Abstract
Objective
Since the implementation of a series of blood donation safety improvements in Kenya, information about seroprevalence and determinants of transfusion transmissible infections among voluntary blood donors especially in high HIV burden regions of Homabay, Kisumu and Siaya counties remain scanty. A cross-sectional study examining HIV, syphilis, hepatitis B and C virus sero-markers and associated determinants was conducted among voluntary blood donors. Their demographic characteristics and previous risk exposure were recorded in a pre-donation questionnaire, while blood samples collected were screened for hepatitis B, hepatitis C, human immunodeficiency viruses by ELISA and RPR (syphilis), then confirmed using CMIA.
Results
Overall TTIs seroprevalence was 114 (9.4%), distributed among HIV, HBV, HCV and syphilis at 14 (1.15%), 42 (3.46%), 39 (3.21%) and 19 (1.56%), respectively, with co-infections of 3 (0.25%). There were no significant differences in proportions distributions among demographic variables. However, high risk sex was significantly associated with higher odds of HBV infections [> 1 partner vs. 0–1 partner; odd ratio (OR) 2.60; 95% confidence interval (CI) 1.098–6.86; p = 0.046]. In conclusion, a substantial percentage of blood donors still harbor transfusion transmissible infections despite recent safety improvements with greater majority cases caused by HBV infections arising from previous exposure to high risk sex.
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3276-y) contains supplementary material, which is available to authorized users.
Keywords: Seroprevalence, HIV, HBV, Donors, Kenya
Introduction
Transfusion transmissible infections especially human immunodeficiency virus and hepatitis viruses, are a constant threat to blood safety for the recipient, and more endemic in Africa, thus making donors in this region vulnerable to risk of exposure [1]. Moreover, co-infection is also common due to similar routes of transmission [2], with prevalence varying with time and regions [3]. Infections with HIV compromises immunological status of a person, hepatitis B and C viral infections cause liver cirrhosis and hepatocellular carcinoma while infections with syphilis results in neonatal mortality [4]; thus, high quality screening for the four major TTIs have become mandatory and irreducible minimum that must be tested in all blood donations to achieve safety threshold [5].
Globally, about 1.6 million blood units are destroyed annually owing to TTIs seropositivity [5], with 10% discarded in sub-Saharan Africa [6]. In the neighbouring Democratic Republic of Congo (DRC), risk of transfusion-transmitted HIV, HBV and HCV was estimated at 0.6, 7.9 and 3.1 infections per 1000 donations, respectively [7]. Here in Kenya, an estimated risk of transfusion-transmitted HIV was reported at 2% [8]. Moreover, despite of recent blood donation safety improvements such as stringent pre-donation screening, enhanced haemovigilance through e-progesa blood computerized system (BECs) and implementation of fourth generation p24 antigen and HIV antibody screening assays [9], a substantial amount of blood units estimated at 5.26%, are still discarded annually owing to TTIs seropositivity [10]. Furthermore, HIV, HBV, HCV and syphilis seropositive blood discards estimated at 2.6, 3.9, 2.2 and 0.5%, respectively, were reported earlier in Kisumu region under Kenya national blood transfusion services (KNBTS) covering Homabay, Kisumu and Siaya counties in western Kenya [11]. The three counties experience the greatest burden of HIV and other co-infections in the region [12], but also serve as a major blood basket supplying most hospital in the region based on their proximity to regional blood donor center of Kisumu and ease for transport during donations. To date, there is no published data on blood donation safety assessment following recent safety improvements.
Socio-demographic characteristics influence the distribution of TTIs among blood donors. In Kenya, national blood transfusion services (NBTS) rely mainly on young voluntary blood donors, particularly secondary schools, colleges and University students, majority in the age range of 15–24 years [13]. Blood donations from school students are preferred over adult donors owing to lower HIV prevalence estimated at 1% compared to 6.6% prevalence recorded in adults aged 30–34 years [13]. However, a study carried out in Kenya using stimmunology still detected a significant number of early pre-seroconversion of HIV carriers both among adults and teenage population [14]. This study sought to establish the dominant demographic and other risk factors still playing a role in TTIs seropositivity recorded in the region despite recent safety interventions.
