Abstract
Background
Mainly because of the diversity of clinical presentations, diagnostic delays in lymphoma can be excessive. The time spent in primary care before referral to the specialist may be relatively short compared with the interval between hospital appointment and diagnosis. Although studies have examined the diagnostic intervals and referral patterns of patients with lymphoma, the time to diagnosis of outpatient compared to inpatient settings and the costs incurred are unknown.
Methods
We performed a retrospective study at two academic hospitals to evaluate the time to diagnosis and associated costs of hospital-based outpatient diagnostic clinics or conventional hospitalization in four representative lymphoma subtypes. The frequency, clinical and prognostic features of each lymphoma subtype and the activities of the two settings were analyzed. The costs incurred during the evaluation were compared by microcosting analysis.
Results
A total of 1779 patients diagnosed between 2006 and 2016 with classical Hodgkin, large B-cell, follicular, and mature nodal peripheral T-cell lymphomas were identified. Clinically aggressive subtypes including large B-cell and peripheral T-cell lymphomas were more commonly diagnosed in inpatients than in outpatients (39.1 vs 31.2% and 18.9 vs 13.5%, respectively). For each lymphoma subtype, inpatients were older and more likely than outpatients to have systemic symptoms, worse performance status, more advanced Ann Arbor stages, and high-risk prognostic scores. The admission time for diagnosis (i.e. from admission to excisional biopsy) of inpatients was significantly shorter than the time to diagnosis of outpatients (12.3 [3.3] vs 16.2 [2.7] days; P < .001). Microcosting revealed a mean cost of €4039.56 (513.02) per inpatient and of €1408.48 (197.32) per outpatient, or a difference of €2631.08 per patient.
Conclusions
Although diagnosis of lymphoma was quicker with hospitalization, the outpatient approach seems to be cost-effective and not detrimental. Despite the considerable savings with the latter approach, there may be hospitalization-associated factors which may not be properly managed in an outpatient unit (e.g. aggressive lymphomas with severe symptoms) and the cost analysis did not account for this potentially added value. While outcomes were not analyzed in this study, the impact on patient outcome of an outpatient vs inpatient diagnostic setting may represent a challenging future research.
Keywords: Lymphoma, Outpatient, Inpatient, Time to diagnosis, Diagnosis, Length of stay, Excisional biopsy, Fine-needle aspiration cytology, Emergency departments, Primary care
Background
In the absence of pathological lumps, lymphomas may pose a difficult diagnostic challenge. Patients presenting with enlarged lymphadenopathy are straightforwardly diagnosed. However, others present with systemic symptoms such as long-lasting fever and progressive weight loss and a worsening performance status. And in the middle of the spectrum are patients who complain of nonspecific or subtle symptoms [1].
Studies investigating the mean duration of intervals on the illness paths of patients with lymphomas have shown that whereas the patient interval (i.e. from onset of symptoms to first medical contact) constitutes the longest individual interval, the primary care (PC) interval (i.e. time spent in PC before referral to the specialist) is highly variable depending not only on the PC physician experience but also on the broad range of presenting symptoms [1, 2]. Because symptoms are often seen in PC in association with other more common and less serious conditions, patients are referred to a wide range of specialists, with a few referred directly to the appropriate secondary specialist [3]. A case series study conducted in patients aged > 25 years with newly diagnosed lymphomas who were first seen in PC revealed that only 12% were directly referred to the hematologist and that this specialist reached a quicker diagnosis than any other specialist [3]. Indeed, diagnostic delays can be excessive, and studies have shown that the PC interval may be relatively short compared with the diagnostic interval (i.e. from first specialist appointment to actual diagnosis) [2]. The heterogeneity of presentations and symptoms severity reflects the heterogeneous assortment of lymphoma subtypes and their aggressive or more indolent behavior [1, 2, 4, 5].
In the 2000 recommendations of the United Kingdom (UK) to improve cancer survival, hematological malignancies were considered as a single entity [6]. When considering referral for specialist assessment, the updated 2015 referral guidelines of the National Institute for Health and Care Excellence (NICE) highlighted unexplained lymphadenopathy and/or splenomegaly as well as other symptoms and signs that may suggest Hodgkin and non-Hodgkin lymphoma and that deserve further investigation [7]. The updated guidelines included separate recommendations for adults and for children and young people (aged 16-24 years) to indicate that there are different referral pathways. However, the significance and ranking of each particular sign and symptom is not disclosed [7]. Although quick access lymph node diagnostic clinics recommended by NICE in 2003 to reduce the time to diagnosis in patients with suspected lymphoma [8] appeared a more useful, simplified referral pathway [9], the challenge of appropriate referral remains in those patients who do not present with lymphadenopathy.
Many of the above findings come from the UK. However, they may well be generalizable to other health care systems where the patient first sees a PC physician [10] such as the Spanish public health care system.
To reduce diagnostic delays of patients with potentially serious diseases, the Spanish health care system created in the mid-2000s the so-called quick diagnosis units (QDUs). These hospital based-outpatient facilities have proven to be cost-effective alternatives to conventional hospitalization for diagnostic workup of disorders such as highly suspected cancer, severe anemia, or fever of unknown origin [11–13].
Largely because of long-delayed investigations ordered by PC physicians, these patients were traditionally hospitalized to speed-up the diagnostic process. However, most of them were too well to justify admission as just waited for examinations without receiving actual therapy [14–16]. While many QDUs were implemented during the recent economic regression to reduce the huge expenses associated with hospitalization [17–19], admission for workup is one of the most common reasons for inappropriate hospitalizations in Spain and elsewhere [19–23].
In contrast to the more focused approach of specialists at units such as the UK one-stop diagnostic clinics [9, 24], the versatility of general internists for the diagnosis of conditions characterized by nonspecific symptoms such as unintentional weight loss, fatigue or unexplained fever explains why QDUs are predominantly run by these physicians [11–13, 25].
Although reported data on these units are limited, several advantages over hospitalization have been established: in addition to ensuring a time to diagnosis similar to the length of stay for the same evaluable conditions, they are known to decrease emergency department (ED) referrals from PC easing ED overcrowding, are associated with higher patient satisfaction scores, and are considerably cost-saving [11–13, 15, 16, 25–29]. Yet to be evaluated, patients’ physical performance should allow them to travel from home to hospital and back for visits and investigations.
Because an acceptable performance status argues in general against admission of subjects who are eventually diagnosed with lymphoma after referral to QDUs for investigation of typical or nontypical manifestations, these outpatient units appear a proper setting for their assessment. However, no study has examined the time to diagnosis and associated costs of an outpatient compared to an inpatient setting. The main purpose of this retrospective study was to investigate the time to diagnosis of a hospital-based outpatient or inpatient setting in four major subtypes of lymphomas and the costs incurred by both clinical settings in the diagnostic process. A further goal was to investigate the frequency, clinical, and prognostic features of each lymphoma subtype according to an outpatient or inpatient diagnosis.
Methods
Settings and study population
The QDU of the Hospital Clínic (QDU (1)), a public 855-bed tertiary university hospital in Barcelona (Spain) with a reference population of almost 550,000, the inpatient wards of the internal medicine department of this hospital, and the QDU of the Hospital of Bellvitge (QDU (2)), a public 750-bed tertiary university hospital near Barcelona with a reference population of almost 350,000, participated in the study. All patients had been referred to the three settings from the respective PCs and EDs between January 2006 and September 2016. The referral criteria of the two outpatient units are essentially the same [14, 15, 30]. For the purpose of this study, both outpatient units were combined, and the analyzed patients were considered a single outpatient cohort (herein referred to as ‘outpatients’). The study was approved by the Comitè d’ Ètica de la Investigació Clínica (Clinical Research Ethics Committee) of the Hospital Clínic and the Comitè d’ Ètica i Assajos Clínics (Ethics and Clinical Assays Committee) of the University Hospital of Bellvitge. The ethics committees waived the need for written informed consent because no intervention was involved and the retrospective analysis of clinical data.
To analyze a homogeneous and representative sample of major lymphoma subtypes, cases included were patients aged ≥ 18 years with classical Hodgkin lymphoma, large B-cell lymphoma, follicular lymphoma, and mature nodal peripheral T-cell lymphoma (comprising peripheral T-cell lymphoma, not otherwise specified [NOS], angioimmunoblastic T-cell lymphoma, and anaplastic large cell lymphoma, anaplastic lymphoma kinase [ALK]-negative). All cases were diagnosed according to the 2008 World Health Organization classification of lymphoid neoplasms [31] and codified according to the International Classification of Diseases for Oncology (3rd edition; ICD-O-3) [32].
Pathologists at the Hospital Clínic and Hospital of Bellvitge selected and reviewed histopathological data of consecutive cases with these diagnoses, and attending and resident physicians from the outpatient and inpatient units reviewed the medical records of all patients and entered the following data into an electronic database: 1) individual frequency of lymphoma subtypes; 2) general demographic and clinical information including clinical manifestations at presentation (lymphadenopathy, systemic, pain and chest symptoms, or other symptoms or signs), relevant laboratory data (white blood cell count [including absolute lymphocyte count], erythrocyte count, platelet count, hemoglobin level, serum liver enzymes and lactate dehydrogenase [LDH], serum albumin, erythrocyte sedimentation rate, and C-reactive protein), and imaging reports; and 3) specific lymphoma information including Ann Arbor stage, Eastern Cooperative Oncology Group (ECOG) performance score, B symptoms, extranodal disease, bulky disease (maximum tumor dimension ≥ 10 cm) for classical Hodgkin and large B-cell lymphomas, international prognostic score (IPS) for advanced-stage classical Hodgkin lymphoma [33], international prognostic index (IPI) score for large B-cell and nodal peripheral T-cell lymphomas [34], follicular lymphoma international prognostic index (FLIPI) score for follicular lymphoma [35], and histologic grading of follicular lymphoma. The medical records of patients without explicit registered information on ECOG performance, bulky disease, and IPS, IPI, and FLIPI scores were carefully assessed to determine these parameters when all individual required data were available. Otherwise, the actual number and percentage of cases relative to the total number of cases with complete data for each parameter was entered into the database.
