Abstract
Background
Countries in the Southeast Asia region have a high prevalence of soil-transmitted helminth, such as roundworm, whipworm, and hookworms [Ancylostoma duodenale, Necator americanus, Ancylostoma ceylanicum]. Recent molecular-based surveys have revealed that A. ceylanicum, a zoonotic hookworm, is likely the second most prevalent hookworm species infecting humans in that part of the world, while others have noted that this infection is an emerging public health risk not only for indigenous people but also for visitors from other countries.
Case presentation
We recently encountered four cases of A. ceylanicum infection in Japanese individuals who returned from Southeast Asia and Papua New Guinea. Case 1 was a 25-year-old male who stayed in a rainforest in Malaysia for 4 weeks, where he developed abdominal pain and diarrhea in the third week. Eleven adult worms (five males, six females) were expelled after treatment with pyrantel pamoate and identified as A. ceylanicum based on morphological characteristics and DNA sequences of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene. Case 2 was a 26-year-old male who spent 2 years as an overseas cooperation volunteer for agriculture in Papua New Guinea. He did not note any symptoms at that time, though eggs were detected in feces samples at a medical check-up examination after returning. Although collection of adult worms was unsuccessful, DNA analysis of the eggs for cox1 and the ribosomal internal transcribed spacer (ITS)-1 and ITS-2 genes demonstrated that they were A. ceylanicum. Case 3 was a 47-year-old male who spent 1 month in a rural village in Lao People’s Democratic Republic and began suffering from watery diarrhea from the third week. A total of nine adult worms (three males, six females) were collected by endoscopic procedures and following treatment with pyrantel pamoate. Morphological examination and molecular analyses of the cox1 gene showed that they were A. ceylanicum. Case 4 was a 27-year-old male who participated in group travel to India for 5 days. Three weeks after returning, he developed abdominal pain and diarrhea. Hookworm eggs were found in feces samples and developed into larvae in culture, which were identified as A. ceylanicum based on molecular analysis of the cox1 gene. Eosinophilia was observed in all of the cases prior to treatment.
Conclusions
A. ceylanicum should be recognized as an important etiologic pathogen of hookworm diseases in travelers to countries in the Southeast Asia and West Pacific Ocean regions.
Electronic supplementary material
The online version of this article (10.1186/s41182-018-0087-8) contains supplementary material, which is available to authorized users.
Keywords: Ancylostoma ceylanicum, Soil-transmitted helminth, Imported parasitosis, Southeast Asia, Papua New Guinea, Traveler’s diarrhea, Hookworm diseases
Background
Human hookworm infections are mainly attributed to Necator americanus and Ancylostoma duodenale [1, 2]. Ancylostoma ceylanicum can also cause a patent human infection. Although high prevalence rates of A. ceylanicum in domestic, stray, and community-raised dogs and cats have been reported in Asia, human infection has been largely ignored. Nevertheless, recent molecular-based surveys have revealed that A. ceylanicum is likely the second most prevalent hookworm species infecting humans in Asia, especially in the Southeastern Asia region [3–9]. Due to the potential for patent enteric infection by this parasite in humans through percutaneous penetration as well as the fecal-oral route, A. ceylanicum should be recognized as a public health risk, not only for indigenous communities but also for tourists visiting from other countries. We recently encountered four cases of A. ceylanicum infection in Japanese individuals, each of whom had recently visited Southeast Asia or Papua New Guinea (PNG).
