Abstract
Background
Emotional disorders, which include both anxiety and depressive disorders, are the most prevalent psychological disorders according to recent epidemiological studies. Consequently, public costs associated with their treatment have become a matter of concern for public health systems, which face long waiting lists. Because of their high prevalence in the population, finding an effective treatment for emotional disorders has become a key goal of today’s clinical psychology. The Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders might serve the aforementioned purpose, as it can be applied to a variety of disorders simultaneously and it can be easily performed in a group format.
Methods
The study is a multicenter, randomized, non-inferiority controlled clinical trial. Participants will be 220 individuals with emotional disorders, who are randomized to either a treatment as usual (individual cognitive behavioral therapy) or to a Unified Protocol condition in group format. Depression, anxiety, and diagnostic criteria are the primary outcome measures. Secondary measures include the assessment of positive and negative affect, anxiety control, personality traits, overall adjustment, and quality of life. An analysis of treatment satisfaction is also conducted. Assessment points include baseline, post-treatment, and three follow-ups at 3, 6, and 12 months. To control for missing data and possible biases, intention-to-treat and per-protocol analyses will be performed.
Discussion
This is the first randomized, controlled clinical trial to test the effectiveness of a transdiagnostic intervention in a group format for the treatment of emotional disorders in public settings in Spain. Results obtained from this study may have important clinical, social, and economic implications for public mental health settings in Spain.
Trial registration
Retrospectively registered at https://clinicaltrials.gov/. Trial NCT03064477 (March 10, 2017). The trial is active and recruitment is ongoing. Recruitment is expected to finish by January 2020.
Keywords: Unified protocol, Transdiagnostic, Emotional disorders, Group therapy, Public mental health, Multicenter, Randomized, Controlled trial
Background
Emotional disorders (EDs; i.e., depressive and anxiety disorders) have become the most prevalent psychiatric disorders globally [1]. The 12-month prevalence of anxiety and depressive disorders affect 14% and 7.8% (6.9% by major depression) of the population, respectively [2], and comordibity may be as high as 50% [3]. Lifetime prevalence rates in primary care settings in Spain reveal that mood and anxiety disorders, as defined in the DSM-IV-TR) [4], are the most prevalent psychiatric problems as well, with 35.8% and 25.6%, respectively [5].
As a result of their high prevalence in the population, EDs have become a global health problem due to their associated costs. For instance, a study conducted in 36 countries estimated that the annual treatment cost of depressive disorders and anxiety problems amounted to $91 billion and $56 billion, respectively [6]. It has been calculated that more than 12 billion days of productivity loss are attributable to depression and anxiety disorders every year, which results in $925 billion in productivity losses. In Spain, when direct and indirect costs are included, mood and anxiety disorders (again, according to DMS-IV-TR categories), are estimated to cost approximately $10.76 billion and $10.36 billion, respectively [7]. The high prevalence and important burden of EDs indicate that there is an urgent need to enhance the effectiveness of treatments for EDs in public mental health systems.
The Unified Protocol (UP) for the Treatment of Emotional Disorders [8, 9] is a recently developed form of cognitive behavioral therapy (CBT).The UP can be applied to a variety of disorders simultaneously and it can be easily performed in a group format. The UP was created on the basis of the identification of common psychopathological vulnerability factors in EDs [1, 10].In the UP, traditional CBT techniques (i.e., cognitive restructuring) are combined with more novel psychological skills (i.e., increasing awareness) to treat emotion regulation deficits, which are argued to be the underlying common factors in all EDs [11]. The UP is a structured, manual-based treatment [8, 9] which facilitates group delivery when patients present different EDs [11]. This, together with the fact that the UP can be applied simultaneously to individuals with different EDs, might help reduce existent waiting lists and current costs of individual treatment.
So far, studies exploring the effectiveness of the UP in a group format have led to promising findings [12–15].Overall, results suggest that the UP obtains medium-to-large effect sizes on numerous outcomes, including depression, anxiety, positive and negative affect, quality of life, overall adjustment, and avoidance of negative sensations, for both anxiety and mood disorders [12–14]. These studies have also revealed that between half and two thirds of patients ceased to meet diagnostic criteria after the treatment and one investigation, conducted by our team, revealed that changes remained stable 12 months after treatment completion [13]. Despite the previous results are encouraging, conclusions should be interpreted with caution as sample sizes have been small (11 participants in two studies and 47 patients in one investigation). Methodologically-sound, randomized, controlled trials are needed in order to replicate the aforementioned findings and to elucidate whether the UP in group format is indeed an effective treatment option for EDs in public settings.
The present non-inferiority, randomized, controlled trial will compare the efficacy of the UP in group format against traditional individual CBT treatment in a sample of patients with EDs. Ultimately, our goal is to explore whether the UP in group format can be an effective psychological intervention for EDs in the Spanish National Health System, that is, one that generates long-lasting changes in symptoms. To ensure the generalizability of results, our goal is tested in various public mental health centers in Spain.
Methods/design
The current study is a non-inferiority, multicenter, randomized, controlled clinical trial with two treatment groups: UP in group format and traditional CBT in individual format (treatment as usual, TAU). A conservative, non-inferiority design was selected because, to the best of our knowledge, this is the first randomized controlled trial to compare the UP in a group format against individual CBT in public mental health settings.
