Abstract
Objective
Antimicrobial resistance (AMR) is a critical public health issue that involves interrelationships between people, animals, and the environment. Traditionally, interdisciplinary efforts to mitigate AMR in the food chain have involved public health, human and veterinary medicine, and agriculture stakeholders. Our objective was to identify a more diverse range of stakeholders, beyond those traditionally engaged in AMR mitigation efforts, via diagramming both proximal and distal factors impacting, or impacted by, use and resistance along the Canadian food chain.
Results
We identified multiple stakeholders that are not traditionally engaged by public health when working to mitigate AMR in the food chain, including those working broadly in the area of food (e.g., nutrition, food security, international market economists) and health (e.g., health communication, program evaluation), as well as in domains as diverse as law, politics, demography, education, and social innovation. These findings can help researchers and policymakers who work on issues related to AMR in the food chain to move beyond engaging the ‘traditional’ agri-food stakeholders (e.g., veterinarians, farmers), to also engage those from the wider domains identified here, as potential stakeholders in their AMR mitigation efforts.
Keywords: Antimicrobial resistance, Food safety, Participatory epidemiology, Public health policy
Introduction
Antimicrobial resistance (AMR) is a critical public health issue causing increased morbidity and mortality worldwide [1, 2]. Antimicrobial use (AMU) in any sector, including in humans and animals, on crops, in cleaning products, or through environmental contamination during manufacturing, creates selection pressures that favour the survival of microorganisms less affected by, or resistant to, the antimicrobial’s effects [3, 4]. In pathogenic bacteria, AMR leads to infections that are difficult to treat. Alarmingly, such bacteria are exhibiting more serious resistance, including to multiple antimicrobials simultaneously [5], and to the most important antimicrobials for human medicine, including fluoroquinolones, 3rd and 4th generation cephalosporins, and other drugs of last resort (e.g., colistin; [6]).
In pathogenic bacteria transmitted via food, such as Campylobacter and Salmonella, AMR is a complex issue involving interrelationships between people, animals, and the environment, and AMU in food-producing animals (whether for preventive, therapeutic, or growth promotion purposes) is a recognized contributor to resistant human infections [7, 8]. Thus, public health efforts to track the link between on-farm AMU and the emergence of AMR have been implemented, allowing the resistance profiles of pathogenic bacteria from food animals on-farm and at slaughter to be compared to profiles from food products at retail and from subsequent human infection [9–12].
In addition to the direct relationship between AMU and AMR in animals and humans, it is important to assess the role of broader, systemic drivers. To-date, such assessments have evaluated the contribution of governance and corruption to AMR in a variety of pathogens including those transmitted by food [13]; explored the role of regulation related to AMR in the environment [14]; and considered the impact of AMR policy actions that have yet to adequately address the economic situations of farmers [15]. Exploring additional factors such as these enables other stakeholders—beyond those traditionally involved from the medical, public health, veterinary medicine and agri-food sectors—to be identified and engaged in creating sustainable actions to reduce AMU and AMR, and ultimately maintain the effectiveness of antimicrobials for human and veterinary medicine. Therefore, our objective was to diagram the range of potential proximal and distal factors impacting, or impacted by, AMU and AMR along the Canadian food chain, and to use this diagram to identify additional stakeholders who are not currently engaged in the effort to reduce AMR in foodborne pathogens in Canada, with whom researchers and policymakers working on AMR in foodborne pathogens can engage in future transdisciplinary activities.
Main text
We diagrammed factors related to AMU and AMR in the Canadian food chain, drawing on our own expertise in foodborne disease, AMR, and the Canadian food system, and informed by both group model building [16] and expert elicitation [17] approaches. We created a conceptual model that illustrated such factors, and the interrelationships between them where possible, through an iterative series of in-person brainstorming sessions. The model was created in Vensim® PLE Plus for Macintosh (version 6.3; Ventana Systems, Inc.), and was drawn using a systems dynamics model format, which has been used for other public health issues to depict the underlying set of complex factors and interrelationships that drive the issue [18, 19]. We started by diagramming the most traditionally-considered source of AMR in the food chain, on-farm AMU in food-producing animals, and its links to AMR and human illness (Fig. 1). Next, we added other potential factors that could impact, or be impacted by, on-farm AMU. We continued expanding the conceptual model, by iteratively identifying additional factors, and adding specificity so that the factors aptly represented the Canadian food chain context, until no new changes were noted.
Fig. 1.