Patients without a biopsy-proven diagnosis and those with human immunodeficiency virus infection, a prior diagnosis of another lymphoma, incomplete clinical or pathological information, lost to follow-up, or dead before initial staging were excluded.
Fine-needle aspiration cytology
Although all study patients underwent an excisional biopsy, we calculated in a separate analysis the number of fine-needle aspiration cytologies (FNACs) performed and the proportion of suspected or compatible diagnosis of lymphoma according to cytomorphology and/or flow cytometry studies. In particular, following a systematic approach [36], a fast FNAC was commonly performed in patients (principally outpatients) with easily accessible lumps, then followed by a mandatory excisional biopsy in those with a suspicious/compatible lymphoma diagnosis. While both FNAC and biopsy were ordered by physicians from the inpatient and outpatient settings, to save time, outpatients with a positive FNAC result were immediately referred to the hematology (for QDU (1)) and the oncology (for QDU (2)) outpatient clinics, without need to wait for the biopsy being performed at time of referral. In contrast, irrespective of FNAC, all inpatients were discharged once the biopsy had been performed and its results were available. For consistency, cytologists at the two hospitals reviewed cytologic data of patients with a positive FNAC result and compared the cytologic and histopathological findings on an individual basis.
Activities of the inpatient and outpatient settings
The activities of the inpatient and outpatient units during the diagnostic evaluation were reviewed and registered. These included intervals between referral and first outpatient appointments/inpatient admissions, intervals between first outpatient appointments/inpatient admissions and dates of FNACs and excisional biopsies, successive/first visit ratio and time to diagnosis in outpatients, length of stay in inpatients, and onward referrals upon discharge of outpatients and inpatients. Additionally, although disease staging was systematically performed by the hematologist or the oncologist, physicians from the inpatient and outpatient units ordered a positron emission tomography-computed tomography (PET-CT) scan when lymphoma was suspected or confirmed.
QDU time for diagnosis and admission time for diagnosis
As mentioned, outpatients with a positive FNAC diagnosis were discharged from the outpatient environment without an excisional biopsy still being done. To allow for a better meaning and equivalent measure of time to diagnosis between outpatients and inpatients, we defined QDU time for diagnosis as the time elapsed between the first outpatient visit and the excisional/diagnostic biopsy, and admission time for diagnosis (instead of length of stay) as the actual time elapsed between inpatient admission and the excisional biopsy. This was made to try to deal with the differences in patient- and lymphoma-related characteristics that may influence comparison between results. More specifically, because the duration of hospitalization is likely impacted by inpatient factors (i.e. inpatients may be older, sicker, and have presented with more symptoms and advanced stages of disease than outpatients), length of stay may not be a precise reflection of the time needed to evaluate and diagnose a patient with lymphoma. Indeed, inpatients may require a longer hospitalization both to expedite lymphoma diagnosis and for symptom management and, as such, they are more prone than outpatients to incur greater care-related costs.
Resource use data collection and cost analysis
Costs of outpatients and inpatients were analyzed and compared with the microcosting method, often considered as a paradigm of hospital service costs, since all relevant cost components are precisely determined [37–39]. The microcosting methodology used by us for other disorders has been described elsewhere [11–13, 27]. In brief, resource use for each patient evaluated was obtained. Resource use data collected included all laboratory tests, cytologies, biopsies, imaging studies, and any other diagnostic procedure performed. The excisional biopsy requested for outpatients who were discharged with an onward referral if the FNAC result was positive was also included in the cost analysis. Data were also gathered on pharmaceuticals and consumables, therapeutic procedures, adverse events, and consultations. Only treatments other than lymphoma-specific treatments (i.e. treatment of patient’s symptoms) were included in the analysis. Thus, lymphoma-specific therapies such as chemotherapy and biologic agents were started by the hematologist or the oncologist after their first patient assessment once inpatients and outpatients had been discharged and referred to them. Costs of all individual resource items of outpatients and inpatients were obtained from the institutional information systems of the Hospital Clínic and Hospital of Bellvitge (bottom-up microcosting). For outpatients, the cost of an average outpatient consultation was determined according to officially established Catalan Health Service fees. In addition, the cost of each type of diagnostic investigation corresponded to hospital tariffs for QDU (1) outpatients and Catalan Health Service fees for QDU (2) outpatients. For inpatients, the costs of diagnostic tests, which were based on the same costs used for QDU (1) outpatients, were also computed.
The microcosting analysis also incorporated fractions of all staff wages. Particularly, QDU (1) is integrated in the internal medicine department of the Hospital Clínic and its staff includes a full-time consultant internist, a senior internal medicine resident, a full-time nurse, a part-time nurse coordinator, and two part-time secretaries. The unit is open 5 h a day, 4 days a week. Furthermore, QDU (2) is also integrated in the internal medicine department of the Hospital of Bellvitge and its staff includes a part-time consultant internist and a part-time nurse. This unit is open 7 h a day, 2 days a week. Finally, staff in the inpatient setting (three wards) includes two full-time consultant internists, three residents, a full-time nurse coordinator, three teams of three full-time nurses and three teams of two full-time nursing assistants (8-h daily shifts), and a full-time secretary.
With an outpatient approach, patients (and any caregiver) may incur in more costs. Thus, indirect costs and costs borne by patients were not included in the cost analysis. Depreciation of fixed costs was included in the final analysis.
Mean number of visits, cost per visit and cost per patient from QDU (1) and (2), and mean admission time for diagnosis, cost per day of stay and cost per patient from the inpatient wards were computed and compared. Costs of referring agencies were excluded. All costs were adjusted for the year of collection (2006-2016) to reflect 2016 Euros (€). Final costs and cost differences are presented in 2016 Euros.
Statistical analysis
Categorical data were compared using the chi-square test or the Fisher’s exact test, as appropriate, and are expressed as absolute frequencies (%). Continuous variables with a normal distribution were compared using the t-test, and are expressed as means with standard deviations (SD). The nonparametric Mann-Whitney U test was used, when appropriate, to compare continuous variables with skewed distributions. The extent and nature of any missing data was also included in the analysis. Statistical significance was set at P < .05. Analyses were performed using the SPSS software (version 21.0) (SPSS, Chicago, USA).
Results
General characteristics of study population
Of 2047 eligible patients, 328 were excluded. Figure 1 shows the number of initially eligible patients from QDU (1), QDU (2), and inpatient wards and the causes for their exclusion. The main reasons for exclusion were incomplete clinical or pathological information and absence of a conclusive pathological diagnosis. After exclusion, 1719 patients comprising 1184 outpatients (688 from QDU (1) and 496 from QDU (2)) and 535 inpatients were available for the analysis (Fig. 1). The general characteristics of the whole population is shown in Table 1. Mean age was 63.4 (17.4) years and 55.4% were males. There were minor differences in patient characteristics between QDU (1) and QDU (2) outpatient cohorts (Tables 1 and 2). Inpatients were older and more likely to be males than outpatients. Nearly 65% of patients presented with lymphadenopathy, which was more frequent in outpatients than in inpatients. Just above 25% of patients had systemic symptoms and these were more common in inpatients (35.7%) than in outpatients (21.8%). The main referral source of inpatients was ED (i.e. emergency admissions) (68.4%) and it was PCs in outpatients (75.5%). Expectedly, inpatients waited less than 24 h to be admitted, whereas time to first visit in outpatients was significantly longer (0.6 [0.3] vs 1.7 [1.1) days; P < .001) (Table 1). The admission time for diagnosis of inpatients was significantly shorter than the QDU time for diagnosis of outpatients (12.3 [3.3] vs 16.2 [2.7] days; P < .001). An FNAC was performed in 935 (54.4%) patients (766 [64.7%] outpatients and 169 [31.6%] inpatients) yielding an overall suspicious/compatible lymphoma diagnosis in 65.3% with no false positive result in any patient after considering the biopsy findings. The rate of positive FNAC results was slightly, albeit nonsignificantly, higher in outpatients than in inpatients. Specifically, 65.7% of outpatients vs 63.9% of inpatients had a suspected/compatible lymphoma by FNAC (P = .102). The mean time to biopsy was substantially longer in outpatients (7.4 [1.8] days) than in inpatients (3.5 [1.1] days) (P < .001). After diagnosis, most patients were referred to outpatient specialist clinics and inpatients more often received direct palliative care after discharge than outpatients. There was ≤ 3% of missing data on the variables waiting time to first QDU visit, waiting time to admission, time to FNAC, and time to excisional biopsy (see footnote of Table 1).
Fig. 1.