In the present investigation, all consecutive patients with EDs attending any of the collaborating centers (see “Sample and recruitment” section) are asked to participate. Once inclusion criteria are met (see “Inclusion and exclusion criteria” section), each patient is randomly assigned to one of the two experimental groups: TAU or UP (see “Procedure” section). The study includes 5 assessment points (baseline, post-treatment, and three follow-ups at 3, 6, and 12 months after treatment completion). The flow chart of study design is shown in Fig. 1. Also, a schedule of enrolment, interventions, and assessments is reported following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Fig. 2). Note that, despite treatment occurs at different frequencies between conditions (approximately once a month in the TAU condition and weekly in the UP group), assessment points are the same for both groups.
Fig. 1.
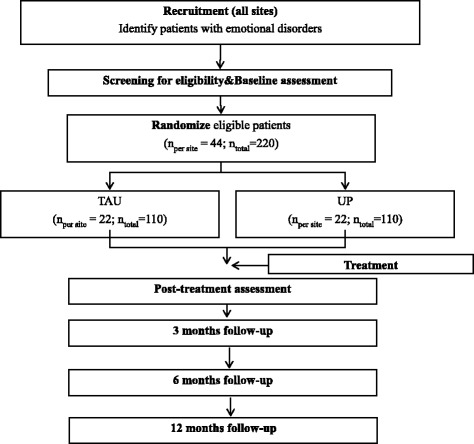
Study flow chart
Fig. 2.
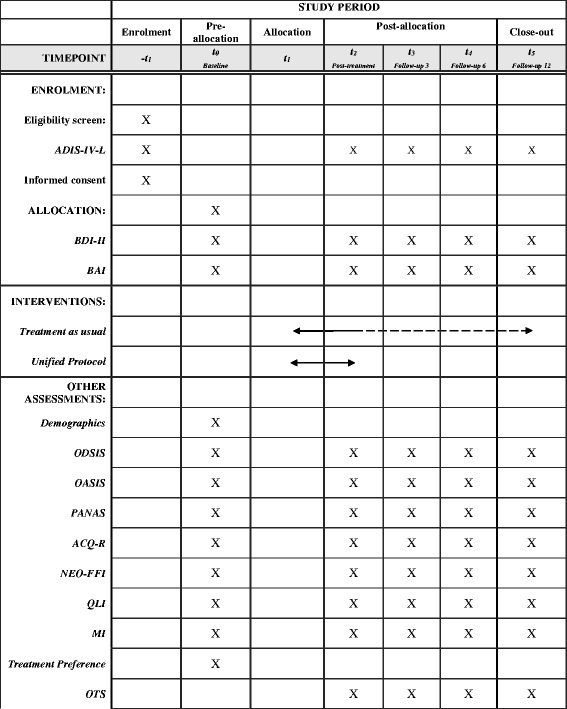
Study schedule of enrolment, interventions, and assessments
Sample and recruitment
The trial started the recruitment in September 2014 and it is still active. The study is conducted in five different Public Mental Health Centers and Primary Care Centers in Spain, namely, the Elda Clinical General Hospital, the Hospital Clínico Universitario de Valencia, the Dr. Peset University Hospital, the PCC IIA Milagrosa of Pamplona, and the Hospital Comarcal de Vinaròs. Participants are individuals over 18 years of age seeking psychological assistance in the Spanish Public Health System. Sample size was set to 220 participants to ensure enough statistical power when testing our hypotheses (see “Sample size” section).
Patients are referred to the study by licensed psychologists, psychiatrists, and clinical psychology residents working at the collaborating centers, who also assess for current DSM diagnoses (See “measures” section) and the remaining eligibility criteria (see “Inclusion and exclusion criteria” section). Individuals with comorbid diagnosis of several EDs are also enrolled in the study. The existence of such comorbidities might be important for assessment scores. However, in the present study we did not stratify considering comorbidities because this could significantly increase the complexity of the design (i.e., some patients might have more than one comorbid diagnosis). Due to the difficulties derived from conducting an RCT in different public mental health settings, we cannot guarantee a 100% of independent evaluations. We expect between 60 to 75% of independent evaluations. The rest of evaluations will probably be made by recruiters who are also the therapists in one of the conditions. However, this information is collected and considered in the interpretation of results.
Patients meeting eligibility criteria are asked to participate and are provided with an information sheet, information on data confidentiality, and an informed consent form. After participation acceptance, patients complete the baseline assessment protocol in their correspondent health center (see “Measures” section). Next, participants are informed of the condition they have been randomly assigned to, that is, TAU or UP.
Enrollment period and waiting time are comparable across participants (2–3 weeks). When the time from assessment, diagnosis, and randomization to the onset of the intervention takes more than 2 weeks we establish a new period of assessment. For ethical reasons, if a patient feels uncomfortable with the group format at any time during the study, he/she is allowed to join the TAU condition. This information is collected and considered in the analyses.
Sample size
To calculate the sample size required, the non-inferiority margin (i.e., tolerable amount of effect difference between TAU and the UP) has to be set. This was done according to the US Food and Drug Administration (FAD) [16] guidelines. First, the effect of the active control (i.e., CBT) compared with placebo needs to be established, based on previous research. Meta-analyses indicate medium-to-large effect sizes (between .4 and .8) of individual CBT on EDs [17–19]. Then, an amount of minimum preserved effect needs to be set. According to FDA guidelines, a preserved effect of a 50% of the effect of the active control (individual CBT) is recommended, so this was set to .3 in the present study. Because, to the best of our knowledge, no meta-analysis has reported effect sizes for each outcome included in the study, the margin has been defined according to the pooled estimate of overall effectiveness of CBT.