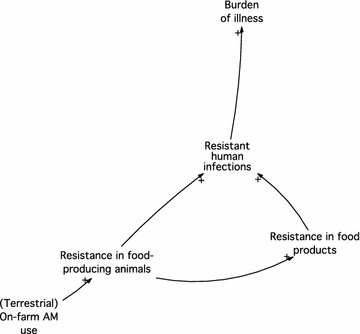
Model showing the traditionally-considered source of antimicrobial use and resistance in foodborne pathogens. AM antimicrobial; + represents the positive association between factors
We then applied a case scenario to our draft model: the voluntary withdrawal of ceftiofur (a Category I antimicrobial, the most important category for human medicine [20]) in chicken hatcheries in the province of Québec, Canada, and the subsequent return to partial use, as described by Dutil et al. [10]. Briefly, 2003 data from the Canadian Integrated Program for Antimicrobial Resistance Surveillance showed that resistance to ceftiofur in human infections of Salmonella Heidelberg in the province of Québec was high, and tracked well against the level of resistance found in Salmonella Heidelberg isolated from retail chicken samples collected from Québec. Upon receiving these data in 2005, Québec hatcheries responded and voluntarily stopped using ceftiofur to treat and prevent E. coli omphalitis in chicks, and in 2006 levels of resistance in both S. Heidelberg infections in humans and S. Heidelberg isolated from retail chicken decreased. However, in 2007, Québec hatcheries returned to partial use of ceftiofur to treat and prevent E. coli omphalitis, and resistance in human infections and retail chicken began to increase. By 2013, levels of resistance had returned to the 2003 levels.
Using this case scenario, we further refined our model by examining factors that may have: (1) been positively or negatively impacted when the voluntary withdrawal was in place; (2) been impacted had the withdrawal continued, either with or without any other changes in the underlying system of factors; or (3) contributed to the withdrawal being non-sustainable, that is, that might have driven hatcheries to start using ceftiofur again. For example, during the full withdrawal period we considered: did producers see a re-emergence of disease within flocks, and did this arise from the fact they were still producing chickens in the same manner as before, with no other changes in the broader production environment (e.g., economic drivers)? We used our knowledge of the industry and production practices to hypothesize about wider forces and added these factors to the model. We also examined the potential implications of the 2014 voluntary ban by the Canadian poultry industry, that “eliminates the preventative use of Category I antibiotics in Canadian chicken production” [21]. Through these explorations we added and removed factors and relationships, to yield the final conceptual model (Fig. 2).
Fig. 2.
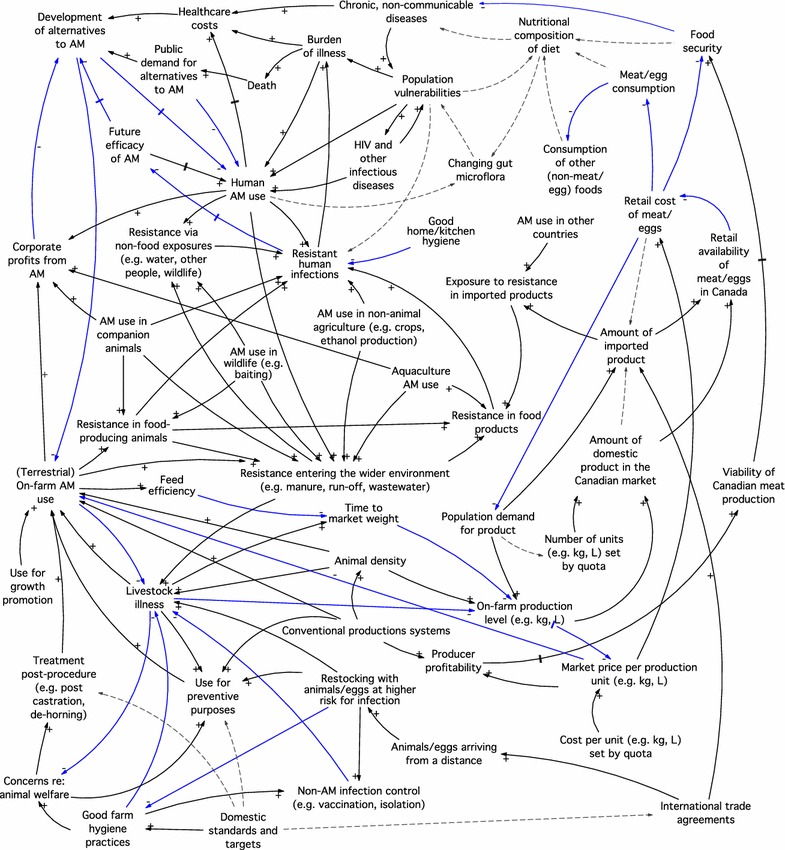
Final conceptual model, showing factors related to antimicrobial use and resistance in foodborne pathogens. AM antimicrobial; +/black arrow and −/blue arrow signs represent the positive and negative directions of association between factors (where possible); dashed lines show potential associations or complex pathways that cannot be summarized with a ± relationship; double hash marked lines show time-delayed pathways
We then used the model to identify potential stakeholders by determining the individuals and organizations who act upon the different factors in the model. We classified stakeholders by whether or not they had been commonly engaged by public health practitioners in mitigating and reducing AMU and AMR in the Canadian food chain (Table 1). Interestingly, this process allowed us to identify a wide range of potential stakeholders not typically engaged in the issue, many of whom exist as individual experts who may not necessarily be represented by organized groups. Historically, multidisciplinary approaches to mitigating and reducing AMU and AMR in the Canadian food chain context have engaged organizations; such entities are typically easier to identify and approach, and often represent large groups of individuals with a stake in the issue (e.g., Chicken Farmers of Canada, representing individual chicken farmers). Our model highlighted that such approaches to engagement, although important, may be missing key stakeholders for whom formal or organized entities do not exist, and that an important next step is to initiate conversations with a wider range of individuals with diverse expertise and experience, to explore potential roles and impacts of non-traditional stakeholders in the issue of AMR.