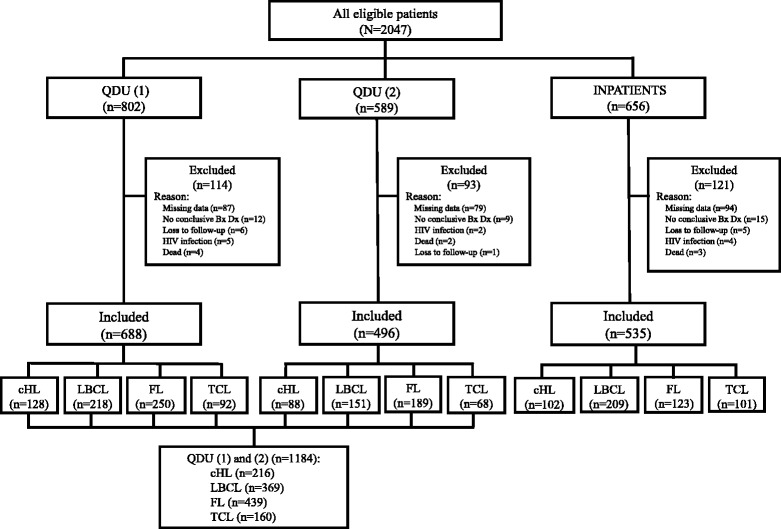
Flowchart of patients included in the study. Abbreviations: QDU (1) cohort of patients from the quick diagnosis unit of the Hospital Clínic, QDU (2) cohort of patients from the quick diagnosis unit of the Hospital of Bellvitge, QDU (1) and (2) combined cohort of patients from the quick diagnosis units of the Hospital Clínic and the Hospital of Bellvitge, Bx biopsy, Dx diagnosis, HIV human immunodeficiency virus, cHL classical Hodgkin lymphoma, LBCL large B-cell lymphoma, FL follicular lymphoma, TCL mature nodal peripheral T-cell lymphoma
Table 1.
General characteristics of study patientsa
| Characteristic | Total (N = 1719) | QDU (1) OPs (n = 688) | QDU (2) OPs (n = 496) | Total OPs 1 (n = 1184) | IPs 2 (n = 535) | P value 1 vs 2 |
|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 63.4 (17.4) | 62.7 (13.8) | 61.8 (11.5) | 62.3 (14.7) | 65.6 (12.2) | <.001 |
| Sex, n (%) | ||||||
| Females | 768 (44.6) | 318 (46.2) | 227 (45.8) | 545 (46.0) | 223 (41.7) | <.001 |
| Males | 951 (55.4) | 370 (53.8) | 269 (54.2) | 639 (54.0) | 312 (58.3) | <.001 |
| Clinical manifestations, n (%)b | ||||||
| Lymphadenopathy | 1112 (64.7) | 487 (70.8) | 358 (72.2) | 845 (71.4) | 267 (49.9) | <.001 |
| Systemic symptomsc | 449 (26.1) | 155 (22.5) | 103 (20.8) | 258 (21.8) | 191 (35.7) | <.001 |
| Pain symptomsd | 89 (5.2) | 26 (3.8) | 17 (3.4) | 43 (3.6) | 46 (8.6) | <.001 |
| Chest symptomse | 45 (2.6) | 14 (2.0) | 12 (2.4) | 26 (2.2) | 19 (3.6) | .128 |
| Other symptoms/signsf | 24 (1.4) | 6 (0.9) | 6 (1.2) | 12 (1.0) | 12 (2.2) | .139 |
| Referral sources, n (%) | ||||||
| Emergency department | 656 (38.2) | 182 (26.5) | 108 (21.8) | 290 (24.5) | 366 (68.4) | <.001 |
| Primary care | 1063 (61.8) | 506 (73.5) | 388 (78.2) | 894 (75.5) | 169 (31.6) | <.001 |
| Waiting time to first QDU visit/admission (days), mean (SD)g | 1.7 (0.8) | 1.8 (0.7) | 1.7 (1.1) | 0.6 (0.3) | <.001 | |
| Successive/first visit ratio | 2.29 | 2.12 | 2.22 | |||
| QDU time for diagnosis/admission time for diagnosis (days), mean (SD)h | 16.8 (2.5) | 15.4 (2.2) | 16.2 (2.7) | 12.3 (3.3) | <.001 | |
| Suspected/compatible lymphoma by FNAC, n (%)/total ni | 611 (65.3)/935 | 302 (67.3)/449 | 201 (63.4)/317 | 503 (65.7)/766 | 108 (63.9)/169 | .102 |
| Time to FNAC (days), mean (SD)j | 1.2 (0.9) | 1.5 (0.8) | 1.0 (0.5) | 1.3 (0.9) | 1.2 (0.6) | .183 |
| Time to excisional biopsy (days), mean (SD)k | 5.7 (1.7) | 7.5 (1.6) | 7.1 (1.4) | 7.4 (1.8) | 3.5 (1.1) | <.001 |
| Onward referrals, n (%) | ||||||
| Outpatient specialist clinics | 1622 (94.4) | 654 (95.1) | 476 (96.0) | 1130 (95.4) | 492 (92.0) | .046 |
| Primary care | 47 (2.7) | 21 (3.1) | 15 (3.0) | 36 (3.0) | 11 (2.1) | .169 |
| Palliative care | 50 (2.9) | 13 (1.9) | 5 (1.0) | 18 (1.5) | 32 (6.0) | <.001 |
Abbreviations: QDU (1) OPs cohort of outpatients from the quick diagnosis unit of the Hospital Clínic; QDU (2) OPs cohort of outpatients from the quick diagnosis unit of the Hospital of Bellvitge; Total OPs total number of outpatients from the quick diagnosis units of the Hospital Clínic and the Hospital of Bellvitge; IPs cohort of hospitalized patients of the Hospital Clínic; FNAC fine-needle aspiration cytology
aLymphoma subtypes are not included; bSingle or combined; cMainly include intense tiredness, abnormal sweating at night, unintentional weight loss, nausea, and anorexia; dMainly abdominal pain; eMainly include shortness of breath, cough, and sore throat; fInclude, among others, pruritus, bowel symptoms, unusually thirsty, fever, and incidental findings on imaging studies; gIntervals between referral and first QDU appointment and hospital admission, respectively; hIntervals between first QDU visit/hospital admission and excisional biopsies; in (%)/total n: actual number and percentage of cases relative to the total number of available cases; jInterval between ordering FNAC and the procedure being done; kInterval between ordering biopsy and the procedure being done Missing data: variables ‘waiting time to first QDU visit’ (total OPs = 19), ‘waiting time to admission’ (IPs = 10), ‘time to FNAC’ (total OPs = 24, IPs = 16), ‘time to excisional biopsy’ (total OPs = 6, IPs = 4)
Table 2.
Frequency of lymphoma subtypes in outpatient and inpatient cohorts
| Subtypea | Total (N = 1719) | QDU (1) OPs (n = 688) | QDU (2) OPs (n = 496) | Total OPs 1 (n = 1184) | IPs 2 (n = 535) | P value 1 vs 2 |
|---|---|---|---|---|---|---|
| Classical Hodgkin lymphoma | 318 (18.5) | 128 (18.6) | 88 (17.7) | 216 (18.2) | 102 (19.1) | .164 |
| Nodular sclerosis, NOSb | 235 (73.9) | 96 (75.0) | 66 (75.0) | 162 (75.0) | 73 (71.6) | .045 |
| Mixed cellularity, NOSc | 37 (11.6) | 16 (12.5) | 10 (11.4) | 26 (12.0) | 11 (10.8) | .135 |
| Lymphocyte richd | 13 (4.1) | 5 (3.9) | 3 (3.4) | 8 (3.7) | 5 (4.9) | .140 |
| Lymphocyte depletion, NOSe | 4 (1.3) | 1 (0.8) | 0 (0.0) | 1 (0.5) | 3 (2.9) | .078 |
| Classical Hodgkin lymphoma, NOSf | 29 (9.1) | 10 (7.8) | 9 (10.2) | 19 (8.8) | 10 (9.8) | .156 |
| Large B-cell lymphomag | 578 (33.6) | 218 (31.7) | 151 (30.4) | 369 (31.2) | 209 (39.1) | <.001 |
| Follicular lymphoma, NOSh | 562 (32.7) | 250 (36.3) | 189 (38.1) | 439 (37.1) | 123 (23.0) | <.001 |
| Mature nodal peripheral T-cell lymphomai | 261 (15.2) | 92 (13.4) | 68 (13.7) | 160 (13.5) | 101 (18.9) | <.001 |
| Peripheral T-cell lymphoma, NOSj | 147 (56.3) | 50 (54.3) | 37 (54.4) | 87 (54.4) | 60 (59.4) | <.001 |
| Angioimmunoblastic T-cell lymphomak | 77 (29.5) | 28 (30.4) | 21 (30.9) | 49 (30.6) | 28 (27.7) | .076 |
| Anaplastic large cell lymphoma, ALK-negativel | 37 (14.2) | 14 (15.2) | 10 (14.7) | 24 (15.0) | 13 (12.9) | .086 |
Abbreviations: NOS not otherwise specified; ALK anaplastic lymphoma kinase. For other abbreviations, see Table 1
aThe ICD-O-3 codes of the International Classification of Diseases for Oncology, 3rd edition [32], are shown for each lymphoma subtype; b9663/3; c9652/3; d9651/3; e9653/3; f9650/3; g9680/3, 9684/3, 9735/3, 9688/3, and 9679/3 (anaplastic large B-cell lymphoma, diffuse large B-cell lymphoma associated with chronic inflammation, primary diffuse large B-cell lymphoma of the CNS, primary cutaneous DLBCL leg type, and EBV positive diffuse large B-cell lymphoma of the elderly were excluded); h9690/3; i9702/3; j9702/3; k9705/3; l9702/3
Frequency of lymphoma subtypes in outpatient and inpatient settings
Table 2 shows the total number of lymphoma subtypes and their frequency in each cohort of patients. Overall, large B-cell lymphoma was the leading subtype of lymphoma (33.6%) and follicular lymphoma ensued (32.7%). Large B-cell lymphoma was more frequent in inpatients than in outpatients (39.1% vs 31.2%; P < .001). In contrast, follicular lymphoma was less common in inpatients than in outpatients (23% vs 37.1%; P < .001). Classical Hodgkin lymphoma made up 18.5% of lymphomas, without differences in its frequency between cohorts. Nodal peripheral T-cell lymphomas were diagnosed in 15.2% of patients, and were more prevalent in inpatients than in outpatients (18.9% vs 13.5%; P < .001). Peripheral T-cell lymphoma, NOS, was the main subtype of nodal peripheral T-cell lymphomas (56.3%) and it was more frequent in inpatients than in outpatients (59.4% vs 54.4%; P < .001). No differences were observed in the frequency of angioimmunoblastic T-cell lymphoma and anaplastic large cell lymphoma, ALK-negative, between cohorts (Table 2).