Case presentation
Case 1
A 25-year-old male biologist from Japan visited a rainforest in Malaysia in 2009 to collect spiders for study. He developed abdominal pain and diarrhea during the third week. The symptoms did not improve, and he returned to Japan after the fourth week. Because of intermittent abdominal pain and watery diarrhea, the patient visited Kyoto Prefectural University of Medicine, where hookworm eggs were detected in a feces sample prepared by formalin-ethyl ether sedimentation (Fig. 1a) along with an increased number of peripheral eosinophils (3.0 × 103/μL), while neither pathogenic protozoa nor bacteria were identified in fecal samples or culture. Filariform larvae of hookworms were found in a Harada-Mori culture (Fig. 1b). Eleven adult worms, five males and six females, were expelled after treatment with a single dose (10 mg/kg) of pyrantel pamoate and identified as A. ceylanicum based on morphological characteristics [10, 11], such as one set of prominent outer and one set of much smaller inner teeth on a cutting plate in the mouthpart (Fig. 1c) and parallel mediolateral and posterior-lateral rays of the copulatory bursa in the males (Fig. 1d). Later, DNA was extracted from one of the adult worms and preserved in ethanol solution using a DNeasy Blood & Tissue Kit (QIAGEN) and subjected to a PCR assay specific for the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene. The primer sets used are shown in Additional file 1: Table S1. The cox1 region was analyzed using an ABI Prism 3100-Avant Genetic analyzer (Applied Biosystems) and shown to have the A. ceylanicum sequence (GenBank: accession no. LC271155.1).
Fig. 1.
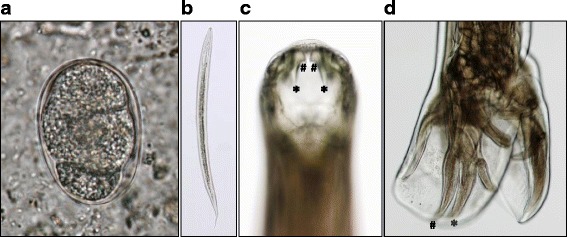
Case 1. a Representative four-cell stage egg obtained from feces sample (55.9 × 36.6 μm). b A single filariform larva (750 × 25.0 μm), one of many recovered in Harada-Mori culture of feces. c Anterior of an adult Ancylostoma ceylanicum, showing prominent sets of outer (asterisk) and small inner (number sign) teeth on a cutting plate in the buccal cavity. d Posterior of an adult male Ancylostoma ceylanicum, showing mediolateral (asterisk) and posterior-lateral (number sign) rays of the copulatory bursa of the running parallel
Case 2
A 26-year-old Japanese male spent 2 years as an overseas cooperation volunteer for agriculture in PNG and was examined as part of a medical check-up after returning to Japan in 2014. Although there were no health problems noted, such as dermatitis, fever, and respiratory or abdominal symptoms, during his stay in PNG, laboratory examinations following the return revealed hookworm eggs in feces in direct microscopy observations and increased peripheral eosinophils (1.6 × 103/μL). A Harada-Mori fecal culture failed to obtain fully developed filariform larvae of hookworm. Eggs were obtained by a floatation method with saturated sodium chloride solution, then DNA was extracted and subjected to PCR assays specific for the ribosomal internal transcribed spacer (ITS)-1 and ITS-2 genes and cox1 (Additional file 1: Table S1). Analyses with ITS-1 and ITS-2 (GenBank accession no. LC036567) and cox1 (GenBank accession no. LC036568) sequences identified the eggs as A. ceylanicum. The patient was treated with pyrantel pamoate, after which eggs became undetectable in feces and the eosinophil count was normalized. Collection of adult worms from feces was unsuccessful. Details of case 2 have been previously reported [12].
Case 3
A 47-year-old male was presented with abdominal pain and watery diarrhea in the fourth week of his stay in a rural village in Lao People’s Democratic Republic. He returned to Japan and visited a local clinic. Laboratory examination showed increased eosinophiles (43%) with normal leukocyte count (7600 cells/μL), but any pathogenic protozoa or bacteria organisms were not detected in fecal samples and culture. The symptoms were improved, but he suffered from intermittent watery diarrhea for a month and referred to Nara Prefectural General Medical Center. Hookworm eggs were found in a feces sample prepared using a formalin-ethyl ether sedimentation method, and filariform larvae of hookworm were successfully obtained in a Harada-Mori fecal culture. Capsule endoscopy findings led us to suspect small nematodes in the jejunum (Fig. 2a). Double-balloon enteroscopy confirmed the presence of worms, including one with its head hooked into the intestinal mucosa and sucking blood (Fig. 2b). A total of three worms (two females, one male) were removed, each of which was identified as Ancylostoma ceylanicum based on morphological and genetic examinations using a PCR assay specific for the cox1 gene followed by sequencing (GenBank accession no. LC271184.1). The patient was treated with pyrantel pamoate and six worms (four females, two males) were collected from feces. Figure 2c shows one each of the female and male adult worms. The patient had eaten local food, worn sandals on bare feet, and lived as a local native in a Laotian village for approximately 1 month in August 2015. Details of case 3 have been previously reported [13].
