R, 5′-TCTCCCGGACTCCAGTCTG-3′), msp4 for A. centrale (F, 5′-CATGGGGCATGAATCTGTG-3′; R, 5′-AATTGGTTGCAGTGAGCGC-3′), and msp4 for A. marginale (F, 5′-CATCTCCCATGAGTCACGAAGTGGC-3′; R, 5′-GCTGAACAG GAATCTTGCTCC-3′). PCR was performed under the cycling conditions: 98 °C for 5 min, followed by 35 cycles of 10 s at 98 °C, annealing at 58 °C for 30 s for 16S rRNA gene [3], 55 °C for 1 min for A. bovis [20], 53 °C for 30 s for A. centrale, and 53 °C for 30 s for A. marginale [21], 72 °C for 1 min, and final extension at 72 °C for 5 min. Distilled water was used as negative control for each PCR. The expected sizes of the 16S rRNA gene, A. bovis, A. centrale, and A. marginale were 429, 551, 395, and 252 bp, respectively. PCR products were visualized under UV light after 1.5% agarose gel electrophoresis and ethidium bromide staining.
The amplicons were purified using the Accupower Gel Extraction kit (Bioneer, Daejeon, ROK) and cloned into the pGEM®-T Easy vector (Promega, Madison, WI, USA), which was directly sequenced (Bioneer). Sequences were analyzed using the BioEdit version 7.2.5 sequence alignment software. A phylogenetic tree was constructed using the neighbor-joining method in MEGA 6.0 software [22] and bootstrapping with 1000 replicates. The two representative sequences obtained in this study were deposited in the GenBank database under accession numbers MF197897 and MF197898.
Anaplasma bovis was not detected in cattle from Namwon region, while three of 71 animals (4.2%) from Jeju Island tested positive (Table 1). No samples were positive for either A. centrale or A. marginale. None of the A. bovis-positive cattle showed hematological signs of infection, such as anemia and leukocytosis. A. bovis infection in cattle from Jeju Island was observed only after the animals had been on pasture consisted with having been exposed to ticks. This is the first study to report A. bovis infection in cattle in the ROK. The observed difference between the regions may be due to differences in climate. Unlike the Namwon region, Jeju Island has a subtropical climate with seasonal variations in precipitation, humidity, and temperature, which are more suitable for the reproduction and activity of ticks. Several studies have reported that the prevalence of Anaplasma spp. differs among climatic zones and is associated with suitability of tick habitats and animal management methods [13, 23].
Table 1.
Comparison of Anaplasma infections before and after grazing in Namwon and Jeju Island
| Region | Namwon | Jeju Island | ||
|---|---|---|---|---|
| Date of sample collection | April 27, 2016 | July 1, 2016 | May 16, 2016 | August 4, 2016 |
| Grazing type/no. of samples | Housing (n = 40) | Grazing (n = 40) | Housing (n = 58) | Grazing (n = 13) |
| A. bovis | 0 | 0 | 0 | 3 |
| A. centrale | 0 | 0 | 0 | 0 |
| A. marginale | 0 | 0 | 0 | 0 |
To detect A. bovis DNA in the blood, we first performed PCR using primers for the 16S rRNA gene shared by all Anaplasma spp. To identify A. bovis-infected cattle, PCR products were then amplified using A. bovis-specific primers (Table 1). Of the three A. bovis gene amplicons, two high-quality sequences were obtained (MF197897 and MF197898), which showed 98.1% homology. Phylogenetic analysis of the partial 16S rRNA gene was performed by aligning the obtained A. bovis sequences with selected Anaplasma spp. sequences found in GenBank. The MF197897 and MF197898 sequences were closely related to A. bovis and were distinct from A. centrale, A. marginale, A. ovis, A. phagocytophilum, and unspecified Anaplasma sp. included in GenBank (Fig. 2). The Korean cattle isolates had 99.7% homology to sequences from A. bovis strains originating from a Korean spotted deer (EU682764) and H. longicornis ticks (GU064902 and KC311344), respectively. They were also 97.1% homologous to sequences of A. bovis isolated from H. longicornis ticks (EU181142) from the Jeju Island and H. longicornis ticks (AF470698) collected from a different province (Gyeonggi) in the ROK (Fig. 2).
Fig. 2.