Characteristics of lymphoma subtypes in outpatient and inpatient settings
The characteristics of each subtype of lymphoma in inpatients and outpatients are shown in Tables 3 and 4. In all lymphoma subtypes, inpatients were significantly older than outpatients. Also in all subtypes, lymphadenopathy was less common in inpatients than in outpatients. Although systemic symptoms were more frequent in inpatients than in outpatients, differences did not reach significance in follicular lymphoma. Remarkably, systemic symptoms were the leading clinical manifestation in inpatients with nodal peripheral T-cell lymphoma (46.5%), surpassing lymphadenopathy (38.6%) (Table 4). Furthermore, pain symptoms were more common in inpatients than in outpatients with large B-cell, follicular, and nodal peripheral T-cell lymphomas. In all lymphoma subtypes, the QDU time for diagnosis of outpatients was significantly longer than the admission time for diagnosis of inpatients as it was the time to biopsy of the former compared to the latter (Tables 3 and 4). In addition, inpatients with all subtypes of lymphoma were more likely than outpatients to have a worse performance status, a III-IV Ann Arbor stage, and B symptoms. Whereas an increased LDH was more frequently observed in inpatients than in outpatients with large B-cell, follicular, and nodal peripheral T-cell lymphomas, bulky disease was more common in inpatients than in outpatients with classical Hodgkin and large B-cell lymphomas. Although extranodal disease was more likely in inpatients than in outpatients with all lymphoma subtypes, differences were only statistically significant in large B-cell and nodal peripheral T-cell lymphomas. Inpatients with all subtypes were also more likely than outpatients to have high-risk prognostic scores. Lastly, in all subtypes, there was a minimal rate (≤ 4.1%) of missing data on the variables time to FNAC, time to excisional biopsy, and B symptoms (see footnotes of Tables 3 and 4).
Table 3.
Characteristics of classical Hodgkin and large B-cell lymphomas in outpatient and inpatient cohorts
| Classical Hodgkin lymphoma (N = 318) | Large B-cell lymphoma (N = 578) | |||||
|---|---|---|---|---|---|---|
| Characteristic | OPs 1 (n = 216) | IPs 2 (n = 102) | P value 1 vs 2 | OPs 1 (n = 369) | IPs 2 (n = 209) | P value 1 vs 2 |
| Age (years), mean (SD) | 43.6 (15.8) | 51.7 (13.3) | <.001 | 65.1 (17.5) | 69.7 (15.5) | <.001 |
| < 45, n (%) | 113 (52.3) | 44 (43.1) | <.001 | |||
| ≥ 45, n (%) | 103 (47.7) | 58 (56.9) | <.001 | |||
| ≤ 60, n (%) | 121 (32.8) | 28 (13.4) | <.001 | |||
| > 60, n (%) | 248 (67.2) | 181 (86.6) | <.001 | |||
| Sex, n (%) | ||||||
| Females | 98 (45.4) | 42 (41.2) | .069 | 156 (42.3) | 84 (40.2) | .104 |
| Males | 118 (54.6) | 60 (58.8) | .074 | 213 (57.7) | 125 (59.8) | .091 |
| Clinical manifestations, n (%) | ||||||
| Lymphadenopathy | 152 (70.4) | 51 (50.0) | <.001 | 246 (66.7) | 95 (45.5) | <.001 |
| Systemic symptoms | 51 (23.6) | 39 (38.2) | <.001 | 101 (27.4) | 86 (41.1) | <.001 |
| Pain symptoms | 9 (4.2) | 8 (7.8) | .084 | 11 (3.0) | 16 (7.7) | .025 |
| Chest symptoms | 3 (1.4) | 2 (2.0) | .190 | 8 (2.2) | 8 (3.8) | .120 |
| Other symptoms/signs | 1 (0.0) | 2 (2.0) | .131 | 3 (0.8) | 4 (1.9) | .135 |
| Successive/first visit ratio | 2.10 | 2.33 | ||||
| QDU time for diagnosis /admission time for diagnosis (days), mean (SD) | 15.5 (1.7) | 11.3 (2.7) | .030 | 16.7 (2.2) | 13 (2.5) | .001 |
| Suspected/compatible lymphoma by FNAC, n (%)/total n | 72 (52.9) /136 | 21 (52.5)/40 | .203 | 156 (71.9)/217 | 35 (71.4)/49 | .155 |
| Time to FNAC (days), mean (SD) | 1.3 (0.6) | 1.1 (0.4) | .130 | 1.3 (0.7) | 1.2 (0.5) | .125 |
| Time to excisional biopsy (days), mean (SD) | 7.3 (1.3) | 3.3 (0.9) | <.001 | 7.1 (1.3) | 3.6 (0.9) | .003 |
| ECOG performance score > 1, n (%)/total n | 31 (15.6)/199 | 21 (21.2)/99 | .045 | 70 (20.8)/336 | 53 (27.5)/193 | <.001 |
| B symptoms, n (%)a | 68 (31.4) | 39 (38.2) | .002 | 107 (29.0) | 71 (34.0) | .021 |
| Serum LDH > UNL, n (%) | 30 (13.9) | 18 (17.6) | .082 | 184 (49.9) | 119 (56.9) | <.001 |
| Bulky disease, n (%)/total nb | 44 (22.4)/196 | 27 (28.7)/94 | .010 | 86 (24.9)/345 | 63 (31.5)/200 | .001 |
| Extranodal disease, n (%)c | 33 (15.3) | 20 (19.6) | .061 | |||
| > 1 extranodal site, n (%) | 99 (26.8) | 69 (33.0) | .009 | |||
| Ann Arbor stage, n (%) | ||||||
| I-II | 142 (65.7) | 60 (58.8) | .002 | 157 (42.5) | 71 (34.0) | <.001 |
| III-IV | 74 (34.3) | 42 (41.2) | .001 | 212 (57.5) | 138 (66.0) | <.001 |
| IPS score (advanced-stage diseasedd), n (%)/total n | ||||||
| Low risk (≤ 3) | 166 (84.7)/196 | 74 (78.7)/94 | .035 | |||
| High risk (≥ 4) | 30 (15.3)/196 | 20 (21.3)/94 | .042 | |||
| IPI score, n (%)/total n | ||||||
| Low risk (0-1) | 114 (33.9)/336 | 52 (26.9)/193 | <.001 | |||
| Intermediate risk (2-3) | 158 (47.0)/336 | 92 (47.7)/193 | .144 | |||
| High risk (4-5) | 64 (19.0)/336 | 49 (25.4)/193 | .004 | |||
Abbreviations: OPs total number of outpatients from the quick diagnosis units of the Hospital Clínic and the Hospital of Bellvitge; ECOG Eastern Cooperative Oncology Group; LDH lactate dehydrogenase; UNL upper normal limit; IPS international prognostic score; IPI international prognostic index. For other abbreviations, see Table 1
aRecurrent fever, night sweats, or > 10% weight loss; b ≥ 10 cm largest diameter; cInvolvement of extra lymphatic tissue; dAdvanced-stage disease was defined as stage III or IV disease or stage I or II with bulky disease or stage II disease with B symptoms [59]
For other definitions and explanations, see Table 1 Missing data: variables ‘time to FNAC’ (classical Hodgkin lymphoma: OPs = 5, IPs = 3; large B-cell lymphoma: OPs = 7, IPs = 5), ‘time to excisional biopsy’ (classical Hodgkin lymphoma: OPs = 0, IPs = 1; large B-cell lymphoma: OPs = 3, IPs = 1), ‘B symptoms’ (classical Hodgkin lymphoma: OPs = 2, IPs = 1; large B-cell lymphoma: OPs = 4, IPs = 3)
Table 4.