With regards to treatment satisfaction, a 35% non-inferiority margin was established. This is based on a meta-analysis of computer CBT, in which between 70% and 100% of depressed patients were satisfied with treatment [20]. To the best of our knowledge, there is no meta-analysis of treatment satisfaction with individual, face-to-face CBT and its study is rare. However, similar to group UP, computer CBT has been argued to be an alternative to traditional CBT [20], especially when resources are limited, so the aforementioned satisfaction rates of computer CBT might be relevant in the present study.
We obtained a sample size of 95 participants for each group with 80% power, an alpha level of .05 (one-sided), a standard deviation of .83 in the CBT group compared with placebo, and a non-inferiority limit of .30 [21, 22]. Considering a drop-out of 15%, we decided to recruit 110 subjects in each group. When registering the study, a sample size of 200 individuals was calculated. However, this was increased by 20 patients after considering non-inferiority sample size calculations.
Procedure
Randomization has been performed by a researcher unrelated to the study using a computer-generated sequence (Randomizer). In the program, the researcher generated 1 set of numbers with 220 numbers, which had a 1-to-2 range. Randomization is stratified according to the severity of the primary measures of depression (BDI-II) [23, 24] and anxiety (BAI) [25, 26], using the recommended cut-off in manuals. In the BDI-II, these are 0–13 for normal, 14–19 for mild, 20–28 for moderate, and for 29–63: severe depressive symptoms [24]. As for anxiety symptoms (BAI), cut-off are 0–7 for normal, 10–18 for mild, 16–25 for moderate, and 26–63 for severe [26]. Stratification is made to ensure a comparable proportion of severely depressed and anxious individuals in each group. For each subgroup (i.e., severe or less severe depression and/or anxiety), participants are randomly assigned to the UP or to TAU. In the UP condition, there are between 8 and 10 participants and 2 clinicians (therapist and co-therapist) per group. In the TAU condition, participants receive individual sessions with one therapist.
Eligibility criteria
Inclusion and exclusion criteria were set following the recommendations made by the authors of the UP [27] (Table 1).
Table 1.
Eligibility criteria
| Inclusion criteria | |
| 1 | Anxiety disorder, mood disorder, or adjustment disorder is the main diagnosis (most interfering and severe)a |
| 2 | The patient is over 18 years of age |
| 3 | The patient is fluent in the language in which therapy is performed (Spanish in the present study) |
| 4 | The patient is able to attend to the evaluation and treatment sessions and signs the informed consent form |
| 5 | Patients taking pharmacological treatment for their emotional disorder are asked to maintain the same dosages and medications for at least 3 months prior to enrolling in the study and during the whole treatmentb |
| Exclusion criteria | |
| 1 | The patient presents a severe condition that would require to be prioritized for treatment, so that an interaction between both interventions cannot be ruled out. These include a severe mental disorder (bipolar disorder, schizophrenia, or an organic mental disorder),suicide risk at the time of assessment, or substance use in the last three months (excluding cannabis, coffee, and / or nicotine) |
| 2 | The patient has previously received 8 or more sessions of psychological treatment with clear and identifiable CBT principles within the past 5 years. |
aThe following disorders will be included based on DSM-IV-TR diagnostic criteria [21]: major depression disorder, dysthymic disorder, panic disorder, agoraphobia, obsessive-compulsive disorder, generalized anxiety disorder, posttraumatic stress disorder, social anxiety disorder, hypochondria, and adjustment disorders. Patients with unspecified anxiety disorders and unspecified depressive disorders will also be included as they are frequent in public settings
bIf medication stability is not possible, the participant’s data will be treated separately in the analyses
Ethics
This study is carried out in accordance with the study protocol, the Helsinki Declaration, and good clinical practice. This non-inferiority, multicenter, randomized, controlled clinical trial has been approved by ethical and research committees of all collaborating centers: Hospital General Clínico de Elda (N°. PUG-02-14), Hospital Clínico Universitario de Valencia (N°. F-CE-GEva-15), Hospital Universitario Dr. Peset (N°. CEIC 53/15), CSM IIA Milagrosa (N°. Pyto2016/41), and Hospital Comarcal de Vinaròs (N°. 0103–2016). Data handling is carried out according to premises established by Spanish laws [28]. Security and confidentiality of participants’ data are guaranteed by using alphanumeric codes (SUP001) instead of names.
Therapists and interventions
Therapists participating in the study include licensed psychologists with between 8 and 20 years of experience in delivering CBT, clinical psychology residents with 2 to 4 years of experience, and doctoral students in clinical psychology with between 3 and 5 years of experience.
Unified protocol in group format
Our research team has adapted the UP [8, 9] to implement it in group format in a public mental health setting in Spain. This adaptation is composed of 12, two-hour treatment sessions, at a rate of one session per week. Treatment content, split by session, is shown in Table 2.To encourage maximum fidelity to the protocol, all therapists participating in this condition received a UP training course prior to study onset, as well as videoconference supervision before every session. The course consisted of a 20-h group workshop divided in 3 sessions. Next, all therapists received an individual training during 12 therapy sessions. Depending on availability, this individual training consisted of an online supervision before sessions or it involved acting as a co-therapist. Supervision is conducted by the leading author, J.O., who has been certified UP Researcher/Trainer by the Unified Protocol Institute.