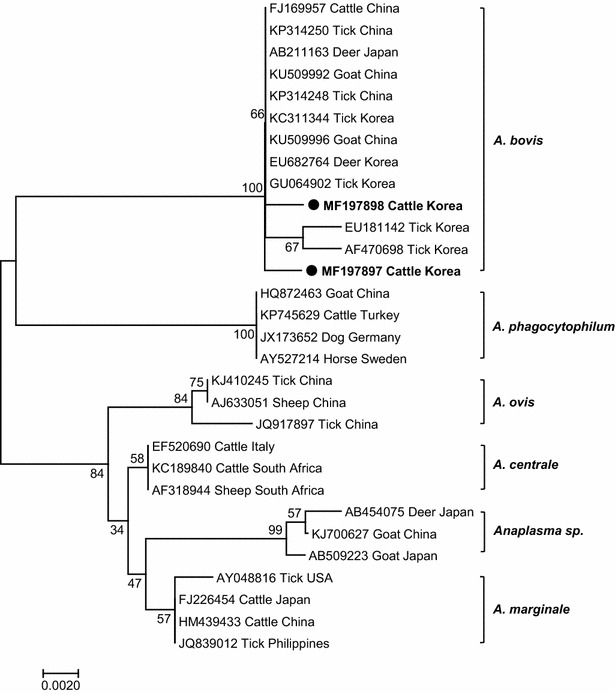
Phylogenetic analysis using the partial 16S rRNA gene (521 bp) sequences of isolates from Holstein cattle and representative Anaplasmataceae species. An unrooted phylogenetic tree was constructed with bootstrap values obtained by 1000 replicates using MEGA 6.0 software and the neighbor-joining method. The sequences found in this study are shown in bold
Knowledge on the epidemiology of A. bovis infection in cattle in the ROK is limited. A. bovis has been detected in H. longicornis ticks [18, 19, 24], the most common tick species in the ROK. This tick species may play an important role in the transmission of A. bovis infection in the ROK. Infection with A. centrale and A. marginale, the most common pathogens causing bovine anaplasmosis, were not found. This may be related to the absence of their vectors in the ROK. Rhipicephalus simus and Dermacentor variabilis are considered as tick vectors for A. centrale in Africa and A. marginale in the USA, respectively [23, 25]; however, in the ROK, these tick species have not been found. Although the clinical significance of A. bovis infection was not evaluated in the present study, extensive epidemiological studies on domestic animals are needed to clarify the pathogenicity and pathogenesis of A. bovis infection.
Authors’ contributions
KSC designed the study and drafted the manuscript. JHP participated in designing the study, coordinated, and revised the manuscript. JBC, JSC, DHY, BKP, and HCK participated in sample collection. DGH, JHR, JBC, JSC, and DHY performed the experiments, data analysis, and participated in drafting of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All procedures were performed according to the ethical guidelines for the use of animal samples according to the Chonbuk National University (Institutional Animal Care and Use Committee [IACUC] Decision No. CBU 2014-00026). Consent was obtained from cattle owners.
Funding
This work was performed with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010092), Rural Development Administration, the Republic of Korea.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- A. bovis
Anaplasma bovis
- A. centrale
Anaplasma centrale
- A. marginale
Anaplasma marginale
- H. longicornis
Haemaphysalis longicornis
- PCR
polymerase chain reaction
- ROK
Republic of Korea
Footnotes
Jinho Park and Du-Gyeong Han contributed equally to this work
Contributor Information
Jinho Park, Email: jpark@jbnu.ac.kr.
Du-Gyeong Han, Email: ktf0222@hanmail.net.
Ji-Hyoung Ryu, Email: yjh562@naver.com.
Jeong-Byoung Chae, Email: jbchae117@gmail.com.
Joon-Seok Chae, Email: jschae@snu.ac.kr.
Do-Hyeon Yu, Email: yudh@gnu.ac.kr.
Bae-Keun Park, Email: bkpark@cnu.ac.kr.
Hyeon-Cheol Kim, Email: advs@kangwon.ac.kr.
Kyoung-Seong Choi, Email: kschoi3@knu.ac.kr.