Fig. 2.
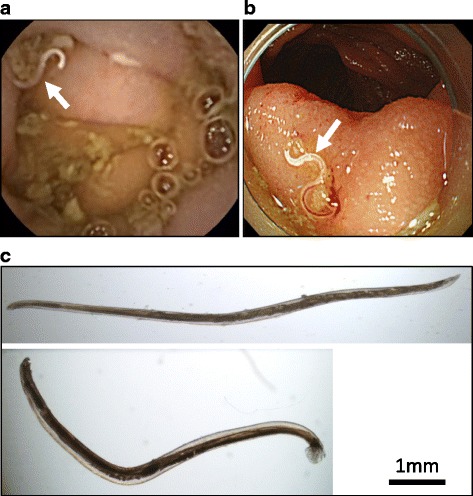
Case 3. a Small nematode (arrow) in jejunum shown by capsule endoscopy. b Worm sucking blood with head hooked into intestinal mucosa (arrow) shown by double-balloon enteroscopy. c One each of female (upper) and male (lower) adult worms obtained in feces after pyrantel pamoate treatment
Case 4
A 27-year-old male attended a 5-day tour to India, including visits to New Delhi, Varanasi, Agra, and Bodhgaya, in September 2016. Fourteen days after returning to Japan, he developed abdominal pain and diarrhea, and the symptoms continued for 2 weeks until initiation of antihelminthic drug treatment. No protozoa or pathogenic bacteria were detected in stool samples or cultures. A direct microcopy examination found hookworm eggs in feces along with increased peripheral eosinophils (7.1 × 103/μL). Larvae were obtained from a Harada-Mori fecal culture and identified as A. ceylanicum by an examination of the cox1 gene (GenBank accession no. LC271185.1). The patient was treated with albendazole (400 mg/day for 7 days), after which eggs were undetected in feces and consequent normalization of eosinophil count in peripheral blood was seen. Collection of adult worms from feces was not performed. Although the patient had been careful to avoid eating raw vegetables and drinking water without first boiling, he noted that he had lied down and exposed the skin of his upper body to the ground soil in Bodhgaya.
Patient information and clinical features of these four cases are summarized in Table 1. None showed a decreased hemoglobin level in laboratory findings.
Table 1.
Patient information and clinical and diagnostic features of four Japanese traveler cases of Ancylostoma ceylanicum infection
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age (years) sex (F・M) | 25・M | 26・M | 47・M | 26・M |
| Destination | Malaysia | PNG | Laos | India |
| Symptoms | Abdominal pain | Asymptomatic | Abdominal pain | Abdominal pain |
| Watery diarrhea | Watery diarrhea | Watery diarrhea | ||
| Hemoglobin (g/dL) | 14.5 | 15.1 | 13.5 | 16.1 |
| Eosinophil count (/μL) | 3000 | 1570 | 20,470c | 7050 |
| Eggs in feces | Detected | Detected | Detected | Detected |
| Larvae isolation (Harada-Mori culture) | Succeeded | Faileda | Succeeded | Succeeded |
| Endoscopy | Capsule | Not performed | Capsule | Not performed |
| DB enteroscopy | ||||
| Adult worms isolated (total, M/F) | 11 (5 M/6 F) | 0 | 9 (3 M/6 F) | 0 |
| Treatment | Pirantel pamoate | Pirantel pamoate | Pirantel pamoate | Albendazole |
| GenBank accession number (cox1) | LC271155.1 | LC036567.1b | LC271184.1 | LC271185.1 |
| LC036568 |
aOnly single larva was obtained. Unfortunately, it had failed to grow fully into a filariform larva
bLC036567.1 shows sequences for the gene of 18S rRNA, ITS1, 5.8S rRNA, ITS2, and 28S rRNAva
cThe value at the visit to Nara Prefectural General Medical center
Discussion
Although N. americanus and A. duodenale are generally considered to be the most common species related to human hookworm disease [1, 2], a recent molecular-based copro-diagnostic survey revealed that following N. americanus, A. ceylanicum is the second most common in human cases and responsible for between 6 and 23% of total patent hookworm infections in Asia [14]. A. ceylanicum is commonly found in dogs and cats in Asia, and high rates of infection with that species in domestic, stray, and community-raised dogs and cats have been reported in Malaysia [3], Laos [5], India [15, 16], Thailand [6, 7, 17], Cambodia [8], Vietnam [18], Indonesia [19], and southern China [20]. In addition, infections in dogs as well as humans have also been reported in the Solomon Islands [21, 22] and northern Australia [23–27]. Since A. ceylanicum causes patent human enteric infections, those living among stray or community-raised dogs and cats have an increased zoonotic risk. On the other hand, human prevalence seems to differ depending on other factors, such as socioeconomic status, general educational attainment in the region, and recognition of the importance of personal hygiene.