Table 2.
Treatment content split by session
| Session number | Content |
|---|---|
| Session1 | Motivation for change and commitment to treatment |
| Session2 | Understanding the adaptability of emotions |
| Session3 | Recognition and analysis of emotions |
| Session4 | Emotional awareness training - I |
| Session5 | Emotional awareness training - II |
| Session6 | Cognitive flexibility- I |
| Session7 | Cognitive flexibility- II |
| Session8 | Emotional avoidance and emotion-driven behaviors |
| Session9 | Consciousness and tolerance to physical sensations |
| Session10 | Interoceptive and situational emotional exposure- I |
| Session11 | Interoceptive and situational emotional exposure- II |
| Session12 | Achievements, maintenance, and relapse prevention |
Participants who miss one or more UP sessions receive a phone call from the group therapist to explore the reasons for non-attendance, as well as to encourage reading the materials that summarize missed sessions. Also, at the beginning of each session the therapist reviews important contents of the past session to minimize this effect of missed sessions.
Treatment as usual (TAU)
Individual CBT is the treatment of choice by psychologists and psychiatrists at the collaborating Public Mental Health Centers and Primary Care Centers, together with pharmacological treatment (i.e., antidepressants and / or anxiolytics). Clinicians in this condition complete a self-report sheet describing the characteristics of their interventions with treatment modules as cues (psychoeducation module, identification of negative thoughts, breathing training, etc.), the average duration of sessions, number of sessions delivered, and end-of-treatment date, as well as information on the number of appointments with the psychiatrist and pharmacological treatment prescribed during the study. Information on the number of appointments with the psychiatrist and pharmacological treatment prescribed during the study is also collected for patients in the UP group following the same procedure described for the CBT condition.
As opposed to the UP condition, in which the duration of treatment is fixed (12 sessions), the usual practice in Spanish public settings is that treatment ends when both the clinician and the patient agree that the intervention has been effective. This is not necessarily guided by questionnaires or a diagnostic interview, but on clinical judgment. Because the study goal is to compare a new treatment (UP in a group format) with the usual practice in Spanish public settings, the same procedure described above will be used to determine the end of treatment in the TAU condition and no specific end points (i.e., a decrease in questionnaire scores) or number of sessions will be imposed. Because this is likely to yield different treatment durations between study conditions, the treatment duration (number of sessions) will be used as a covariate in the analyses.
Measures
The evaluation protocol is administered by therapists in a paper and pencil format at the participant’s health center in 5 different times: baseline, post-treatment, and follow-ups at 3, 6, and 12 months. To minimize biases, every administration of the diagnostic interview is done by two different psychologists, one face-to-face and the other by phone. Inter-rater reliability will be calculated. Assessment instruments include demographic characteristics (age, sex, education, marital status, and work status), a diagnostic interview, and well-established questionnaires for both primary and secondary outcomes.
Primary outcomes
Severity of depression and anxiety symptoms are assessed with the Beck Depression Inventory (BDI-II) [23, 24] and the Beck Anxiety Inventory (BAI) [25, 26], respectively.
In addition to symptoms, a current diagnosis of anxiety and/or mood disorder is made with the lifetime version of the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV-L) [4, 29]. The ADIS-IV-L is a semi-structured interview based on the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders -4th ed. (DSM-IV-TR) [4]. We have not used the ADIS-5 because it is not available in Spanish yet.
Secondary outcomes
We administer the Overall Depression Severity and Impairment Scale (ODSIS) [30] and the Overall Anxiety Severity and Impairment Scale (OASIS) [31] weekly to assess the severity of depressive and anxiety symptoms, respectively. These measures are included to complement the results obtained with the BDI-II and the BAI, as well as to obtain a continuous assessment. The Positive and Negative Affect Scale (PANAS) [32, 33] is administered to evaluate positive and negative affect. The perception of control over anxiety is assessed by means of the Anxiety Control Questionnaire-Revised (ACQ-R) [34, 35]. Personality is measured with the NEO Five-Factor Inventory (NEO-FFI) [36], which offers a rapid and general measure of the Big Five personality traits: Neuroticism, Extraversion, Openness to experience, Agreeableness, and Conscientiousness. The Quality of Life Index (QLI) [37] is used to evaluate several aspects of health-related quality of life (i.e., physical disability, emotional well-being, self-care and independent functioning, occupational functioning, interpersonal functioning, social emotional support, community and services support, personal fulfillment, spiritual fulfillment, and overall quality of life). Similarly, the Maladjustment Inventory (MI) [38] is used to evaluate the extent to which the subject’s current problems impact negatively on different areas of daily life, namely, work, social life, leisure time, relationship with the partner, family life, and overall adjustment in daily activities. Additionally, we created an ad hoc questionnaire, the Treatment Opinion Scale (OTS), which is administered to participants in both conditions. Our ad hoc questionnaire evaluates the quality of the intervention (i.e., “How would you rate the quality of the treatment program you have received?”) and that of its components (i.e., “Have the techniques and exercises that we have practiced helped you regulate your emotions properly?”). Also, it measures the amount of discomfort experienced during treatment (i.e., “To what extent has this treatment caused you discomfort?”) and the experience of participating in a group format (i.e., “If you were to seek help again, would you choose a group treatment program?”). Some questions in the OTS use a4-point Likert scale (0 = “poor” or “nothing” to 3 = “excellent” or “very much”), while an 11-point response scale is selected in some items (0 = “nothing” to 10 = “very much”).