Main text
Methods
Using Yamane formula, and based on KNBTS selection protocol, blood samples of 1215 voluntary blood donors aged 16–65 years from Homabay, Kisumu and Siaya counties (see Additional file 1), was determined for a cross-sectional study by random sampling. Laboratory tests were conducted at Kisumu Regional Blood Donor Center, Kenya, from November 2015 to October 2016 based on its proximity to study sites and quality standards available. Ethical approval was obtained from Maseno University Ethical Review Committee (MUERC). Blood donors were enrolled under informed written consent or with guardian consent in case of donors less than 18 years. A self administered pre-donation questionnaire (see Additional file 2), was used to screen potential donors for health fitness and to collect demographic details such as age. Recent exposure to high risks such as unprotected sex in a period less than 3 months were noted and excluded. Baseline hemoglobin test and body weight measurements was performed by nurse counselor to exclude individuals with anemia (hemoglobin < 12.5 g/dl) or body weight below 50 kg. After blood collection, about 10 ml of donor blood was transferred into a plain vacutainer tube (BD Franklin Lakes, USA) for HIV-1/2, HBV, HCV and syphilis serological testing.
Sera were tested for antigen or/antibody to HIV-1/2 using Vironostika HIV Uni-Form II Ag/Ab test kit (Biomerieux SA, Microelisa system), antibodies to hepatitis C (Anti-HCV) using Abbott Murex Anti-HCV version 4.0 (Murex Biotech SA (Pty) Ltd) and Hepatitis B surface antigen (HBsAg) using Hepanostika HBsAg Ultra test (Biomerieux SA, Microelisa system). Sera were also tested for presence of treponemal antibodies using rapid plasma reagin (RPR) test (Omega diagnostics, UK). All tests were done according to manufacturers’ instructions. All reactive samples were confirmed by chemiluminescent immunoassay. A result was considered positive if the first test and confirmatory test were all seroreactive.
Statistical analysis was performed using SPSS version 20. Seroprevalence distribution in subcategories were compared using Chi square test while logistic regression was used to determine the association between TTIs seroprevalence and various risk factors with a p-value < 0.05 considered statistically significant.
Results
The baseline characteristics of the study participants are summarized in Table 1. Of 1215 voluntary blood donors recruited, 700 (57.6%) were males and 515 (42.4%) females. The overall seroprevalence of TTIs was 114 (9.4%), distributed among HIV, HBV, HCV and syphilis variables at 14 (1.15%), 42 (3.46%), 39 (3.21%) and 19 (1.56%), respectively, with co-infections detected in only 3 (0.25%). There were no significant differences in proportions distributions among demographic variables tested. Demographic and risk factors among donors were evaluated as shown in Table 2. High risk sex was significantly associated with higher odds of HBV infections [> 1 partner vs. 0–1 partner; odd ratio (OR) 2.60; 95% confidence interval (CI) 1.098–6.86; p = 0.046], rather than other infections (see Additional file 3). All other characteristic variables tested had no significant association with infections.
Table 1.
Demographic characteristics of reactive and non-reactive participants
| Characteristics | Non-reactive, n (%) | Reactive, n (%) |
|---|---|---|
| Age groups | ||
| Teenage (16–19) | 894 (91.6%) | 82 (8.4%) |
| Adults (20–65) years | 211 (88.28%) | 28 (11.72%) |
| Gender | ||
| Females | 475 (92.23%) | 40 (7.77%) |
| Males | 630 (90.0%) | 70 (10.0%) |
| Marital status | ||
| Married | 49 (98.0%) | 1 (2.0%) |
| Single | 1056 (90.64%) | 109 (9.36%) |
| Number of donations | ||
| First | 813 (90.33%) | 87 (9.76%) |
| Repeats | 292 (92.7%) | 23 (7.30%) |
| Counties | ||
| Homabay | 293 (89.0%) | 36 (10.94%) |
| Kisumu | 469 (92.69%) | 37 (7.31%) |
| Siaya | 343 (90.26%) | 37 (9.74%) |
Data shown are numbers (n) and proportions (%) of TTIs seronegative (1105) and seropositive (110) as distributed among demographics
Table 2.