Table 1.
Stakeholders in the issue of antimicrobial use and resistance in the Canadian food chain
| Stakeholder | Traditionally engaged by public health organizations in the issue of AMR in the food chain? | |
| Yes | No | |
| Organizations | Federal Agriculture, Food, Health, and Trade Ministries Provincial Ministries of Agriculture, Health/Public Health Animal and Human Health Organizations Animal Industry Organizations Vet/Human Medical Associations Codex Alimentarius Commission Food and Agriculture Organization of the United Nations (FAO) World Organisation for Animal Health (OIE) World Health Organization (WHO) Drug Manufacturers (R&D, marketing) |
Consumers, consumer advocacy groups Political organizations Urban, municipal planners |
| Individuals | Farmers, veterinarians | Nutrition, food security experts Farm, international market economists The public Educators, parents Health communication, program evaluation experts Lawyers Animal, human welfare advocates Academia, network/systems experts Political strategists, lobbyists Demographers Futurists, social innovators Environmentalists, urban agriculture workers |
Conclusions
Generating a conceptual model of the factors underlying AMR in foodborne pathogens was a useful process that allowed us to identify stakeholders who are not traditionally engaged by public health, to mitigate AMR in the food system. In addition to identifying stakeholders beyond the traditional agri-food partners, our model also identified that traditional methods for stakeholder engagement, that focus on engaging organizations, may be missing key stakeholders for whom formal or organized entities do not exist. Future efforts to engage a broader range of stakeholders are needed, in ways that allow for dialogue and exploration of how they act within and impact the system, in order to identify multi-pronged and sustainable approaches to mitigate and reduce AMR and its impacts across humans, animals, and the environment. Specifically, such dialogue is necessary to: (1) identify additional relevant model factors from domains like land use management, the media, access to health care and services; (2) explore other case scenarios to further identify factors that hold the current system ‘in place’ (e.g., support keeping the current level of AMU); (3) co-define potential courses of action, including roles and key influencers; and (4) explore potential ramifications, both intended and unintended, of possible actions. The ideal expansion of this work would involve revising the model to include all additional relevant factors and inter-relationships, and assigning quantities and functions to all factors and relationships, respectively, so that predictive modelling can occur. To achieve this, expert engagement with the range of stakeholders identified here is needed, to determine where quantitative data exist, and where approaches like expert elicitation are needed to specify such a model in the absence of empirical evidence.
Limitations
We recognize that this work is subject to several limitations, most notably that it relies on our own expertise. However, this should not diminish the utility of the findings as a first depiction of the diversity of factors related to AMR in the Canadian food chain context, that allows us to identify additional domain experts with whom to engage in future model expansion and validation. Our approach was guided by expert elicitation, a method particularly useful for addressing questions that are difficult to answer via any other means [17, 22–25], that has been used previously to both qualitatively to rank pathways and build models [26, 27], and to produce quantitative estimates [24, 25, 28]. In the field of enteric pathogen source attribution, for example, when quantitative data are incomplete or unavailable, expert elicitation represents the only possible method for synthesizing knowledge about pathogen transmission [29]. In expanding the model presented here, expert elicitation involving diverse domain experts will undoubtedly be key to specifying the larger model structure (as we have begun, here), to identifying empirical evidence and data that can be used to quantify model components (e.g., to build structural equation models), and to determining values for model components where quantitative data are missing or incomplete.