All measures used in the study have been standardized in Spanish. Administration time is between 90 and120 minutes for the ADIS-IV-L and approximately 90 min for the primary and secondary outcomes altogether.
Analyses
First, we will calculate between-group differences in baseline measures, to ensure that randomization resulted in comparable groups. When normally distributed, continuous data will be analyzed via t test. If the Kolmogorov-Smirnov test indicates a non-normal distribution, a Mann-Whitney U test will be preferred. A chi-square test will be performed for categorical data. Then, to compare the efficacy of treatments, we will use a mixed-effects regression modeling with full information maximum likely hood estimation. This method is recommended over repeated-measures ANOVAs due to its flexibility in handling missing data [39]. Group (TAU or UP) will be used as the between-subject factor and time (baseline, post-treatment, and follow-ups) as the within-subject factor. Standardized effect sizes (Cohen’s d) will be calculated both for within and between-group analyses. Covariates will be demographic and clinical variables that are not comparable between groups, as exposed in the beginning of this section. Effect estimators in the UP group will be adjusted to control for the impact of group dependencies (i.e., homogenization of response to treatment due to shared environment), following the recommendations of Baldwin et al. [40].
To address problems of missing data or premature dropouts, an intention-to-treat analysis will be used and the most recent data will be imputed (last observation carried forward approach). This analytic strategy will only be performed with patients attending at least 50% of sessions, as recommended in past research [41], and with those responding to at least one of the post-treatment assessments (i.e., end of treatment or follow-ups) so that treatment effect may be captured by the last observation. In addition to the last observation forward, missing data in the UP group will also be replaced with the CBT mean +/− the non-inferiority margin and missing data in the CBT group will be replaced with the UP mean (i.e., bias toward inferiority) [42]. Results using a last observation carried forward approach and a bias toward inferiority imputation will be compared against a completers-only strategy and all of them will be reported. To ensure non-inferiority, a per-protocol analysis will also be conducted. Following FDA guidelines [16], non-inferiority will be concluded if the upper bound of the 95% CI for the effect estimate in the UP condition is smaller than the non-inferiority margin (50% of the effect estimate of individual CBT when compared to placebo). Due to the non-inferiority design (i.e., the Unified Protocol in a group format is not worse than individual CBT), a one-sided confidence interval will be used in the analyses. A post-hoc power analysis with the primary outcomes will be conducted at post-treatment and follow-ups using the sample size and obtained effect size.
The researcher analyzing data, C.S.R., will be blind to arm allocation. To ensure that, arms will be given arbitrary letters (A and B) in the database and only the lead researcher, J.O., will know the correspondence between study allocation arms and assigned arbitrary letters.
Reporting of results will follow the Consolidated Standards of Reporting Trials (CONSORT) recommendations [43].
Discussion
EDs have become a matter of public concern due to their high prevalence and associated costs [6, 7, 44]. The UP has emerged as a promising form of CBT to be used across diagnostic categories both in individual and group format [8, 9]. Therefore, the study of a therapeutic approach that can be administered to several patients with different disorders simultaneously, as in the present study, may have important clinical implications for countries.
The use of the UP in group format in public mental health settings may have some advantages over traditional individual CBT: 1) it may allow clinicians to use the same treatment for a variety of psychological disorders [8, 9, 12]; 2) it might help reduce existing waiting lists as between 6 and 8 patients can be treated simultaneously, which would probably reduce the duration of suffering in unattended patients; 3) in contexts similar to the Spanish public system, where individual CBT is the norm and therapy sessions occur at long intervals (i.e., once a month) due to waiting lists, a UP-based treatment program in group format can help increase the frequency of sessions because more patients are treated simultaneously, which may facilitate a more rapid detection of patients’ problems during treatment; 4) finally, group therapy is known to report benefits for the patient that are not obtained with individual treatment, such as reducing isolation, facilitating social support, and learning from others’ experiences [45, 46], which might enhance its efficiency. The present RCT taps into the aforementioned aspects of treatment satisfaction and effectiveness.
Our study has also some limitations. First, some people who ask for treatment in the public mental health system prefer individual treatment, which could be a barrier when randomly assigned to a group format (i.e., dropout or decreased satisfaction and efficacy of the UP). To control for this, we will explore the effect of format preference and format assignment on treatment adherence and effectiveness. Additionally, as conditions differ in terms of type of therapy (CBT vs. UP) and format (individual vs. group), it will be difficult to ascertain what was responsible for the results. Despite the present study only seeks to find whether the UP in a group format can be an alternative to the traditional psychological treatment in public settings in Spain (i.e., individual CBT), results should be interpreted with caution and no conclusions can be drawn on whether results are due to differences in the type of therapy, its format, or both. In line with the previous, it is important to note that the usual treatment was not stabilized across clinicians at centers (i.e., duration of sessions and treatment) precisely to ensure that the newly tested treatment, namely the UP in a group format, was compared against the usual treatment in Public Mental Health Settings in Spain. It is also important to note that, due the multicenter nature of the study, interventions are conducted by different clinicians, so implementation could differ across centers. In order to minimize this effect, a certified UP Researcher/Trainer, J.O., has been in charge of the full treatment for the first two groups at all collaborating centers. All clinicians will act as co-therapists of one group at their working center. Also, when they start treatment they will receive a videoconference supervision before every session by J.O. Despite this is done to promote fidelity to the protocol, it is important to note that assessment of fidelity is not performed by an independent rater, so true fidelity cannot be ensured. Finally, another shortcoming is that interviewers using the ADIS are not blind to treatment arm. This was not possible due to legal reasons (data collection at participating hospitals had to be done by a psychologist/psychiatrist working at the center). Due to time restrictions of psychiatrists, this was done by psychologists, who were also in charge of treatment. Despite this limitation, it is important to note that the ADIS is an objective, structured interview, and that on each assessment two interviews are independently made by two different psychologists, which should reduce the likelihood of biases.