Demographic variables of individual infections among reactive and non-reactive blood donors
| Characteristics | HIV (n = 14) | p-value | HBV (n = 42) | p-value | HCV (n = 39) | p-value | Syphilis (n = 19) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-reactive | Reactive | Non-reactive | Reactive | Non-reactive | Reactive | Non-reactive | Reactive | |||||
| Counties | ||||||||||||
| Homabay | 322 | 7 | 0.062 | 317 | 12 | 0.824 | 318 | 11 | 0.633 | 323 | 6 | 0.192 |
| Kisumu | 503 | 3 | ref. | 489 | 17 | ref. | 492 | 14 | ref. | 502 | 4 | ref. |
| Siaya | 376 | 4 | 0.45 | 367 | 13 | 0.96 | 366 | 14 | 0.441 | 371 | 9 | 0.66 |
| Age groups years | ||||||||||||
| Teenagers (16–19) | 965 | 11 | ref. | 945 | 31 | ref. | 947 | 29 | ref. | 961 | 15 | ref. |
| Adults (20–65) | 236 | 3 | 0.863 | 228 | 11 | 0.282 | 229 | 10 | 0.348 | 235 | 4 | 0.879 |
| Gender | ||||||||||||
| Female | 510 | 5 | ref. | 501 | 14 | ref. | 498 | 17 | ref. | 508 | 7 | ref. |
| Male | 691 | 9 | 0.613 | 672 | 28 | 0.23 | 678 | 22 | 0.877 | 688 | 12 | 0.623 |
| Marital status | ||||||||||||
| Married | 50 | 0 | 49 | 1 | ref. | 50 | 0 | 50 | 0 | |||
| Single | 1151 | 14 | – | 1124 | 41 | 0.57 | 1126 | 39 | – | 1146 | 19 | – |
| Drug use | ||||||||||||
| No | 1189 | 14 | 1161 | 42 | 1164 | 39 | 1185 | 18 | ref. | |||
| Yes | 12 | 0 | – | 12 | 0 | – | 12 | 0 | – | 11 | 1 | 0.095 |
| High risk sex | ||||||||||||
| No | 1139 | 13 | ref. | 1115 | 37 | ref. | 1116 | 36 | ref. | 1134 | 18 | ref. |
| Yes | 62 | 1 | 0.741 | 58 | 5 | 0.046 | 60 | 3 | 0.476 | 62 | 1 | 0.988 |
| No. donations | ||||||||||||
| First | 888 | 12 | ref. | 869 | 32 | ref. | 869 | 31 | ref. | 884 | 16 | ref. |
| Repeats | 313 | 2 | 0.329 | 305 | 10 | 0.75 | 307 | 8 | 0.435 | 312 | 3 | 0.317 |
| Blood transfusion | ||||||||||||
| No | 1180 | 14 | 1153 | 41 | ref. | 1157 | 37 | ref. | 1175 | 19 | ||
| Yes | 21 | 0 | – | 20 | 1 | 0.742 | 19 | 2 | 0.118 | 21 | 0 | – |
High risk sex was significantly associated with HBV infections p = 0.046
Data shown are: ref., reference category; n, numbers; HIV-1/2Ag/Ab, human immunodeficiency virus 1 and 2 antigen and antibody; HBVsAg, hepatitis B surface antigen; Anti-HCV, antibody to hepatitis C virus; –, no analysis done due to lack of cases; No, not exposed; Yes, exposed; Reactive, seropositive; Non-reactive, seronegative
Discussion
The present study was set to determine the seroprevalence and determinants of HIV, HBV, HCV and syphilis among voluntary blood donors in Homabay, Kisumu and Siaya counties in western Kenya. Results established that, overall seroprevalence of TTIs among voluntary blood donors was 9.4%. Similar results have been reported in studies done in Ethiopia showing 9.5% seroprevalence [1], and in Benin, Nigeria showing 12.5% seroprevalence [15]. However, different result were reported in some parts of Nigeria showing seroprevalence of 19.0% [16], and in Burkina Faso showing seroprevalence of 24% [17]. This observation relates to HIV endemicity and intermediate endemicity of HBV and HCV in the region. In this study, overall TTIs seroprevalence in the three study sites was relatively higher compared to earlier reports of 5.26% national seroprevalence [10]. This variation may be attributed to HIV endemicity experienced in the three study sites as compared to the rest of the country showing difference in HIV/STIs profile. Meanwhile, co-infection of 0.25% detected in the study population was comparable to 0.02% reported earlier in a local study [18], and 0.8% reported in the neighbouring Ethiopia [1].