Characteristics of follicular and mature nodal peripheral T-cell lymphomas in outpatient and inpatient cohorts
| Follicular lymphoma (N = 562) | Nodal peripheral T-cell lymphoma (N = 261) | |||||
|---|---|---|---|---|---|---|
| Characteristic | OPs 1 (n = 439) | IP 2 (n = 123) | P value 1 vs 2 | OPs 1 (n = 160) | IP 2 (n = 101) | P value 1 vs 2 |
| Age (years), mean (SD) | 61.1 (18.4) | 65.6 (13.6) | <.001 | 62.1 (14.3) | 68.5 (12.4) | <.001 |
| ≤ 60, n (%) | 212 (48.3) | 40 (32.5) | <.001 | 75 (46.9) | 21 (20.8) | <.001 |
| > 60, n (%) | 227 (51.7) | 83 (67.5) | <.001 | 85 (53.1) | 80 (79.2) | <.001 |
| Sex, n (%) | ||||||
| Females | 224 (51.0) | 59 (48.0) | .071 | 67 (41.9) | 38 (37.6) | .066 |
| Males | 215 (49.0) | 64 (52.0) | .074 | 93 (58.1) | 63 (62.4) | .068 |
| Clinical manifestations, n (%) | ||||||
| Lymphadenopathy | 356 (81.1) | 82 (66.7) | <.001 | 91 (56.9) | 39 (38.6) | <.001 |
| Systemic symptoms | 52 (11.8) | 19 (15.4) | .058 | 54 (33.8) | 47 (46.5) | <.001 |
| Pain symptoms | 13 (3.0) | 9 (7.3) | .037 | 10 (6.3) | 13 (12.9) | <.001 |
| Chest symptoms | 12 (2.7) | 8 (6.5) | .054 | 3 (1.9) | 1 (1.0) | .211 |
| Other symptoms/signs | 6 (1.4) | 5 (4.1) | .086 | 2 (1.3) | 1 (1.0) | .235 |
| Successive/first visit ratio | 2.10 | 2.66 | ||||
| QDU time for diagnosis /admission time for diagnosis (days), mean (SD) | 15.8 (1.8) | 11.5 (2.3) | .002 | 18.3 (2.4) | 14.9 (3.0) | .007 |
| Suspected/compatible lymphoma by FNAC, n (%)/total n | 230 (68.5)/336 | 33 (70.2)/47 | .143 | 45 (58.4)/77 | 19 (57.6)/33 | .215 |
| Time to FNAC (days), mean (SD) | 1.4 (0.7) | 1.3 (0.5) | .120 | 1.1 (0.6) | 1.2 (0.4) | .184 |
| Time to excisional biopsy (days), mean (SD) | 7.6 (1.5) | 3.8 (1.0) | .006 | 7.2 (1.3) | 2.9 (0.8) | <.001 |
| ECOG performance score > 1, n (%)/total n | 24 (5.7)/418 | 12 (10.4)/115 | .031 | 48 (31.6)/152 | 37 (37.8)/98 | .020 |
| B symptoms, n (%) | 61 (13.9) | 24 (19.5) | .020 | 90 (56.3) | 64 (63.4) | <.001 |
| Serum LDH > UNL, n (%) | 92 (21.0) | 34 (27.6) | .003 | 96 (60.0) | 69 (68.3) | <.001 |
| Extranodal disease, n (%) | 38 (8.7) | 14 (11.4) | .093 | |||
| > 1 extranodal site, n (%) | 63 (39.4) | 52 (51.5) | <.001 | |||
| Histologic grading, n (%) | ||||||
| 1 | 123 (28.0) | 33 (26.8) | .157 | |||
| 2 | 172 (39.2) | 48 (39.0) | .208 | |||
| 3A | 114 (26.0) | 36 (29.3) | .063 | |||
| Unspecified | 30 (6.8) | 6 (4.9) | .135 | |||
| Ann Arbor stage, n (%) | ||||||
| I-II | 156 (35.5) | 38 (30.9) | .033 | 36 (22.5) | 14 (13.9) | <.001 |
| III-IV | 283 (64.5) | 85 (69.1) | .034 | 124 (77.5) | 87 (86.1) | <.001 |
| FLIPI score, n (%)/total n | ||||||
| Low risk (0-1) | 165 (37.6)/439 | 41 (33.3)/123 | .040 | |||
| Intermediate risk (2) | 153 (34.9)/439 | 43 (35.0)/123 | .214 | |||
| High risk (3-5) | 121 (27.6)/439 | 39 (31.7)/123 | .049 | |||
| IPI score, n (%)/total n | ||||||
| Low risk (0-1) | 30 (19.7)/152 | 13 (13.3)/98 | .008 | |||
| Intermediate risk (2-3) | 75 (49.3)/152 | 47 (48.0)/98 | .179 | |||
| High risk (4-5) | 47 (30.9)/152 | 38 (38.8)/98 | <.001 | |||
Abbreviations: FLIPI follicular lymphoma international prognostic index
For other abbreviations, definitions, and explanations, see Tables 1 and 3
Missing data: variables ‘time to FNAC’ (follicular lymphoma: OPs = 8, IPs = 5; nodal peripheral T-cell lymphoma: OPs = 4, IPs = 3), ‘time to excisional biopsy’ (follicular lymphoma: OPs = 2, IPs = 2; nodal peripheral T-cell lymphoma: OPs = 1, IPs = 0), ‘B symptoms’ (follicular lymphoma: OPs = 2, IPs = 0; nodal peripheral T-cell lymphoma: OPs = 3, IPs = 2)
Frequency and characteristics of lymphomas according to referral sources
Potential differences between lymphoma subtypes and their main characteristics between outpatients and inpatients were determined according to the referral sources (Table 5). Outpatients referred from ED were more commonly diagnosed with large B-cell and nodal peripheral T-cell lymphomas than those referred from PC. In contrast, classical Hodgkin and follicular lymphomas were more common among outpatients referred from PC. Moreover, outpatients referred from ED were more likely than those referred from PC to have an age > 60 years, a higher frequency of systemic and B symptoms, a worse performance status, and an increased LDH. Also, a III/IV Ann Arbor stage was slightly more common in outpatients referred from ED than from PC, yet without statistically significant differences. Regarding inpatients, those referred from ED were more often diagnosed with large B-cell and nodal peripheral T-cell lymphomas than those referred from PC. In contrast, classical Hodgkin and follicular lymphomas were a more common diagnosis in inpatients referred from PC. Inpatients referred from ED were also more likely than those referred from PC to have an increased LDH. Although the former had a higher frequency of systemic and B symptoms, a worse performance status, and a more advanced Ann Arbor stage than the latter, differences did not reach statistical significance (Table 5).
Table 5.
Frequency and main characteristics of lymphoma subtypes in outpatient and inpatient cohorts according to referral sources
| OPs (N = 1184) | IPs (N = 535) | |||||
|---|---|---|---|---|---|---|
| PC (n = 894) | ED (n = 290) | P value | PC (n = 169) | ED (n = 366) | P value | |
| Classical Hodgkin lymphoma, n (%) | 183 (20.5) | 33 (11.3) | <.001 | 41 (24.3) | 61 (16.7) | <.001 |
| Large B-cell lymphoma, n (%) | 242 (27.1) | 127 (43.8) | <.001 | 54 (32.0) | 155 (42.3) | <.001 |
| Follicular lymphoma, n (%) | 370 (41.4) | 69 (23.8) | <.001 | 50 (29.6) | 73 (19.9) | <.001 |
| Nodal peripheral T-cell lymphoma, n (%) | 106 (11.9) | 54 (18.6) | <.001 | 24 (14.2) | 77 (21.0) | <.001 |
| Age > 60 years, n (%) | 451 (50.4) | 160 (55.2) | <.001 | 123 (72.8) | 260 (71.0) | .108 |
| ECOG performance score > 1, n (%) | 120 (13.4) | 53 (18.3) | <.001 | 36 (21.3) | 87 (23.8) | .089 |
| B symptoms, n (%) | 244 (27.3) | 93 (32.1) | .001 | 61 (36.1) | 143 (39.1) | .052 |
| Systemic symptoms, n (%) | 186 (20.8) | 72 (24.8) | .012 | 57 (33.7) | 134 (36.6) | .060 |
| Pain symptoms, n (%) | 33 (3.7) | 10 (3.4) | .197 | 14 (8.3) | 32 (8.7) | .210 |
| Serum LDH > UNL, n (%) | 287 (32.1) | 115 (39.7) | <.001 | 68 (40.2) | 172 (47.0) | <.001 |
| Ann Arbor III/IV stage, n (%) | 520 (58.2) | 173 (59.7) | .103 | 108 (63.9) | 244 (66.7) | .066 |
| High risk IPI score (LBCL), n (%) | 42 (17.4) | 22 (17.3) | .230 | 13 (24.1) | 36 (23.2) | .167 |
| High risk IPI score (NPTCL), n (%) | 32 (30.2) | 15 (27.8) | .081 | 9 (37.5) | 29 (37.7) | .231 |
| High risk FLIPI score, n (%) | 101 (27.3) | 20 (29.0) | .098 | 16 (32.0) | 23 (31.5) | .195 |
| High risk IPS score, n (%) | 25 (13.7) | 5 (15.2) | .106 | 8 (19.5) | 12 (19.7) | .252 |
Results of cost analysis
Table 6 shows the mean costs per day of hospitalization for diagnosis, per outpatient visit, and per patient in inpatients and outpatients. Considering that the mean admission time for diagnosis of inpatients was 12.3 (3.3) days and that the mean number of visits of outpatients (corresponding to the mean QDU time for diagnosis) was 3.26 (1.2), the total cost per hospitalized patient was €4039.56 (513.02), with 69.5% being attributable to personnel salaries and 25.4% to diagnostic tests, and the total cost per outpatient was €1408.48 (197.32), with 50.6% being attributable to diagnostic tests, 29.5% to outpatient visits, and 18.6% to personnel salaries. According to the analysis, the total saving from hospitalization was €2631.08 per patient. There was some degree of missing data on the variables diagnostic tests, therapeutic procedures, pharmaceuticals and consumables, and consultations (see footnote of Table 6).