Travelers in addition to indigenous individuals are at risk for infection with A. ceylanicum. We examined four different Japanese individuals who became infected after visiting countries in the Southeast Asia and West Pacific Ocean regions. The patient in case 1 visited Malaysia, where 9.1% of the population is reported to be infected with hookworms, with A. ceylanicum responsible for 23.4% of those infections [3]. For academic study purposes, he remained for an extended period in a rainforest, where the moist soil is known to be suitable for development and growth of filariform larvae. In case 2, the patient spent 2 years as an agricultural volunteer in PNG, which has a wet equatorial tropical environment and high annual rainfall. Although N. americanus was previously considered to be the only species present in PNG [28], infection with A. ceylanicum was reported in two Australian soldiers returning home from service in PNG during World War II [22] (described as A. brasiliensis, synonymous with A. ceylanicum at that time). Furthermore, nine service personnel from the Netherlands who had returned from West New Guinea in the early 1960s [29] and an Australian soldier who had stayed in the Solomon Islands, to the east of PNG in Oceania, in 2003 [21], were found to be infected. In the Solomon Islands, a high prevalence of A. ceylanicum infections in humans was recently noted and those comprised 18.2% of all hookworm disease cases reported in the region [22], suggesting a similarly high prevalence of A. ceylanicum in PNG. The patient in case 3 lived a reclusive country life in a Laotian rural village, where stray and domesticated dogs and cats were often seen, and ate local food and wore sandals with bare feet. The prevalence of hookworm disease is reported to be 46.3% in Laos, and A. ceylanicum infection has been shown in 3 (17.6%) of 17 molecularly characterized samples from local individuals with hookworm disease [5]. As for case 4, the patient was mainly in northeast India, which is classified as a humid temperate climate zone. He reported having lied down with the skin of his upper body exposed to the ground soil in Bodhgaya. In a recent study of distinct geographical and climatic locations in India [30], A. ceylanicum was identified in dogs as either a single or mixed infection with A. caninum in districts located in a humid temperate or tropical climate zone, though that species was not seen in semiarid or arid mountainous zones. Another study reported a high prevalence of A. ceylanicum in dogs in Assam, located in northeast India in a humid temperate zone (originally erroneously reported as A. braziliense [15] and later corrected [16]).
Clinical signs in infected individuals have been reported to range from asymptomatic to heavy symptoms, including anemia, lethargy, and excessive hunger [29]. Three of the present four cases noted major symptoms of abdominal pain and diarrhea, while the other had no symptoms. In observations following experimental infection of human volunteers, abdominal symptoms such as discomfort, pain, flatulence, and diarrhea appeared with eosinophilia within approximately 3 to 4 weeks after infection, regardless of oral or percutaneous route [31, 32]. In other reports that investigated natural infections in travelers [33–35], affected patients had such symptoms as nausea, abdominal pain, watery diarrhea, and bloody diarrhea with eosinophilia. Abdominal pain and mild to severe diarrhea seem to be frequent during the early period following infection. One of the affected patients in a previous report, a French traveler to Myanmar, recalled the development of pruritic erythematous macules on the buttocks after sitting in a public park in Rangoon while wearing short pants [35]. Although the route of infection was not determined in the present cases, a percutaneous route seems to be more likely as compared to an oral route.