Despite the aforementioned shortcomings, the present study may have important clinical implications as it is the first randomized, controlled clinical trial to test the effectiveness of a transdiagnostic intervention in group format for the treatment of EDs in public settings in Spain. Results will reveal whether the use of the UP in group format may serve to reduce existent waiting lists without decreasing the effectiveness of interventions (non-inferior to TAU).This would have important implications for patients, as it would possibly allow treatments to be more intensive (i.e., on a weekly basis instead of monthly) thanks to the simultaneous treatment of an increased number of patients.
Acknowledgements
We would like to thank all collaborating centers, all healthcare professionals, and all volunteers and participants that have made this study possible. Special thanks to the collaborators who are in charge of recruiting study participants: Cristian Castellano, Paco Pérez; Paula Palencia; Sara Diago; Nieves Hermosín; Mª Ángeles Escudero; Ana Cortés; Olga Domínguez; Luisa Luna; Lorena Pallás; Laura Fernández; Jordi de Luna.
Funding
Funding for the study was provided by the PI17/00320 project integrated in Plan Estatal de I+D+I 2013-2016 and co-funded by the "ISCIII-Subdirección General de Evaluación y Fomento de la investigación del Fondo Europeo de Desarrollo Regional (FEDER). Otra manera de hacer Europa", the Ministerio de Educación y Ciencia, Spain, Project FIS, Ministerio de Economía y competitividad. ISCIII (PI13/00982), “CIBER of Physiopathology of Obesity and Nutrition an initiative of ISCIII”, the University of Zaragoza (Vicerrectorado de Política Científica. N°: JIUZ-2016-SOC-10), the Antonio Gargallo University Foundation (N°:2016/B003), and by the following Awards: "Research and Psychological Development Chair 2015 Award" by the Official College of Psychologists of the Valencian Community and the "VII Call for Research Awards 2016" by the Spanish Association for Clinical Psychology and Psychopathology.
Availability of data and materials
Not applicable.
Abbreviations
- A
Agoraphobia
- ACQ-R
Anxiety Control Questionnaire-Revised
- AD
Adjustment Disorder
- ADIS
Anxiety Disorders Interview Schedule
- ADNOS
Anxiety disorder not otherwise specified
- BAI
Beck Anxiety Inventory
- BDI
Beck Depression Inventory
- DD
Dysthymic disorder
- DSM-IV-TR
Diagnostic and statistical manual of mental disorders, fourth text-revised edition
- EDs
Emotional Disorder
- GAD
Generalized anxiety disorder
- H
Hypochondria
- IA
Inventory for Agoraphobia
- LQI
Life Quality Index
- MDD
Major depression disorder
- MDNOS
Mood Disorder Not Otherwise Specified
- MI
Maladjustment Inventory
- NEO-FFI
NEO-Five Factor Inventory
- OASIS
Overall Anxiety Severity and Impairment Scale
- OCD
Obsessive-Compulsive Disorder
- ODSIS
Overall Depression Severity and Impairment Scale
- OTS
Opinion of Treatment Scale
- PANAS
Positive and Negative Affect Schedule– Negative Affectivity
- PCC
Primary Care Center
- PD
Panic Disorder
- PMHC
Public Mental Health Center
- POST-T
Post-treatment
- RCT
Randomized Controlled Trial
- SAD
Social Anxiety Disorder
- TAU
Treatment As Usual
- UP
Unified Protocol
Authors’ contributions
JO and AGP designed the study protocol with important contributions from ECD and CSR. JO and ECD translated and published the original UP manuals into Spanish, with important contributions from AGP JO, CRF, ASG,VFG, LPA, AMF, MATA, and all their collaborators are in charge of recruiting study participants at several mental health and primary health centers in Spain. JO and CSR drafted the manuscript. All authors participated in the review and revision of the manuscript and have approved the final manuscript to be published.
Authors’ information
J.O. is an Associate professor and Director of the General Health Psychology Master at the Psychology and Sociology Department of the University of Zaragoza. He is the PI of this project and an effective member of the “Psychological research and technological tools” (INPAT) research group (S-116 Aragon Government) and the Instituto de Investigación Sanitaria de Aragón. C.S.R. is a Postdoctoral researcher at the Department of Basic and Clinical Psychology and Psychobiology of the Universitat Jaume I. A.G.P. is Full professor of Psychopathology at the Department of Basic and Clinical Psychology and Psychobiology of the Universitat Jaume I. E.C.D. is an Assistant professor at the Department of Basic and Clinical Psychology and Psychobiology of the Universitat Jaume I. C.R.F. and A.S.G. are Clinical Psychologists at the La Fuente de San Luís Mental Health Unit in Valencia. V.F.G. is a Clinical Psychologist at the Hospital Comarcal de Vinaròs in Castellón. L.P.A. is a Clinical Pshychologist at the Centro de Salud Mental La Milagrosa in Pamplona. A.M.F. is a Clinical Pshychologist at the La Malvarrosa Mental Health Center in Valencia. M.A.T.A. is a Clinical Pshychologist at the Hospital General Universitario de Alicante.