The HIV seroprevalence of 1.15% detected among blood donors is indicative of low seroprevalence and decreased susceptibility to HIV infections. Similar studies in DRC reported similar result of 1.1% seroprevalence [7]. However, in Ethiopia, a relatively higher seroprevalence of 3.8% was reported [1]. These findings were comparable to the previous national estimates of 0.96–1.43% seroprevalence reported for the periods 2007–2010 [19], and 0.5–1.5% seroprevalence for the periods 2011–2012 [20]. Likewise, the result was also comparable to other national estimates of 1.2–2.5% seroprevalence reported earlier in a local study [21]. This study observation could be results of exclusion of family replacement donors (FRDs) from the study, and the milestones of beyond zero campaign initiatives in the country. In contrast, the findings were relatively low compared to 2.6% national seroprevalence reported in a similar local study [13], as well as 2.6% seroprevalence observed earlier from same study sites [11], (Fig. 1).
Fig. 1.
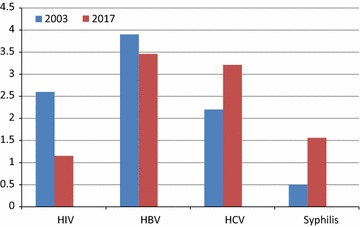
TTIs seroprevalence of 2003 [11] was compared with current study 2017 using test of proportions. HIV infections decreased significantly (p < 0.001), whereas HBV, HCV and syphilis remained similar
In this study, HBV seroprevalence of 3.46% detected among blood donors is indicative of intermediate endemicity and increased susceptibility to HBV infections. Similar results have been reported in Ethiopia showing seroprevalence of 4.7% [1], and in Egypt showing seroprevalence of 4.3% [22]. The findings were comparable to 3.9% seroprevalence reported earlier from the same study sites [11], but relatively higher compared to 1.97–2.77% national estimates for the periods 2006–2010 [19]. In contrast, these study findings were relatively lower compared to the national seroprevalence peak of 5.2% reported in the period 2011–2012 [20]. Thus, it is evident from the study that, despite of recent blood donation safety improvements, HBV infection remains a safety concern in blood donation.
The HCV seroprevalence of 3.21% detected among voluntary blood donors is indicative of intermediate endemicity and increased susceptibility to HCV infections. Similar studies reported relatively lower seroprevalence of 2.7% in Egypt [22], and 1.8% in Cameroon [23] compared to this study results. The variation observed in HCV seroprevalence may be attributed to difference in geographical settings and study methodology adopted by different authors. In this study, HCV seroprevalence was relatively higher compared 0.79–0.99% national estimates for the period 2007–2010 [19]. In addition, this study result was relatively higher compared 2.2% seroprevalence previously reported in the same study sites [11]. Thus; it is evident from the study that HCV infection remains a challenge in blood donation safety.
Syphilis seroprevalence of 1.56% detected among blood donors is indicative of low seroprevalence and decreased susceptibility to syphilis infection. Similar studies in Ethiopia reported similar results of 1.3% seroprevalence [1]. However, different results were reported in Ghana showing seroprevalence of 13.5% [24], and in Cameroon showing 9.1% seroprevalence [25]. Variation observed in seroprevalence may be attributed to difference in geographical setting. In this study, syphilis seroprevalence was similar to the national estimates of 0.15–0.28% [19], and 0.5% seroprevalence reported earlier in the same study area [11]. This observation may be attributed to milestones of beyond zero campaign initiatives.