A case of neuroretinitis caused by larval migration has also been recently reported [36] and molecular analysis using DNA isolated from intraoperative rinsing fluid determined the larva to be A. ceylanicum. In that case, a molecular-based technique was the only method available to reach a diagnosis, since the worm was completely destroyed during surgical removal. In the present case 2, eggs from feces were the only evidence obtained. Although morphological examinations of adult and larval worms are important, a molecular approach is of great help for making a diagnosis of hookworm disease including the infecting worm species, especially in cases that lack suitable specimens for morphological diagnosis.
The present cases were only found because of the local network of physicians engaged in parasitic disease treatment and study in Nara, Kyoto, and Osaka prefectures; thus, additional individuals in Japan may have been infected with A. ceylanicum while visiting countries in the Southeast Asia and West Pacific Ocean regions. We consider that increased attention is needed in regard to A. ceylanicum infection in patients who are presented with abdominal pain and diarrhea, or even more subtle abdominal symptoms, after traveling to Southeast Asia and West Pacific Ocean countries, as well as northeastern Australia.
Conclusions
A. ceylanicum should be recognized as an important etiologic pathogen of hookworm diseases that may infect travelers who visit countries in the Southeast Asia and West Pacific Ocean regions.
Additional file
Table S1. List of gene-specific primers used for PCR. (PDF 60 kb)
Acknowledgements
We sincerely thank Dr. Hideo Hasegawa, former Professor in the Department of Biology, Faculty of Medicine, Oita University, Japan, who performed the molecular study in case 2.
Funding
No funding received.
Availability of data and materials
All the data and information are included in the manuscript.
Abbreviations
- cox1
Mitochondrial cytochrome c oxidase subunit 1
- ITS
Ribosomal internal transcribed spacer
- PDR
Lao People’s Democratic Republic
- PNG
Papua New Guinea
Authors’ contributions
MYo contributed to the collection of cases’ information, surveyed literatures, and prepared the manuscript with YO, NH, and FNU. MYa, NAr, NAk, and TY contributed to the management of case 1. DK, TN, and EK contributed to the management of case 3. YK and KS contributed to the management of case 3. RM, YM, and YO contributed to the management of case 4. KK and KM contributed to the critical discussion. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This is not a research project involving people or animals, only a case report. Therefore, the requirement in our local setting is to get consent for publication.
Consent for publication
Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s41182-018-0087-8) contains supplementary material, which is available to authorized users.
Contributor Information
Masahide Yoshikawa, Phone: 81-744-29-8847, Email: myoshika@naramed-u.ac.jp.
Yukiteru Ouji, Email: oujix@naramed-u.ac.jp.
Nobuyasu Hirai, Email: nobuyasuhirai1986@gmail.com.
Fukumi Nakamura-Uchiyama, Email: fukumi_nakamura@tokyo-hmt.jp.
Minoru Yamada, Email: minori@koto.kpu-m.ac.jp.
Naoki Arizono, Email: arizonon@koto.kpu-m.ac.jp.
Naoaki Akamatsu, Email: naoaki-akamatsu@nifty.com.
Takaharu Yoh, Email: kyou@koto.kpu-m.ac.jp.
Daisuke Kaya, Email: kklogist@hotmail.com.
Toshiya Nakatani, Email: toshiyan@sa2.so-net.ne.jp.
Eiryo Kikuchi, Email: eiryokikuchi@mac.com.
Yuichi Katanami, Email: yuichi.katanami@gmail.com.
Kimitoshi Satoh, Email: sezec6@gmail.com.
Ryosuke Maki, Email: realtreeinnmu@gmail.com.
Yusuke Miyazato, Email: yusuke.m4.ss@gmail.com.
Yuichiro Oba, Email: oobau@mbj.ocn.ne.jp.
Kei Kasahara, Email: kassan@naramed-u.ac.jp.
Keiichi Mikasa, Email: mikasak1@naramed-u.ac.jp.