We also recognize that taking a higher level systems view of AMR, as we have done here, necessitates ignoring nuance and detail, and reduces entire disciplines to a summary arrow or label in our model. This is another reason that domain experts across the factors identified here must now be engaged to ensure model completeness and accuracy, particularly given that we recognize our perspective is biased towards public health, foodborne disease, and veterinary medicine and livestock-associated factors. As well, to manage the size of this model, we focused on AMR in foodborne pathogens; however, micro-organisms transmitted via different pathways interact to shape the resistance landscape, and it will be important to explore how AMR in non-foodborne pathogens (e.g., AMR in Neisseria gonorrhea; [30]) may link or add to the factors identified here. Nevertheless, we feel that presenting our process, our model and our identified list of stakeholders provides a transparent starting point for more in-depth model building and stakeholder engagement processes, and that taking a broad approach to identifying model factors compliments existing models of AMR, that are often built specific to one domain and that typically focus on a limited number of proximate factors (e.g., [31]).
Authors’ contributions
SEM and EJP conceived the study; all authors contributed equally to the model building and stakeholder identification; SEM wrote the manuscript with input from EJP, CC, and KP. All authors reviewed and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank their numerous colleagues, with whom watercooler conversations helped spur and clarify the ideas presented here, and the Guelph Grotto Indoor Climbing Gym for providing the right climate for conceiving this idea.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was unfunded.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AMU
antimicrobial use
- AMR
antimicrobial resistance
Contributor Information
Shannon E. Majowicz, Phone: +1 519 888 4567, Email: smajowicz@uwaterloo.ca
E. Jane Parmley, Email: jane.parmley@canada.ca.
Carolee Carson, Email: carolee.carson@canada.ca.
Katarina Pintar, Email: katarina.pintar@canada.ca.
References
- 1.Pumart P, Phodha T, Thamlikitkul V, Riewpaiboon A, Prakongsai P, Limwattananon S. Health and economic impacts of antimicrobial resistance in Thailand. J Health Syst Res. 2012;6:352–360. [Google Scholar]
- 2.World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization. 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/. Accessed 14 Mar 2017.
- 3.O’Brien TF. Emergence, spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002;34:S78–S84. doi: 10.1086/340244. [DOI] [PubMed] [Google Scholar]
- 4.Fick J, Söderström H, Lindberg RH, Phan C, Tysklind M, Larsson DG. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem. 2009;28:2522–2527. doi: 10.1897/09-073.1. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. U.S. Department of Health and Human Services. 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 22 Mar 2017.
- 6.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 7.Review on Antimicrobial Resistance. Antimicrobials in agriculture and the environment: reducing unnecessary use and waste. 2015. http://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf. Accessed 17 Mar 2017.
- 8.World Bank Group. Drug-resistant infections: a threat to our economic future (discussion draft). Washington, DC: World Bank. License: creative commons attribution cc BY 3.0 IGO. 2016. http://pubdocs.worldbank.org/en/689381474641399486/1701381-AMR-Lab-Report-Web.pdf. Accessed 19 June 2017.
- 9.Skjøt-Rasmussen L, Ethelberg S, Emborg HD, Agersø Y, Larsen LS, Nordentoft S, Olsen SS, Ejlertsen T, Holt H, Nielsen EM, Hammerum AM. Trends in occurrence of antimicrobial resistance in Campylobacter jejuni isolates from broiler chickens, broiler chicken meat, and human domestically acquired cases and travel associated cases in Denmark. Int J Food Microbiol. 2009;131(2–3):277–279. doi: 10.1016/j.ijfoodmicro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Dutil L, Irwin R, Finley R, Ng LK, Avery B, Boerlin P, Bourgault AM, Cole L, Daignault D, Desruisseau A, Demczuk W, Hoang L, Horsman GB, Ismail J, Jamieson F, Maki A, Pacagnella A, Pillai DR. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis. 2010;16(1):48–54. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folster JP, Pecic G, Singh A, Duval B, Rickert R, Ayers S, Abbott J, McGlinchey B, Bauer-Turpin J, Haro J, Hise K, Zhao S, Fedorka-Cray PJ, Whichard J, McDermott PF. Characterization of extended-spectrum cephalosporin-resistant Salmonella enterica serovar Heidelberg isolated from food animals, retail meat, and humans in the United States 2009. Foodborne Pathog Dis. 2012;9(7):638–645. doi: 10.1089/fpd.2012.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. NARMS integrated report: 2014. 2014. https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM528861.pdf. Accessed 15 Mar 2017.