In the analysis of demographic and risk factors, HBV infection was significantly associated with previous exposure to high risk sex. This may be explained by the low economic status initiating young adolescents to multiple sexual relationships, thus making them vulnerable to HBV and other co-infections. The findings corroborate local studies that found sex for cash payment associated with HIV and other co-infections [12]. In contrast, different results were observed in Egypt showing that TTIs was not associated with high risk sex [26]. In relation to age and gender, none was associated with any of the TTIs seroprevalence, although previous local studies had reported different results showing that adults’ aged ≥ 20 years were more likely to get infected by HIV compared to young adolescents aged 10–19 years [12]. Moreover, females were more likely to test HIV positive compared to males [13]. The variation observed may be related to the difference in study population. In relation to blood transfusion history, there was no association with any of the TTIs. However, a similar study in Egypt reported different results showing HCV seroprevalence of 72% among donors with transfusion history [26]. Meanwhile, a previous local study had reported HIV transfusion transmission risk of 2% among recipients [8]. On the contrary, this study did not find any association between TTIs seroprevalence and blood transfusion history. Recent implementation of high quality screening assays, coupled with stringent pre-donation screening, and enhanced haemovigilance may have influenced decrease in transfusion risk observed earlier. Meanwhile, previous exposure to illicit drug use was not associated with TTIs seroprevalence. However, different results have been reported in Egypt showing 47.5% of blood donors with drug use history testing HCV positive [26].
In conclusion, a substantial percentage of blood donors still harbor transfusion transmissible infections despite recent blood donation safety improvements with greater majority cases caused by HBV infection arising from previous exposure to high risk sex. Promoting safe sex education in learning institutions and early uptake of HBV self testing with subsequent vaccination would help reduce TTIs burden observed among blood donors.
Limitation
Detection of HBV was based on HBVsAg marker without considering IgM and IgG antibodies to the core protein ideal for a complete diagnosis of infection stages [27]; hence low seropositive estimate was possible. Additionally, questions on illicit drug use and sex life style are all associated with stigma with a possibility of influence on overall risk estimate.
Additional files
Additional file 1. Participants’ distribution.
Additional file 2. Pre-donation questionnaire.
Additional file 3. Excel dataset for regression analysis.
Authors’ contributions
CGO, LO and BG conceived and designed the study. CGO, LO and FH performed data analysis while VO, PO and CS critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank our study participants and the management and staffs of NBTS-Kenya for their participation in sample collection, experimental and data analysis. In addition, we appreciate Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) for institutional approval of the study. JOOTRH collaborates with RBTC-Kisumu on matters of research and treatment operations.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Baseline data were attached in additional files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval of the study protocol was obtained from Ethical Review Committee (ERC) of Maseno University Number MSU/DRPI/MUERC/00286/16, while institutional approval was obtained from Jaramogi Oginga Odinga Teaching and Referral Hospital Number ERC.IB/VOL.1/254. Informed written consent was obtained from all participants including parental or guardian consent where participant was less than 18 years.
Funding
This research was in part supported by Higher Education Loans Board Awards HELB/45/003/.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CMIA
chemiluminescent micro particle immunoassay
- CDC
Center for Disease Control and Prevention
- ELISA
enzyme linked immunosorbent assay
- FRD
family replacement donors
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIVAg/Ab
human immunodeficiency virus antigen/antibody
- JOOTRH
Jaramogi Oginga Odinga Teaching and Referral Hospital
- KNBTS
Kenya national blood transfusions services
- NACC
National AIDS Control Council
- RPR
rapid plasma regain
- RBTC
Regional Blood Transfusion Center
- TTIs
transfusion transmissible infections
- WHO
World Health Organization
- NASCOP
National AIDS/STI Control Program
- BECs
blood establishment computerized system
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3276-y) contains supplementary material, which is available to authorized users.
Contributor Information
Calleb George Onyango, Email: calleb.onyango@yahoo.com.