References
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Ngui R, Lim YA, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis. 2012;6:e1522. doi: 10.1371/journal.pntd.0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngui R, Ching LS, Kai TT, Roslan MA, Lim YA. Molecular identification of human hookworm infections in economically disadvantaged communities in Peninsular Malaysia. Am J Trop Med Hyg. 2012;86:837–842. doi: 10.4269/ajtmh.2012.11-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, Sripa B, Blacksell SD, Fenwick S, Thompson RC. Soil-transmitted helminthiasis in Laos: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg. 2012;86:624–634. doi: 10.4269/ajtmh.2012.11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RC. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008;155:67–73. doi: 10.1016/j.vetpar.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naaglor T, Taamasri P, Leelayoova S. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 2011;84:594–598. doi: 10.4269/ajtmh.2011.10-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inpankaew T, Schar F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P, Traub RJ. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis. 2014;20:976–982. doi: 10.3201/eid2006.131770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pa Pa Aung W, Htoon TT, Tin HH, Sanpool O, Jongthawin J, Sadaow L, Phosuk I, Ropai R, Intapan PM, Maleewong W. First molecular identifications of Necator americanus and Ancylostoma ceylanicum infecting rural communities in lower Myanmar. Am J Trop Med Hyg. 2017;96:214–216. doi: 10.4269/ajtmh.16-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida Y, Okamoto K, Chiu JK. Ancylostoma ceylanicum infection in dogs, cats, and man in Taiwan. Am J Trop Med Hyg. 1968;17:378–381. doi: 10.4269/ajtmh.1968.17.378. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y: Differential diagnosis of some species of hookworms in Southeast Asia and the Far East. Diagnostic methods for important helminthiasis and amoebiasis in Southeast Asia and the Far East, The Central Coordinating Board, Seameo-Troped Project, Bangkok 41975:78–86.
- 12.Katanami Y, Nakamra-Uchiyama F, Sato M. A Japanese patient with Ancylostoma ceylanicum infection returning from Papua New Guinea diagnosed by molecular identification of the parasite eggs. J J A Inf D. 2017;91:759–763. [Google Scholar]
- 13.Kaya D, Yoshikawa M, Nakatani T, Tomo-Oka F, Fujimoto Y, Ishida K, Fujinaga Y, Aihara Y, Nagamatsu S, Matsuo E, et al. Ancylostoma ceylanicum hookworm infection in Japanese traveler who presented chronic diarrhea after return from Lao People’s Democratic Republic. Parasitol Int. 2016;65:737–740. doi: 10.1016/j.parint.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43:1009–1015. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RC. Application of a species-specific PCR-RFLP to identify Ancylostoma eggs directly from canine faeces. Vet Parasitol. 2004;123:245–255. doi: 10.1016/j.vetpar.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Traub RJ, Hobbs RP, Adams PJ, Behnke JM, Harris PD, Thompson RC. A case of mistaken identity—reappraisal of the species of canid and felid hookworms (Ancylostoma) present in Australia and India. Parasitology. 2007;134:113–119. doi: 10.1017/S0031182006001211. [DOI] [PubMed] [Google Scholar]
- 17.Setasuban P, Vajrasthira S, Muennoo C. Prevalence and zoonotic potential of Ancylostoma ceylanicum in cats in Thailand. Southeast Asian J Trop Med Public Health. 1976;7:534–539. [PubMed] [Google Scholar]
- 18.Ng-Nguyen D, Hii SF, Nguyen VA, Van Nguyen T, Van Nguyen D, Traub RJ. Re-evaluation of the species of hookworms infecting dogs in Central Vietnam. Parasit Vectors. 2015;8:401. doi: 10.1186/s13071-015-1015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margono SS, Koesharjono C, Kosin E. Hookworm in dogs and cats in the area of Jakarta. Trop Geogr Med. 1979;31:257–261. [PubMed] [Google Scholar]