- 13.Collignon P, Athukorala P-C, Senanayake S, Khan F. Antimicrobial resistance: the major contribution of poor governance and corruption to this growing problem. PLoS ONE. 2015 doi: 10.1371/journal.pone.0116746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer AC, Shaw H, Rhodes V, Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front Microbiol. 2016;7:1728. doi: 10.3389/fmicb.2016.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lhermie G, Gröhn YT, Raboisson D. Addressing antimicrobial resistance: an overview of priority actions to prevent suboptimal antimicrobial use in food-animal production. Front Microbiol. 2016;7:2114. doi: 10.3389/fmicb.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bérard C. Group model building using system dynamics: an analysis of methodological frameworks. Electron J Bus Res Methods. 2010;8(1):35–45. [Google Scholar]
- 17.Butler AA, Thomas MK, Pintar KDM. Systematic review of expert elicitation methods as a tool for source attribution of enteric illness. Foodborne Pathog Dis. 2015;12(5):367–382. doi: 10.1089/fpd.2014.1844. [DOI] [PubMed] [Google Scholar]
- 18.Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006;96(3):452–458. doi: 10.2105/AJPH.2005.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Government Office for Science. Reducing obesity: Obesity System Map. 2007. https://www.gov.uk/government/publications/reducing-obesity-future-choices. Accessed 14 Feb 2018.
- 20.Government of Canada. Categorization of antimicrobial drugs based on importance in human medicine, April 2009. https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html. Accessed 04 Dec 2017.
- 21.Chicken Farmers of Canada (n.d.) Questions and Answers. http://www.chickenfarmers.ca/what-we-do/antibiotics/faq/. Accessed 14 Mar 2017.
- 22.Cressey P, Lake R. Ranking food safety risks: development of NZFSA policy 2004–2005. Client Report FW0563:23. Christchurch, New Zealand.
- 23.Havelaar AH, Galindo AV, Kurowicka D, Cooke RM. Attribution of foodborne pathogens using structured expert elicitation. Foodborne Pathog Dis. 2008;5:649–659. doi: 10.1089/fpd.2008.0115. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann SA, Fischbeck PS, Krupnick AJ, McWilliams M. Attributing foodborne illnesses to their food sources: Using large expert panels to capture variability in expert judgment. Washington, DC: Resources for the Future, 2006; Discussion Paper 06-17-REV.
- 25.Vally H, Glass K, Ford L, Hall G, Kirk MD, Shadbolt C, Veitch M, Fullerton KE, Mustro J, Becker N. Proportion of illness acquired by foodborne transmission for nine enteric pathogens in Australia: an expert elicitation. Foodborne Pathog Dis. 2014;11:727–733. doi: 10.1089/fpd.2014.1746. [DOI] [PubMed] [Google Scholar]
- 26.Tan K, Baxter G, Newell S, Smye S, Dear P, Brownlee K, Darling J. Knowledge elicitation for validation of a neonatal ventilation expert system utilising modified Delphi and focus group techniques. Int J Hum-Comput Stud. 2010;68:344–354. doi: 10.1016/j.ijhcs.2009.08.003. [DOI] [Google Scholar]
- 27.de Jong A, Wardekker JA, van der Sluijs JP. Assumptions in quantitative analyses of health risks of overhead power lines. Environ Sci Policy. 2012;16:114–121. doi: 10.1016/j.envsci.2011.11.012. [DOI] [Google Scholar]
- 28.Cooke RM, Wilson AM, Tuomisto JT, Morales O, Tainio M, Evans JS. A probabilistic characterization of the relationship between fine particulate matter and mortality: elicitation of European experts. Environ Sci Technol. 2007;41:6598–6605. doi: 10.1021/es0714078. [DOI] [PubMed] [Google Scholar]
- 29.Butler AA, Thomas MK, Pintar KDM. Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne Pathog Dis. 2015;12(4):335–344. doi: 10.1089/fpd.2014.1856. [DOI] [PubMed] [Google Scholar]
- 30.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alban L, Ellis-Iversen J, Andreasen M, Dahl J, Sönksen UW. Assessment of the risk to public health due to use of antimicrobials in pigs—an example of pleuromutilins in Denmark. Front Vet Sci. 2017;4:74. doi: 10.3389/fvets.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