Lilian Ogonda, Email: lilianogonda@gmail.com.
Bernard Guyah, Email: guyah.bernard@yahoo.com.
Peter Okoth, Email: okoth.peter2@gmail.com.
Clement Shiluli, Email: clementshiluli@yahoo.com.
Felix Humwa, Email: fhmpooz@gmail.com.
Vallarie Opollo, Email: vopollo@kemricdc.org, Email: valarie.opollo@gmail.com.
References
- 1.Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, Sack U. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010;10:111. doi: 10.1186/1471-2334-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muriuki BM, Gicheru MM, Wachira D. Prevalence of hepatitis B and C viral co-infections among HIV-1 infected individuals in Nairobi, Kenya. BMC Res Notes. 2013;6:363. doi: 10.1186/1756-0500-6-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jehuda-Cohen. The HIV seronegative window period: diagnostic challenges and solution. ISBN: 978-953-307-719-2. Tech. 2011.
- 4.CDC. Progress towards prevention of transfusion transmissible hepatitis B and C virus in sub Saharan Africa; 2000–2011. 2014. http://www.cdc.gov/mmwr. Accessed 11 Dec 2017.
- 5.WHO. Global data base on blood safety: Summary report 2011. 2011. http://www.who.int/bloodsafety/global-database. Accessed 11 Dec 2017.
- 6.Murphy EL. Transfusion transmitted viral infections. 2007; 1–31. http://www.who.int/bloodsafety/transfusionservices/en/. Accessed 11 Dec 2017.
- 7.Kabinda JM, Bulabula AN, Donnen P, Fiase R, Van-den-Ende J, Sandag-Tull D, Duamaix-Wilmet M. Residual risk of transmission of HIV, hepatitis B and C by blood transfusion in Bukavu in Democratic Republic of Congo. Open J Epidemiol. 2014;4:157–163. doi: 10.4236/ojepi.2014.43021. [DOI] [Google Scholar]
- 8.Moore A, Herrera G, Nyamongo J, Lackritz E, Granade T, Nahlen B, Janssen R. Estimated risk of HIV transmission by blood transfusion in Kenya. Lancet. 2001;358(9282):657–660. doi: 10.1016/S0140-6736(01)05783-X. [DOI] [PubMed] [Google Scholar]
- 9.KNBTS. Progress on NBTS in availing safe blood to all Kenyans. In: A presentation at a breakfast meeting at crown plaza on 14th. August 2017, in Nairobi, Kenya, 2015. http://www.knbs.or.ke. Accessed 11 Dec 2017.
- 10.WHO-CDC. Interregional workshop on blood donor selection and donor counseling for priority countries in Africa and Eastern Mediterranean regions, 27th–30th June 2011, Nairobi, Kenya. 2011;8–10. www.who.int/bloodsafety/who-cdc. Accessed 11 Dec 2017.
- 11.NASCOP. Ministry of Health, Kenya, AIDS in Kenya: trends, interventions and Impacts. 7th ed. 2005. www.aidskenya.org. Accessed 11 Dec 2017.
- 12.NACC, N.A.C.C. Kenya HIV country profile. 2016; 2016: 39, 83, 188. http://www.nacc.or.ke, http://www.nascop.or.ke. Accessed 11 Dec 2017.