- 20.Hu W, Yu XG, Wu S, Tan LP, Song MR, Abdulahi AY, Wang Z, Jiang B, Li GQ. Levels of Ancylostoma infections and phylogenetic analysis of cox 1 gene of A. ceylanicum in stray cat faecal samples from Guangzhou, China. J Helminthol. 2016;90:392–397. doi: 10.1017/S0022149X15000413. [DOI] [PubMed] [Google Scholar]
- 21.Speare R, Bradbury RS, Croese J. A case of Ancylostoma ceylanicum infection occurring in an Australian soldier returned from Solomon Islands. Korean J Parasitol. 2016;54:533–536. doi: 10.3347/kjp.2016.54.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradbury RS, Hii SF, Harrington H, Speare R, Traub R. Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerg Infect Dis. 2017;23:252–257. doi: 10.3201/eid2302.160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer CS, Traub RJ, Robertson ID, Hobbs RP, Elliot A, While L, Rees R, Thompson RC. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet Parasitol. 2007;145:304–313. doi: 10.1016/j.vetpar.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Smout FA, Skerratt LF, Butler JRA, Johnson CN, Congdon BC, Thompson RCA. The hookworm Ancylostoma ceylanicum: an emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health. 2017;3:66–69. doi: 10.1016/j.onehlt.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon C, Kurscheid J, Jones M. Soil-transmitted helminths in tropical Australia and Asia. Trop Med Infect Dis. 2017;2:56. doi: 10.3390/tropicalmed2040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koehler AV, Bradbury RS, Stevens MA, Haydon SR, Jex AR, Gasser RB. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning-coupled approach. Electrophoresis. 2013;34:1720–1728. doi: 10.1002/elps.201300100. [DOI] [PubMed] [Google Scholar]
- 27.Bradbury R, Traub RJ. Hookworm infection in Oceania. Neglected Tropical Diseases-Oceania. Switzerland: Springer International Publishing; 2016. p. 33–68. doi:10.1007/978-3-319-43148-2.
- 28.Kline K, McCarthy JS, Pearson M, Loukas A, Hotez PJ. Neglected tropical diseases of Oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2013;7:e1755. doi: 10.1371/journal.pntd.0001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anten JF, Zuidema PJ. Hookworm infection in Dutch servicemen returning from West New Guinea. Trop Geogr Med. 1964;16:216–224. [PubMed] [Google Scholar]
- 30.Traub RJ, Pednekar RP, Cuttell L, Porter RB, Abd Megat Rani PA, Gatne ML. The prevalence and distribution of gastrointestinal parasites of stray and refuge dogs in four locations in India. Vet Parasitol. 2014;205:233–238. doi: 10.1016/j.vetpar.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Carroll SM, Grove DI. Experimental infection of humans with Ancylostoma ceylanicum: clinical, parasitological, haematological and immunological findings. Trop Geogr Med. 1986;38:38–45. [PubMed] [Google Scholar]
- 32.Yoshida Y, Okamoto K, Chiu JK. Experimental infection of man with Ancylostoma ceylanicum Looss, 1911. Chin J Microbiol. 1971;4:157–167. [Google Scholar]
- 33.Chung CS, Lin CK, Su KE, Liu CY, Lin CC, Liang CC, Lee TH. Diagnosis of Ancylostoma ceylanicum infestation by single-balloon enteroscopy (with video) Gastrointest Endosc. 2012;76:671–672. doi: 10.1016/j.gie.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Hsu YC, Lin JT. Images in clinical medicine. Intestinal infestation with Ancylostoma ceylanicum. N Engl J Med. 2012;366:e20. doi: 10.1056/NEJMicm1101717. [DOI] [PubMed] [Google Scholar]
- 35.Brunet J, Lemoine JP, Lefebvre N, Denis J, Pfaff AW, Abou-Bacar A, Traub RJ, Pesson B, Candolfi E. Bloody diarrhea associated with hookworm infection in traveler returning to France from Myanmar. Emerg Infect Dis. 2015;21:1878–1879. doi: 10.3201/eid2110.150695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poppert S, Heideking M, Agostini H, Fritzenwanker M, Wuppenhorst N, Muntau B, Henneke P, Kern W, Krucken J, Junker B, Hufnagel M. Diffuse unilateral subacute neuroretinitis caused by Ancylostoma hookworm. Emerg Infect Dis. 2017;23:343–344. doi: 10.3201/eid2302.142064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of gene-specific primers used for PCR. (PDF 60 kb)
Data Availability Statement
All the data and information are included in the manuscript.