Ethics approval and consent to participate
The study have been performed in accordance with the Declaration of Helsinki and have been approved and registered by the ethical and research committees from the following collaborating centers: Hospital General Clínico de Elda (PUG-02-14), Hospital Clínico Universitario de Valencia (F-CE-GEva-15), Hospital Universitario Dr. Peset (CEIC 53/15), CSM IIA Milagrosa (Pyto2016/41), and Hospital Comarcal de Vinaròs (0103–2016).In addition, the protocol study have been registered retrospectively at https://clinicaltrials.gov/. Trials on March 10, 2017 (NCT03064477).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jorge Osma, Phone: +34 978645343, Email: osma@unizar.es.
Carlos Suso-Ribera, Email: susor@uji.es.
Azucena García-Palacios, Email: azucena@uji.es.
Elena Crespo-Delgado, Email: screspo@uji.es.
Cristina Robert-Flor, Email: crflors@gmail.com.
Ana Sánchez-Guerrero, Email: anasanguerrero@gmail.com.
Vanesa Ferreres-Galan, Email: ferreres_van@gva.es.
Luisa Pérez-Ayerra, Email: luisa.perez.ayerra@navarra.es.
Amparo Malea-Fernández, Email: amparomalea099@gmail.com.
Mª Ángeles Torres-Alfosea, Email: torres.alfosea@gmail.com.
References
- 1.Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: implications for assessment and treatment. Psychol Assess. 2009;21:256–271. doi: 10.1037/a0016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van OJ, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed.) Washington: Author; 2013.
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.) Washington: Author; 2000.
- 5.Roca M, Gili M, Garcia-Garcia M, Salva J, Vives M, Garcia Campayo J, et al. Prevalence and comorbidity of common mental disorders in primary care. J Affect Disord. 2009;119:52–58. doi: 10.1016/j.jad.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Chisholm D, Sweeny K, Sheehan P, Rasmussen B, Smit F, Cuijpers P, et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. 2016;3:415–424. doi: 10.1016/S2215-0366(16)30024-4. [DOI] [PubMed] [Google Scholar]
- 7.Parés-Badell O, Barbaglia G, Jerinic P, Gustavsson A, Salvador-Carulla L, et al. Cost of disorders of the brain in Spain. PLoS One. 2014; 10.1371/journal.pone.0105471. [DOI] [PMC free article] [PubMed]
- 8.Barlow DH, Ellard KK, Fairholme CP, Farchione TJ, Boisseau CL, Allen LB, Ehrenreich-May J. The unified protocol for transdiagnostic treatment of emotional disorders: client workbook. New York, NY: Oxford University Press; 2011. [Google Scholar]
- 9.Barlow DH, Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Allen LB, Ehrenreich-May J. The unified protocol for transdiagnostic treatment of emotional disorders: therapist guide. New York: Oxford University Press; 2011. [Google Scholar]
- 10.Suárez L, Bennett S, Goldstein C, Barlow DH. In: Understanding anxiety disorders from a “triple vulnerabilities” framework. Antony MM, Stein MB, editors. New York: Oxford Handbooks; 2009. pp. 153–172. [Google Scholar]
- 11.Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- 12.Bullis JR, Sauer-Zavala S, Bentley KH, Thompson-Hollands J, Carl JR, Barlow DH. The unified protocol for transdiagnostic treatment of emotional disorders: preliminary exploration of effectiveness for group delivery. Behav Modif. 2015;39:295–321. doi: 10.1177/0145445514553094. [DOI] [PubMed] [Google Scholar]
- 13.Osma J, Castellano C, Crespo E, García-Palacios A. The unified protocol for Transdiagnostic treatment of emotional disorders in format group in a Spanish public mental health setting. Behavioral psychology. Psicol Conduct. 2015;23(3):447–466. [Google Scholar]
- 14.Reinholt N, Aharoni R, Winding C, Rosenberg N, Rosenbaum B, Arnfred S. Transdiagnostic group CBT for anxiety disorders: the unified protocol in mental health services. Cogn Behav Ther. 2017;46:29–43. doi: 10.1080/16506073.2016.1227360. [DOI] [PubMed] [Google Scholar]
- 15.Varkovitzky RL, Sherrill AM, Reger GM. Effectiveness of the unified protocol for Transdiagnostic treatment of emotional disorders among veterans with posttraumatic stress disorder: a pilot study. Behav Modif. 2018;42:210–30. doi: 10.1177/0145445517724539. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CEDER), Center for Biologics Evaluation and Research (CBER) (2016). Non-Inferiority Clinical Trials to Establish Effectiveness Guidance for Industry. Retrieved from https://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf. Accessed 5 Feb 2018.