References
- 1.Chae JS, Adjemian JZ, Kim HC, Ko S, Klein TA, Foley J. Predicting the emergence of tick-borne infections based on climatic changes in Korea. Vector Borne Zoonotic Dis. 2008;8:265–275. doi: 10.1089/vbz.2007.0190. [DOI] [PubMed] [Google Scholar]
- 2.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 3.Choi KS, Yu DH, Chae JS, Park BK, Yoo JG, Park J. Seasonal changes in hemograms and Theileria orientalis infection rates among Holstein cattle pastured in the mountains in the Republic of Korea. Prev Vet Med. 2016;127:77–83. doi: 10.1016/j.prevetmed.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Kang SW, Doan HT, Choe SE, Noh JH, Yoo MS, Reddy KE, Kim YH, Kweon CH, Jung SC, Chang KY. Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol Int. 2013;62:276–282. doi: 10.1016/j.parint.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Seong G, Han YJ, Oh SS, Chae JS, Yu DH, Park J, Park BK, Yoo JG, Choi KS. Detection of tick-borne pathogens in the Korean water deer (Hydropotes inermis argyropus) from Jeonbuk Province, Korea. Korean J Parasitol. 2015;53:653–659. doi: 10.3347/kjp.2015.53.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang JG, Ko S, Kim HC, Chong ST, Klein TA, Chae JB, Jo YS, Choi KS, Yu DH, Park BK, Park J, Chae JS. Prevalence of Anaplasma and Bartonella spp. in ticks collected from Korean water deer (Hydropotes inermis argyropus) Korean J Parasitol. 2016;54:87–91. doi: 10.3347/kjp.2016.54.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 8.Kocan KM, de la Fuente J, Guglielmone AA, Melendez RD. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin Microbiol Rev. 2003;16:698–712. doi: 10.1128/CMR.16.4.698-712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rar V, Golovljova I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000;31:554–560. doi: 10.1086/313948. [DOI] [PubMed] [Google Scholar]
- 11.Stuen S. Anaplasma phagocytophilum—the most widespread tick-borne infection in animals in Europe. Vet Res Commun. 2007;31:79–84. doi: 10.1007/s11259-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 12.Harrison A, Bastos AD, Medger K, Bennett NC. Eastern rock sengis as reservoir hosts of Anaplasma bovis in South Africa. Ticks Tick Borne Dis. 2013;4:503–505. doi: 10.1016/j.ttbdis.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Ma M, Wang Z, Wang J, Peng Y, Li Y, Guan G, Luo J, Yin H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl Environ Microbiol. 2012;78:464–470. doi: 10.1128/AEM.06848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooshiro M, Zakimi S, Matsukawa Y, Katagiri Y, Inokuma H. Detection of Anaplasma bovis and Anaplasma phagocytophilum from cattle on Yonaguni Island, Okinawa, Japan. Vet Parasitol. 2008;154:360–364. doi: 10.1016/j.vetpar.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Li Y, Liu Z, Liu J, Niu Q, Ren Q, Chen Z, Guan G, Luo J, Yin H. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasit Vectors. 2015;8:108. doi: 10.1186/s13071-015-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M, Yu D, Yoon J, Li Y, Lee J, Park J. Natural co-infection of Ehrlichia chaffeensis and Anaplasma bovis in a deer in South Korea. J Vet Med Sci. 2009;71:101–103. doi: 10.1292/jvms.71.101. [DOI] [PubMed] [Google Scholar]
- 17.Kang JG, Ko S, Kim YJ, Yang HJ, Lee H, Shin NS, Choi KS, Chae JS. New genetic variants of Anaplasma phagocytophilum and Anaplasma bovis from Korean water deer (Hydropotes inermis argyropus) Vector Borne Zoonotic Dis. 2011;11:929–938. doi: 10.1089/vbz.2010.0214. [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Chae JS. Molecular detection of Ehrlichia chaffeensis and Anaplasma bovis in the salivary glands from Haemaphysalis longicornis ticks. Vector Borne Zoonotic Dis. 2010;10:411–413. doi: 10.1089/vbz.2008.0215. [DOI] [PubMed] [Google Scholar]
- 19.Doan HT, Noh JH, Choe SE, Yoo MS, Kim YH, Reddy KE, Quyen DV, Nguyen LT, Nguyen TT, Kweon CH, Jung SC, Chang KY, Kang SW. Molecular detection and phylogenetic analysis of Anaplasma bovis from Haemaphysalis longicornis feeding on grazing cattle in Korea. Vet Parasitol. 2013;196:478–481. doi: 10.1016/j.vetpar.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Kawahara M, Rikihisa Y, Lin Q, Isogai E, Tahara K, Itagaki A, Hiramitsu Y, Tajima T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol. 2006;72:1102–1109. doi: 10.1128/AEM.72.2.1102-1109.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shkap V, Leibovitz B, Krigel Y, Molad T, Fish L, Mazuz M, Fleiderovitz L, Savitsky I. Concomitant infection of cattle with the vaccine strain Anaplasma marginale ss centrale and field strains of A. marginale. Vet Microbiol. 2008;130:277–284. doi: 10.1016/j.vetmic.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Belkahia H, Ben Said M, Alberti A, Abdi K, Issaoui Z, Hattab D, Gharbi M, Messadi L. First molecular survey and novel genetic variants’ identification of Anaplasma marginale, A. centrale and A. bovis in cattle from Tunisia. Infect Genet Evol. 2015;34:361–371. doi: 10.1016/j.meegid.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Kim CM, Yi YH, Yu DH, Lee MJ, Cho MR, Desai AR, et al. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72:5766–5776. doi: 10.1128/AEM.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potgieter FT, van Rensburg L. Tick transmission of Anaplasma centrale. Onderstepoort J Vet Res. 1987;54:5–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