- 13.Kimani D, Mwangi J, Mwangi M, Bunnell R, Kellogg TA, Oluoch T, Group KS Blood donors in Kenya: a comparison of voluntary and family replacement donors based on a population-based survey. Vox Sang. 2011;100(2):212–218. doi: 10.1111/j.1423-0410.2010.01376.x. [DOI] [PubMed] [Google Scholar]
- 14.Mumo J, Vansover A, Jehuda-Cohen T. Detecting seronegative-early HIV infections among adult versus student Kenyan blood donors, by using Stimmunology. Exp Biol Med (Maywood) 2009;234(8):931–939. doi: 10.3181/0812-RM-372. [DOI] [PubMed] [Google Scholar]
- 15.Halim NK, Ajayi OI. Risk factors and seroprevalence of hepatitis C antibody in blood donors in Nigeria. East Afr Med J. 2000;77(8):410–412. [PubMed] [Google Scholar]
- 16.Nwankwo E, Momodu I, Umar I, Musa B, Adeleke S. Seroprevalence of major bloodborne infections among blood donors in Kano, Nigeria Turks. Med Sci. 2012;42(2):337–341. [Google Scholar]
- 17.Nagalo BM, Bisseye C, Sanou M, Kienou K, Nebié YK, Kiba A, Dahourou H, Ouattara S, Nikiema JB, Moret R, Zongo JD. Seroprevalence and incidences of transfusion transmissible infectious diseases among blood donors from regional blood transfusion centers in Burkina Faso, West Africa. Trop Med Int Health. 2012;17(2):247–253. doi: 10.1111/j.1365-3156.2011.02902.x. [DOI] [PubMed] [Google Scholar]
- 18.Karuru JW, Lule GN, Joshi M, Anzala O. Prevalence of HCV and HIV/HCV co-infection among volunteer blood donors and VCT clients. East Afr Med J. 2005;82(4):166–169. doi: 10.4314/eamj.v82i4.9275. [DOI] [PubMed] [Google Scholar]
- 19.KNBTS. Progress on NBTS in availing safe blood to all Kenyans. In: A presentation at a conference in Nairobi, Kenya. 2011. http://www.nbtskenya.or.ke. Accessed 11 Dec 2017.
- 20.KNBTS. Blood transfusion services in Kenya. In: A presentation in a conference in Naivasha March, 2012. 2012. kapkenya.org//NBTS.
- 21.Basavaraju SV, Mwangi J, Nyamongo J, Zeh C, Kimani D, Shiraishi RW, Marum LH. Reduced risk of transfusion-transmitted HIV in Kenya through centrally co-ordinated blood centres, stringent donor selection and effective p24 antigen-HIV antibody screening. Vox Sang. 2010;99(3):212–219. doi: 10.1111/j.1423-0410.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 22.El-Gilany AH, El-Fedawy S. Bloodborne infections among student voluntary blood donors in Mansoura University, Egypt. East Mediterr Health J. 2006;12(6):742–748. [PubMed] [Google Scholar]
- 23.Carole EEM, Francoise NS, Estelle GES, Madeline M, Leopold GL. HIV, HBV, HCV and syphilis infections among blood donors and transfusion related complications among recipients at the Laquintinie Hospital in Douala, Cameroon. BMC Hematol Ser. 2014; 10(1187): 2052-1838-14-5. 10.1186/2052-1839-14-5. [DOI] [PMC free article] [PubMed]
- 24.Ampofo W, Nii-Trebi N, Ansah J, Abe K, Naito H, Aidoo S, Ishikawa K. Prevalence of blood-borne infectious diseases in blood donors in Ghana. J Clin Microbiol. 2002;40(9):3523–3525. doi: 10.1128/JCM.40.9.3523-3525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mbaya DN, Takam D, Ndumbe PM. Serological findings amongst first time blood donors in Yaounde, Cameroon. Is safe donors’ a reality or a myth. Transfus Med. 2003;13(5):267–273. doi: 10.1046/j.1365-3148.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 26.Awadalla HI, Ragab MH, Nassar NA, Osman MA. Risk factors of hepatitis C infection among Egyptian blood donors. Cent Eur J Public Health. 2011;19(4):217–221. doi: 10.21101/cejph.a3628. [DOI] [PubMed] [Google Scholar]
- 27.Webale MK, Budambula V, Lihana R, Musumba FO, Muchama AK, Budambula NI, Were T. Hepatitis B virus sero-profiles and genotypes in HIV-1 infected and uninfected injection and non-injection drug users from coastal Kenya. BMC Infect Dis. 2015;15:299. doi: 10.1186/s12879-015-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Participants’ distribution.
Additional file 2. Pre-donation questionnaire.
Additional file 3. Excel dataset for regression analysis.
Data Availability Statement
Baseline data were attached in additional files.