- 17.Beltman MW, Oude Voshaar RC, Speckens AE. Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br J Psychiatry. 2010;197(1):11–19. doi: 10.1192/bjp.bp.109.064675. [DOI] [PubMed] [Google Scholar]
- 18.Gil PJ, Carrillo FX, Meca J. Effectiveness of cognitive-behavioural in social phobia: a meta-analytic review. Psychol Spain. 2001;5(1):17–25. [Google Scholar]
- 19.van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res. 2010;69(1):23–32. doi: 10.1016/j.jpsychores.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Andrews G, Cuijpers P, Craske MG, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS One. 2010;5:e13196. doi: 10.1371/journal.pone.0013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julious SA. Sample sizes for clinical trials with normal data. Statist Med. 2004;23:1921–1986. doi: 10.1002/sim.1783. [DOI] [PubMed] [Google Scholar]
- 22.Sealed Envelope Ltd. 2012. Power calculator for continuous outcome non-inferiority trial. Retrieved from https://www.sealedenvelope.com/power/continuous-noninferior/. Accessed 2 Feb 2018.
- 23.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 24.Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of the Beck's depression inventory-II (BDI-II): 2. Psychometric properties in the general population. Clínica y Salud. 2003;14:249–280. [Google Scholar]
- 25.Beck AT, Steer R. Beck anxiety inventory manual. San Antonio: Psychological Corporation; 1993. [Google Scholar]
- 26.Magán I, Sanz J, García-Vera MP. Psychometric properties of a Spanish version of the Beck anxiety inventory (BAI) in general population. Span J Psychol. 2008;11:626–640. [PubMed] [Google Scholar]
- 27.Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Thompson-Hollands J, Carl JR, Gallagher MW, Barlow DH. The unified protocol for the Transdiagnostic treatment of emotional disorders: a randomized controlled trial. Behav Ther. 2012;(3):666–78. [DOI] [PMC free article] [PubMed]
- 28.Ministerio de Justicia. Boletin Oficial del Estado, n°17 [BOE-A-2008-979].
- 29.Brown TA, Di Nardo PA, Barlow DH. Anxiety disorder interview schedule for DSM-IV (ADIS-IV). Adult and lifetime version. Clinical manual. San Antonio: Psychological Corporation; 1994. [Google Scholar]
- 30.Bentley KH, Gallagher MW, Carl JR, Barlow HD. Development and validation of the overall depression severity and impairment scale. Psychol Assessment. 2014;26:815–830. doi: 10.1037/a0036216. [DOI] [PubMed] [Google Scholar]
- 31.Norman SB, Cissell S, Means-Christensen AJ, Stein MB. Development and validation of an overall anxiety severity and impairment scale (OASIS) Depress Anxiety. 2006;23:245–249. doi: 10.1002/da.20182. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 33.Sandín B, Chorot P, Lostao L, Joiner TE, Santed MA, Valiente RM. PANAS Positive and Negative affect schedule: factorial validation and transcultural convergence. Psicothema. 1999;11:37–51. [Google Scholar]
- 34.Rapee RM, Craske MG, Brown TA, Barlow DH. Measurement of perceived control over anxiety-related events. Behav Ther. 1996;27:279–293. doi: 10.1016/S0005-7894(96)80018-9. [DOI] [Google Scholar]
- 35.Osma J, Barrada JR, García-Palacios A, Navarro-Haro M, Aguilar A. Internal structure and clinical utility of the anxiety control questionnaire-revised (ACQ-R) Spanish version. Span J Psychol. 2016;19:1–9. doi: 10.1017/sjp.2016.69. [DOI] [PubMed] [Google Scholar]
- 36.Costa PT, McCrae RR. Revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) Madrid: TEA; 1999. [Google Scholar]
- 37.Mezzich JE, Ruipérez MA, Pérez C, Yoon G, Liu J, Mahmud S. The Spanish version of the quality of life index: presentation and validation. J Nerv Ment Dis. 2000;188:301–305. doi: 10.1097/00005053-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Echeburúa E, Corral P, Fernández-Montalvo J. Maladjustment inventory (MI): psychometric properties in clinical contexts. Análisis y Modificación de Conducta. 2000;26:325–340. [Google Scholar]
- 39.Davey D, Thompson JA. Assessing sensitivity to assumptions in mixed effects analyses of stepped-wedge trials. Trials. 2015;16(Suppl 2):O46. doi: 10.1186/1745-6215-16-S2-O46. [DOI] [Google Scholar]
- 40.Baldwin SA, Murray DM, Shadish WR. Empirically supported treatments or type I errors? Problems with the analysis of data from group-administered treatments. J Consult Clin Psychol. 2005;73(5):924–935. doi: 10.1037/0022-006X.73.5.924. [DOI] [PubMed] [Google Scholar]
- 41.Luty SE, Carter JD, McKenzie JM, Rae AM, Frampton CM, Mulder RT, Joyce PR. Randomised controlled trial of interpersonal psychotherapy and cognitive-behavioural therapy for depression. Br J Psychiatry. 2007;190:496–502. doi: 10.1192/bjp.bp.106.024729. [DOI] [PubMed] [Google Scholar]
- 42.Koch GG. Comments on ‘current issues in non-inferiority trials’ by Thomas R. Fleming. Stat Med. 2008;27:333–342. doi: 10.1002/sim.2923. [DOI] [PubMed] [Google Scholar]
- 43.Piaggio G, Elbourne D, Pocock S, Evans S, Altman D. Reporting of noninferiority and equivalence randomized trials: extension of the consort 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 44.Schneider KM, O’Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health Qual Life Out. 2009;7:82. doi: 10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yalom ID, Leszcz M. The theory and practice of group therapy. 5. New York: IUP; 2005. [Google Scholar]
- 46.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69(4):717–721. doi: 10.1037/0022-006X.69.4.717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