Table 6.
Mean costs (€) of outpatients (n = 1184) and inpatients (n = 535)
| Items | Inpatients | Outpatients | Cost per patient (€), mean (SD) | ||
|---|---|---|---|---|---|
| One-day stay | One visit | Inpatientsa | Outpatientsb | P value | |
| Staff salaryc | 228.17d | 80.52 | 2806.49 (174.00) | 262.50 (22.92) | <.001 |
| QDU (1) and (2) visitse | na | 127.24 | na | 414.80 (0.11) | |
| Diagnostic testsf | 83.48 | 218.77 | 1026.80 (76.00) | 713.19 (38.85) | <.001 |
| Therapeutic proceduresg | 1.67 | 0.54 | 20.54 (3.53) | 1.76 (0.23) | <.001 |
| Pharmaceuticals and consumables | 9.95 | 0.87 | 122.39 (20.44) | 2.84 (0.59) | <.001 |
| Consultationsh | 2.47 | 0.32 | 30.38 (9.57) | 1.04 (0.37) | <.001 |
| Adverse events | 0.50 | 0.09 | 6.15 (2.37) | 0.29 (0.10) | <.001 |
| Depreciation | 2.18 | 3.70 | 26.81 (2.44) | 12.06 (0.90) | <.001 |
| Total costs | 328.42 | 432.05 | 4039.56 (513.02) | 1408.48 (197.32) | <.001 |
Abbreviations: na not applicable. For other abbreviations, see Table 1
aMean (SD) overall admission time for diagnosis: 12.3 (3.3) days
bMean (SD) overall number of outpatients’ visits during the QDU time for diagnosis: 3.26 (1.2)
cSee Methods for details about salary of staff at inpatient wards
dSalary of staff at inpatient wards in charge of 12.5 patients (each ward has 25 beds)
eThe cost of an average outpatient consultation was based on officially established Catalan Health Service fees
fInclude costs of laboratory testing and any other investigation (e.g. imaging studies or cytologies and biopsies). The costs of each type of diagnostic tests were based on hospital tariffs in QDU (1) and established fees of the Catalan Health Service in QDU (2)
gInclude costs of procedures such as therapeutic paracentesis and thoracentesis performed to drain excessive amounts of ascites and pleural fluid, respectively
hInclude costs of consultations with professionals such as hospital specialists, dieticians, and social workers
Missing data: variables ‘diagnostic tests’ (outpatients = 2.2%, inpatients = 1.5%)¸ ‘therapeutic procedures’ (inpatients = 0.2%)¸ ‘pharmaceuticals and consumables’ (outpatients = 1.7%, inpatients = 1.6%), ‘consultations’ (outpatients = 9.4%, inpatients = 6.8%)
Discussion
This study revealed that, following referral to hospital, diagnosis of lymphoma is more rapidly accomplished by conventional hospitalization than by hospital-based ambulatory quick diagnostic clinics. Yet the outpatient approach appears to be cost-effective and not detrimental. While no previous study has reported the time to diagnosis and associated costs of an outpatient vs inpatient setting in patients with lymphoma, our study is the first to explicitly describe the clinical and prognostic features of major subtypes of lymphoma according to an outpatient or inpatient diagnosis.
The general features of each lymphoma subtype were consistent with well-known distinctive features. However, there were salient differences between the inpatient and outpatient cohorts. First, lymphoma subtypes in inpatients were more aggressive than in outpatients. Second, inpatients were significantly older and less likely to have lymphadenopathy but more likely in general to have systemic and pain symptoms, worse performance status, advanced Ann Arbor stages, B symptoms, increased LDH, bulky disease, extranodal disease, and high-risk prognostic scores than outpatients. It was of note that the admission time for diagnosis of inpatients was significantly shorter than the QDU time for diagnosis of outpatients. However, because the management of patients referred to outpatient clinics or admitted for investigation of clinical manifestations such as to those reported here and who have an eventual diagnosis of lymphoma can be different in other settings, a circumstance that may depend on various factors such as the type of hospital, the available resources, or the institution traditions, the applicability of the study findings outside Spain is limited, which is an intrinsic limitation. Similarly, although oncologists and hematopathologists - including those from the US - agree that histopathology is essential in lymphoma to make therapeutic decisions [31], excisional biopsy in the US is done much less frequently than in our study (i.e. it is usually performed only after FNAC and only if the diagnosis cannot be reached). Indeed, excisional biopsy is a requirement of the hematology (for QDU (1) outpatients) and the oncology (for QDU (2) outpatients) departments. Therefore, our findings about FNAC/biopsy diagnosis of lymphoma may not be generalizable either.
Although the role of FNAC in the diagnosis of lymphoma is highly controversial [40], several investigators support it as a first, time-saving route for patients with a clinical suspicion of malignancy [41–44], mainly to differentiate metastatic solid cancer from lymphoma lymphadenopathy. In these cases, however, the operator experience in obtaining adequate fine needle aspirates for cytomorphology, flow cytometry, and immunocytochemistry studies is essential [42].
Undeniably, evaluating lymphomas by means of FNAC is challenging. Limitations include sampling error, scarce material to effectively perform flow cytometry and further studies, a relatively high rate of false-negative results, and loss of architecture [9, 40, 42, 45–47]. Nevertheless, it can be a reliable procedure when samples are handled by skilled cytopathologists assisted by specialists in flow cytometry/immunocytochemistry [36, 42].
A former study in 372 consecutive patients referred to QDU (1) for evaluation of peripheral lymphadenopathy revealed malignancy in 120 (32.3%), with an initial fast FNAC being highly specific and sensitive for the diagnosis of metastatic disease. Also, while all patients with lymphoma were fully subtyped by biopsy, a suspicious/compatible diagnosis was initially reached by combining cytomorphology and flow cytometry results in over two-thirds of cases. Owing to insufficient material for flow cytometry or cytomorphology in several cases and the need to perform an excisional biopsy as the unique diagnostic approach, time to diagnosis was significantly longer in lymphoma than in metastatic lymphadenopathy [36]. These findings led us to conclude that a quick FNAC rather than a conventional biopsy can be safely recommended as the initial procedure in patients with suspicious malignant lumps, mostly to differentiate metastasis from lymphomas [36]. In fact, our QDU policy establishes that, if metastatic solid cancer is undisputable by FNAC, an immediate referral to the oncologist will be time-saving, preferably after ordering an imaging examination to locate the primary tumor site if not ostensible before referral. At the first oncologist appointment, they may still order further histological studies for molecular characterization, complete the staging, and decide the best treatment for the patient. Alternatively, if a clearly suspicious/compatible diagnosis of lymphoma is beyond any doubt by FNAC, an immediate referral to the hematologist will also be time-saving, ever ensuring that an excisional biopsy (and ideally a PET-CT scan) has been ordered and scheduled after personal communication with the relevant surgeon. At the first hematologist appointment, the biopsy report will hopefully be ready to read and review and the specialist will likely complete the staging, including a bone marrow biopsy if needed, and decide the best treatment.
Although depending on its subtype, biology and aggressiveness, a delay to diagnosis always constitutes a major concern in lymphomas [48]. The diagnostic interval in secondary care can exceed not only the PC interval but even outdo the treatment interval (i.e. from diagnosis to first treatment) [2]. As a matter of example, a distinct referral pathway for patients referred for lymph node biopsy is frequently lacking in hospital centers [3]. Thus, whereas lymphoma patients most commonly present with peripheral lymphadenopathy - otherwise the strongest predictor of non-Hodgkin and Hodgkin lymphoma in patients aged ≥40 years in PC [1, 10, 48] - service delivery for peripheral lymphadenopathy has been reported to be poorly rationalized [49, 50]. An interesting study conducted in a UK hospital in patients with peripheral lymphadenopathy referred for excisional biopsy revealed that waiting times to it were significantly different according to the referral method [51]. Specifically, patients referred from several sources (mainly the hematology department and general practice) waited a median of 51 days before biopsy when referral was made by post compared to 17 days for faxed referrals and just 4 days when referral was made by direct personal request. A surgical outpatient appointment for routine surgical assessment prior to biopsy was the norm for patients referred by letter, whereas those referred following personal request proceeded straight to biopsy. After this study, and with 43% of biopsies revealing malignancy, the authors’ hospital implemented a fast-track, direct-booking pathway to arrange day-case lymph node biopsies without prior discussion with a surgeon [51].
In our study, 68.4% of inpatients vs 24.5% of outpatients with lymphomas were diagnosed via emergency admission. Because inpatients have preferential access to investigations, PC physicians commonly refer patients with potentially serious conditions directly to the ED in the hope of gaining rapid, including invasive, diagnostic procedures [12, 14]. Disturbingly, some patients referred from PC to the ED are in turn referred from ED to QDU (1) both in this study (data not shown) and in others [11]. Referring patients with suspected malignancy, including lymphoma, to the ED to achieve a quicker access to investigations is not uncommon in countries other than Spain including, among others, the United States (US) [52, 53].
In the UK, during 2006-2013, approximately 27 and 17% of all patients with non-Hodgkin and Hodgkin lymphoma, respectively, were diagnosed after ED presentation [54, 55]. While the survival rates of these patients were significantly lower than those observed in all other routes to diagnosis, the proportion of ED presentations increased with increasing age. Thus, the survival differences were partly explained by the higher proportion of ED presentations among older individuals, as has also been observed in several major types of solid cancer [56]. Although outcomes and survival rates were not investigated in the current study, outpatients and inpatients diagnosed after ED presentation had more aggressive lymphomas, with outpatients referred from ED being older than those referred from PC.
Finally, despite the differences in the time to diagnosis, the remarkable cost differences of the diagnostic evaluation were of note and agreed with findings in studies comparing the costs incurred by QDUs vs admission for disorders such as severe anemia, fever of unknown origin, or lung cancer [12, 13, 25]. A recent cost-minimization study in 63 patients with lymphomas diagnosed at QDU (2) revealed a total cost per hospitalized patient of €5457.42 and of €976.01 per outpatient, meaning a total saving from hospitalization of €4481.41 per patient, or €282,328.83 for the overall sample [25]. Although economic evaluation studies of QDUs are scarce [11–13, 15, 25, 26], savings from hospitalization are overwhelmingly associated with health personnel wages when considering the fractions corresponding to the hours worked.
Strengths of our study included the large sample of patients (N = 1779), its duration (10 years), and a design that allowed to analyze representative samples of major lymphoma subtypes according to their aggressive or indolent clinical behavior. Nonetheless, our study should be interpreted in the context of its limitations such as those mentioned above. In addition, and despite the finding of significant savings from hospitalization, there may be multiple factors involved in inpatient admission which were not accounted for in the cost analysis. For instance, aggressive lymphomas are a complex disorder which often require admission for diagnosis due to lymphoma causing severe clinical manifestations other than those described here and that may not be adequately managed in an outpatient unit that is open for 5 h 4 days a week and markedly less staffed. In brief, diagnosis in the hospital may be worth the cost if there are other competing or complementary diagnoses that are managed or treated at the same time. Although detailed clinical, histopathological and cytological information of all inpatients and outpatients were carefully reviewed, certain relevant details might not have been completely captured and potential confounders were not measured - a limitation related to the retrospective design of the study. Furthermore, waiting times to treatment and outcomes were not analyzed, meaning potential differences between cohorts could not be analyzed either. Finally, even though QDUs seem more appropriate for countries with public health care systems, it should be remarked that inpatient admissions in the US - where the health care system is mainly owned by the private sector - also constitute a major component of health costs [57]. A systematic review of Spanish QDUs by US investigators concluded that “[in] our healthcare system with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays” [16]. Although some outpatient care is provided by US hospitals in their EDs and specialty clinics, hospitals primarily exist in this country to provide inpatient care. Moreover, ED physicians commonly hospitalize patients for workup [16, 58].
Conclusion
In summary, among 1779 patients diagnosed with four major subtypes of lymphomas during a 10-year period after referral to hospital-based outpatient diagnostic clinics or hospitalized, clinically aggressive subtypes including large B-cell and nodal peripheral T-cell lymphomas prevailed in admitted patients. For each lymphoma subtype, inpatients were older, had a higher frequency of systemic symptoms than outpatients, and were generally more likely to have a worse performance status, a more advanced Ann Arbor stage, and a higher frequency of B symptoms, increased LDH, bulky disease, extranodal disease, and high-risk prognostic scores. Regardless of the clinical setting, patients referred from EDs had more aggressive lymphoma subtypes than those referred from PCs. Of note, the time to histopathological diagnosis of patients hospitalized for workup was significantly shorter than that of patients evaluated at QDU. Lastly, although there were considerable cost differences between the two settings, there may be other issues associated with admission which may not be properly and safely managed or treated in an outpatient environment and the cost analysis did not account for this potentially added value.
Since outcomes of outpatients and inpatients were not investigated in our study, a challenge of a potential future research is to analyze the impact on outcome of an outpatient vs inpatient diagnostic setting in these patients.
Acknowledgements
The authors thank the pathologists and cytologists at the Hospital Clínic and Hospital of Bellvitge who reviewed the histopathological and cytologic data of study patients. The authors are also indebted to the information system and the department of economics of the Hospital Clínic for providing the costs of individual resource items and for their assistance in the microcosting analysis.
Funding
No funding was obtained for this study.
Availability of data and materials
All data generated and analyzed during this study is available upon request. To request the data, please contact Dr. Xavier Bosch at xavbosch@clinic.ub.es
Abbreviations
- ALK
Anaplastic lymphoma kinase
- ECOG
Eastern Cooperative Oncology Group
- ED
Emergency department
- FLIPI
Follicular lymphoma international prognostic index
- FNAC
Fine-needle aspiration cytology
- ICD-O-3
International Classification of Diseases for Oncology, 3rd edition
- IPI
International prognostic index
- IPS
International prognostic score
- LDH
Lactate dehydrogenase
- NICE
National Institute for Health and Care Excellence
- NOS
Not otherwise specified
- PC
Primary care
- PET-CT
Positron emission tomography–computed tomography
- QDU (1)
Quick diagnosis unit of the Hospital Clínic
- QDU (2)
Quick diagnosis unit of the Hospital of Bellvitge
- QDUs
Quick diagnosis units
- SD
Standard deviation
- UK
United Kingdom
- US
United States
Authors’ contributions
XB, CSA, and ALS designed the work, and analyzed and interpreted data. XB, CSA, OE, EM, JFV, and PM collected data. MGG and NG contributed to data collection and provided material and technical support. XB, CSA, and ALS drafted the paper. All the authors made significant contributions to the conception or design of the study, revised the manuscript for intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The study was approved by the Comitè d’ Ètica de la Investigació Clínica (Clinical Research Ethics Committee) of the Hospital Clínic and the Comitè d’ Ètica i Assajos Clínics (Ethics and Clinical Assays Committee) of the University Hospital of Bellvitge. The ethics committees waived the need for written informed consent because no intervention was involved and the retrospective analysis of clinical data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howell DA, Smith AG, Roman E. Help-seeking behaviour in patients with lymphoma. Eur J Cancer Care (Engl). 2008;17:394–403. doi: 10.1111/j.1365-2354.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 2.Howell DA, Smith AG, Roman E. Lymphoma: variations in time to diagnosis and treatment. Eur J Cancer Care (Engl). 2006;15:272–278. doi: 10.1111/j.1365-2354.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 3.Howell DA, Smith AG, Roman E. Referral pathways and diagnosis: UK government actions fail to recognize complexity of lymphoma. Eur J Cancer Care (Engl) 2007;16:529–532. doi: 10.1111/j.1365-2354.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 4.Fields PA, Wrench DJ. Assessing risk and improving survival in lymphoma. Br J Gen Pract. 2015;65:220–221. doi: 10.3399/bjgp15X684661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell DA, Smith AG, Jack A, Patmore R, Macleod U, Mironska E, et al. Time-to-diagnosis and symptoms of myeloma, lymphomas and leukaemias: a report from the Haematological malignancy research network. BMC Hematol. 2013;13:9. doi: 10.1186/2052-1839-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Referral Guidelines. Referral Guidelines for Suspected Cancer. Health Service Circular. National Health Service. Department of Health. British government. 2000. http://webarchive.nationalarchives.gov.uk/20120503185640/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4012253.pdf. Accessed 8 Mar 2018.
- 7.Suspected cancer: recognition and referral: Recommendations organised by site of cancer: Haematological cancers. National Institute for Health and Care Excellence. United Kingdom. 2015 https://www.nice.org.uk/guidance/ng12/chapter/1-Recommendations-organised-by-site-of-cancer#footnote_4 Accessed 3 Dec 2017.
- 8.Guidance on Cancer Services. Improving Outcomes in Haematological Cancers. The Manual. National Institute for Health and Care Excellence. United Kingdom. 2003.
- 9.Chau I, Kelleher MT, Cunningham D, Norman AR, Wotherspoon A, Trott P, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354–361. doi: 10.1038/sj.bjc.6600738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shephard EA, Neal RD, Rose PW, Walter FM, Hamilton WT. Quantifying the risk of non-Hodgkin lymphoma in symptomatic primary care patients aged ≥40 years: a large case-control study using electronic records. Br J Gen Pract. 2015;65:e281–e288. doi: 10.3399/bjgp15X684793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch X, Moreno P, Ríos M, Jordán A, López-Soto A. Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology. 2012;83:283–291. doi: 10.1159/000341658. [DOI] [PubMed] [Google Scholar]
- 12.Bosch X, Palacios F, Inclán-Iríbar G, Castañeda M, Jordán A, Moreno P, et al. Quick diagnosis units or conventional hospitalisation for the diagnostic evaluation of severe anaemia: a paradigm shift in public health systems? Eur J Intern Med. 2012;23:159–164. doi: 10.1016/j.ejim.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Brito-Zerón P, Nicolás-Ocejo D, Jordán A, Retamozo S, López-Soto A, Bosch X. Diagnosing unexplained fever: can quick diagnosis units replace inpatient hospitalization? Eur J Clin Investig. 2014;44:707–718. doi: 10.1111/eci.12287. [DOI] [PubMed] [Google Scholar]
- 14.Bosch X, Aibar J, Capell S, Coca A, López-Soto A. Quick diagnosis units: a potentially useful alternative to conventional hospitalisation. Med J Aust. 2009;191:496–498. doi: 10.5694/j.1326-5377.2009.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 15.Bosch X, Jordán A, López-Soto A. Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31:114–123. doi: 10.1016/j.ajem.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Sukhal S, Agarwal R, Das K. Quick diagnosis units - an effective alternative to hospitalization for diagnostic workup: a systematic review. J Hosp Med. 2014;9:54–59. doi: 10.1002/jhm.2129. [DOI] [PubMed] [Google Scholar]
- 17.Bosch X, Moreno P, López-Soto A. The painful effects of the financial crisis on Spanish health care. Int J Health Serv. 2014;44:25–51. doi: 10.2190/HS.44.1.c. [DOI] [PubMed] [Google Scholar]
- 18.Bosch X. Reforming Spanish health care: a matter of survival. Health Policy. 2015;119:107–110. doi: 10.1016/j.healthpol.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Pericás JM, Aibar J, Soler N, López-Soto A, Sanclemente-Ansó C, Bosch X. Should alternatives to conventional hospitalisation be promoted in an era of financial constraint? Eur J Clin Investig. 2013;43:602–615. doi: 10.1111/eci.12087. [DOI] [PubMed] [Google Scholar]
- 20.San Román Terán CM, Guil García M, Fernández Sepúlveda S, Lorca Gómez J. Inappropriate admissions and stays in internal medicine. Med Clin (Barc) 2002;118:157. doi: 10.1016/S0025-7753(02)72314-2. [DOI] [PubMed] [Google Scholar]
- 21.Alfonso Sánchez JL, Sentís Vilalta J, Blasco Perepérez S, Martínez Martínez I. Characteristics of avoidable hospitalization in Spain. Med Clin (Barc) 2004;122:653–658. doi: 10.1016/S0025-7753(04)74342-0. [DOI] [PubMed] [Google Scholar]
- 22.MS MD, Smith DH, Goddard M. Measuring appropriate use of acute beds. A systematic review of methods and results. Health Policy. 2000;53:157–184. doi: 10.1016/S0168-8510(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 23.Campbell J. Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;94:628–631. doi: 10.1177/014107680109401206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendall MJ, Toescu V, Wallace DM. QED: quick and early diagnosis. Lancet. 1996;348:528–529. doi: 10.1016/S0140-6736(96)03483-6. [DOI] [PubMed] [Google Scholar]
- 25.Sanclemente-Ansó C, Bosch X, Salazar A, Moreno R, Capdevila C, Rosón B, et al. Cost-minimization analysis favors outpatient quick diagnosis unit over hospitalization for the diagnosis of potentially serious diseases. Eur J Intern Med. 2016;30:11–17. doi: 10.1016/j.ejim.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Capell S, Comas P, Piella T, Rigau J, Pruna X, Martínez F, et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc) 2004;123:247–250. doi: 10.1016/S0025-7753(04)74478-4. [DOI] [PubMed] [Google Scholar]
- 27.Bosch X, Jordán A, Coca A, López-Soto A. Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7:41–47. doi: 10.1002/jhm.931. [DOI] [PubMed] [Google Scholar]
- 28.Bosch X, Escoda O, Nicolás D, Coloma E, Fernández S, Coca A, et al. Primary care referrals of patients with potentially serious diseases to the emergency department or a quick diagnosis unit: a cross-sectional retrospective study. BMC Fam Pract. 2014;15:75. doi: 10.1186/1471-2296-15-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanclemente-Ansó C, Salazar A, Bosch X, Capdevila C, Giménez-Requena A, Rosón-Hernández B, et al. Perception of quality of care of patients with potentially severe diseases evaluated at a distinct quick diagnostic delivery model: a cross-sectional study. BMC Health Serv Res. 2015;15:434. doi: 10.1186/s12913-015-1070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanclemente-Ansó C, Salazar A, Bosch X, Capdevila C, Vallano A, Català I, et al. A quick diagnosis unit as an alternative to conventional hospitalization in a tertiary public hospital: a descriptive study. Pol Arch Med Wewn. 2013;123:582–588. doi: 10.20452/pamw.1966. [DOI] [PubMed] [Google Scholar]
- 31.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3.1). World Health Organization. 2011. http://codes.iarc.fr/codegroup/2. Accessed 3 Dec 2017.
- 33.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International prognostic factors project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 34.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 35.Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 36.Bosch X, Coloma E, Donate C, Colomo L, Doti P, Jordán A, et al. Evaluation of unexplained peripheral lymphadenopathy and suspected malignancy using a distinct quick diagnostic delivery model: prospective study of 372 patients. Medicine (Baltimore) 2014;93:e95. doi: 10.1097/MD.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4. Oxford: Oxford University Press; 2015. [Google Scholar]
- 38.Finkler SA, Ward DM, Baker JJ. Essentials of cost accounting for health care organizations. 3. New York: Jones & Bartlett Learning; 2007. [Google Scholar]
- 39.Tan SS, Rutten FF, van Ineveld BM, Redekop WK, Hakkaart-van RL. Comparing methodologies for the cost estimation of hospital services. Eur J Health Econ. 2009;10:39–45. doi: 10.1007/s10198-008-0101-x. [DOI] [PubMed] [Google Scholar]
- 40.Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046–3052. doi: 10.1200/JCO.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 41.Gregory RK, Cunningham D, Fisher TA, Rhys-Evans P, Middleton GW, Bishop L, et al. Investigating lymphadenopathy--report on the first 12 months of the lymph node diagnostic clinic at the Royal Marsden Hospital. Postgrad Med J. 2000;76:566–568. doi: 10.1136/pmj.76.899.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metzgeroth G, Schneider S, Walz C, Reiter S, Hofmann WK, Marx A, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477–1484. doi: 10.1007/s00277-012-1476-4. [DOI] [PubMed] [Google Scholar]
- 43.Mathiot C, Decaudin D, Klijanienko J, Couturier J, Salomon A, Dumont J, et al. Fine-needle aspiration cytology combined with flow cytometry immunophenotyping is a rapid and accurate approach for the evaluation of suspicious superficial lymphoid lesions. Diagn Cytopathol. 2006;34:472–478. doi: 10.1002/dc.20487. [DOI] [PubMed] [Google Scholar]
- 44.Caraway NP. Strategies to diagnose lymphoproliferative disorders by fine-needle aspiration by using ancillary studies. Cancer. 2005;105:432–442. doi: 10.1002/cncr.21452. [DOI] [PubMed] [Google Scholar]
- 45.Katz RL. Modern approach to lymphoma diagnosis by fine-needle aspiration: restoring respect to a valuable procedure. Cancer. 2005;105:429–431. doi: 10.1002/cncr.21499. [DOI] [PubMed] [Google Scholar]
- 46.Monaco SE, Khalbuss WE, Pantanowitz L. Benign non-infectious causes of lymphadenopathy: a review of cytomorphology and differential diagnosis. Diagn Cytopathol. 2012;40:925–938. doi: 10.1002/dc.21767. [DOI] [PubMed] [Google Scholar]
- 47.Steel BL, Schwartz MR, Ramzy I. Fine needle aspiration biopsy in the diagnosis of lymphadenopathy in 1,103 patients. Role, limitations and analysis of diagnostic pitfalls. Acta Cytol. 1995;39:76–81. [PubMed] [Google Scholar]
- 48.Howell DA, Warburton F, Ramirez AJ, Roman E, Smith AG, Forbes LJ. Risk factors and time to symptomatic presentation in leukaemia, lymphoma and myeloma. Br J Cancer. 2015;113:1114–1120. doi: 10.1038/bjc.2015.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moor JW, Murray P, Inwood J, Gouldesbrough D, Bem C. Diagnostic biopsy of lymph nodes of the neck, axilla and groin: rhyme, reason or chance? Ann R Coll Surg Engl. 2008;90:221–225. doi: 10.1308/003588408X242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsikoudas A. Management pathways and the surgical diagnosis of tuberculous lymphadenitis: can they be improved? The Bradford experience. ORL J Otorhinolaryngol Relat Spec. 2003;65:261–265. doi: 10.1159/000075223. [DOI] [PubMed] [Google Scholar]
- 51.Pannick SA, Ingham Clark CL. Waiting time to lymph node biopsy is dependent on referral method: don't write, phone! Ann R Coll Surg Engl. 2009;91:673–676. doi: 10.1308/003588409X12486167521118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beatty S, Stevens W, Stevens G, Kolbe J, Cox B. Lung cancer patients in New Zealand initially present to secondary care through the emergency department rather than by referral to a respiratory specialist. N Z Med J. 2009;122:33–41. [PubMed] [Google Scholar]
- 53.Sikka V, Ornato JP. Cancer diagnosis and outcomes in Michigan EDs vs other settings. Am J Emerg Med. 2012;30:283–292. doi: 10.1016/j.ajem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 54.Routes to diagnosis 2015 update: non-Hodgkin lymphoma. National Cancer Intelligence Network Short Report. Public Health England’s National Cancer Intelligence Network. 2016.
- 55.Routes to diagnosis 2015 update: Hodgkin lymphoma. National Cancer Intelligence Network Short Report. Public Health England’s National Cancer Intelligence Network. 2016.
- 56.Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, et al. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Squires DA. Explaining high health care spending in the United States: an international comparison of supply, utilization, prices, and quality. Issue Brief (Commonw Fund) 2012;10:1–14. [PubMed] [Google Scholar]
- 58.Schuur JD, Venkatesh AK. The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367:391–393. doi: 10.1056/NEJMp1204431. [DOI] [PubMed] [Google Scholar]
- 59.Hapgood G, Zheng Y, Sehn LH, Villa D, Klasa R, Gerrie AS, et al. Evaluation of the risk of relapse in classical Hodgkin lymphoma at event-free survival time points and survival comparison with the general population in British Columbia. J Clin Oncol. 2016;34:2493–2500. doi: 10.1200/JCO.2015.65.4194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study is available upon request. To request the data, please contact Dr. Xavier Bosch at xavbosch@clinic.ub.es


