Abstract
A scaffold handling device (SHD) has been designed that can switch from gentle suction to positive pressure to lift and place nanofiber scaffolds. In tissue engineering laboratories, delicate fibrous scaffolds, such as electrospun nanofiber scaffolds, are often used as substrates for cell culture. Typical scaffold handling procedures include lifting the scaffolds, moving them from one container to another, sterilization, and loading scaffolds into cell culture plates. Using tweezers to handle the scaffolds can be slow, can damage the scaffolds, and can cause them to wrinkle or fold. Scaffolds may also acquire a static charge which makes them difficult to put down as they cling to tweezers. An SHD has been designed that enables more efficient, gentle lifting, and placement of delicate scaffolds. Most of the parts to make the SHD can be purchased, except for the tip which can be 3D-printed. The SHD enables more reliable handling of nanofiber scaffolds that may improve the consistency of biomanufacturing processes.
I. INTRODUCTION
Electrospun nanofibers are being advanced for tissue engineering applications as scaffolds for cell and tissue culture.1–4 Nanofibers are appealing as scaffolds because they mimic the fibrous structure of native extracellular matrices such as collagen and fibrin.5 Nanofibers can be fabricated from a wide range of synthetic and natural materials, which enables adjustment of their mechanical properties, permeability, and degradation rate.5
Handling of nanofiber scaffolds in a biomanufacturing setting requires consistency and a gentle touch. Lifting and placement of fragile scaffolds for transfer between containers, sterilization, and cell culture can be challenging. Use of tweezers can result in damage to the scaffolds and they can become folded or wrinkled. Also, nanofiber scaffolds and tweezers often acquire a static charge which causes the scaffolds to cling to tweezers, surfaces, and cell culture plates. The static charge makes it difficult to place the scaffolds in culture plates and have them lay flat for cell culture. Herein, a scaffold handling device (SHD) was designed for efficient and gentle lifting and placement of dry nanofiber scaffolds in a laminar flow hood setting. The device uses a gentle vacuum to lift scaffolds and a gentle positive pressure gas stream to place scaffolds in a desired location with reduced perturbations, wrinkling, and folding.
II. MATERIALS AND METHODS
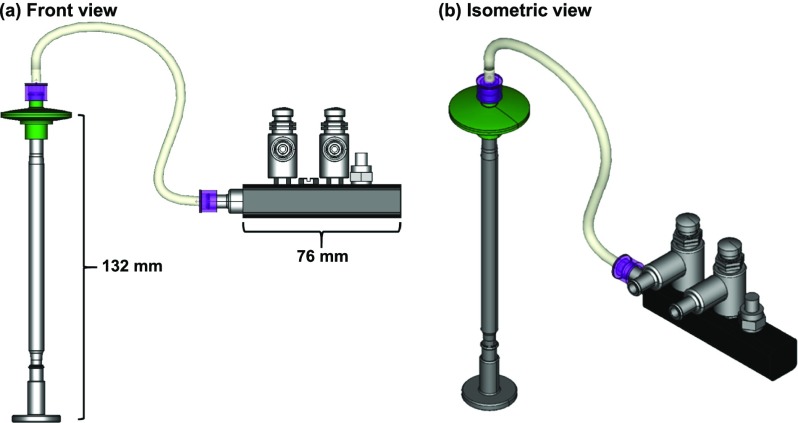
A computer-aided design (CAD) rendering of the scaffold handling device (SHD) is shown in Fig. 1. All but one of the parts for building the SHD can be purchased. See Table I for a parts list. The only custom part is the tip that contacts the nanofiber scaffolds. The tip can be made by fused deposition modeling (FDM) 3D-printing using plastic filament (Fig. 2). Tips were printed on a StrataSys uPrint SE Plus with StrataSys ABSplus thermoplastic filament using the .stl files provided in the supplementary material (Files S1–S3 in the supplementary material). Tips were made in three different nominal sizes to accommodate scaffolds of nominally different sizes: 6.5 mm, 12 mm, and 24 mm [Fig. 2(f)]. The .stl files were created in FreeCAD (www.freecadweb.org).6 The 6.5-mm tip was designed for scaffolds that fit in a 96-well cell culture plate. The 12-mm tip is for scaffolds that fit in a 24-well plate. The 24-mm dia. tip is for scaffolds that fit in a 6-well plate.
FIG. 1.
Images of the assembled scaffold handling device (SHD) with 24-mm dia. tip. (a) Front view. (b) Isometric view. Images were made in FreeCAD.
TABLE I.
Parts list.
| Part no. | Part description | Manufacturer | Manufacturer’s part no. | Quantity |
|---|---|---|---|---|
| 1 | Tip (see Fig. 2) | 3D printed in house | Homemade | 1 |
| 2 | Grip: Stainless steel infusion handle, male | Walcott Rx | Rx0-1051 | 1 |
| luer slip to female luer slip, 10 cm length | ||||
| 3 | Male luer lock to 1/16 in. tube barb | Qosina | LM2130 | 6 |
| (1.5 mm), white, nylon | ||||
| 4 | PVCa tubing, DEHPa-free, 1/16 in. | Qosina | T4303 | 1 |
| IDa (1.5 mm), 2.4 mm ODa | ||||
| 5 | Syringe filter, nylon, 0.2 μm dia. pore, 25 mm dia., | Cole parmer | EW-02915-04 | 1 |
| female luer lock to male luer slip | ||||
| 6 | Vinyl tubing, DEHPa-free, 1/8 in. IDa (3.2 mm), | Cole parmer | EW-06405-02 | 1 |
| 1/4 in. ODa (6.4 mm) | ||||
| 7 | Adapter, chrome-plated, female luer lock | Cole parmer | 41507-71 | 4 |
| to 10-32 UNFa thread male | ||||
| 8 | Adapter, nickel-plated brass, female luer lock | Cole parmer | 41507-86 | 1 |
| to 1/8 in. NPTa thread male | ||||
| 9 | Manifold, 1/8th NPTa thread female to | Pneumadyne | M32-4 | 1 |
| four 10-32 UNFa thread female | ||||
| 10 | Threaded nipples, nickel-plated, 10-32 UNFa thread male | Pneumadyne | SFU-22 | 2 |
| to 10-32 UNFa thread male, with adjustable nut | ||||
| 11 | Input port pressure regulator, | Pneumadyne | R0-RP-3 | 2 |
| 10-32 UNFa thread female inputs | ||||
| 12 | Metal push button control valve, 10-32 UNFa thread | Pneumadyne | C030225 | 2 |
| female input, 10-32 UNFa thread female output, 2-way valve | ||||
| 13 | Bleed valve, nickel-plated, 10-32 UNFa | Pneumadyne | PBV-10/32 | 1 |
| thread male, 5/16 in. hex | ||||
| 14 | Plug fitting, 10-32 UNFa thread male, | Pneumadyne | SPG-10 | 3 |
| slotted hex 5/16 in., nickel-plated brass | ||||
| 15 | Connector, stainless steel, 10-32 UNFa | Pneumadyne | SBF-140-SS | 2 |
| thread male to 1/8 in. barb |
Abbreviations: CAD: computer aided design; PVC: polyvinyl chloride; DEHP: [di-(2-ethylhexyl)phthalate]; ID: internal dia.; OD: outer dia.; UNF: unified form thread designation “unified fine”; NPT: American standard taper pipe thread designation.
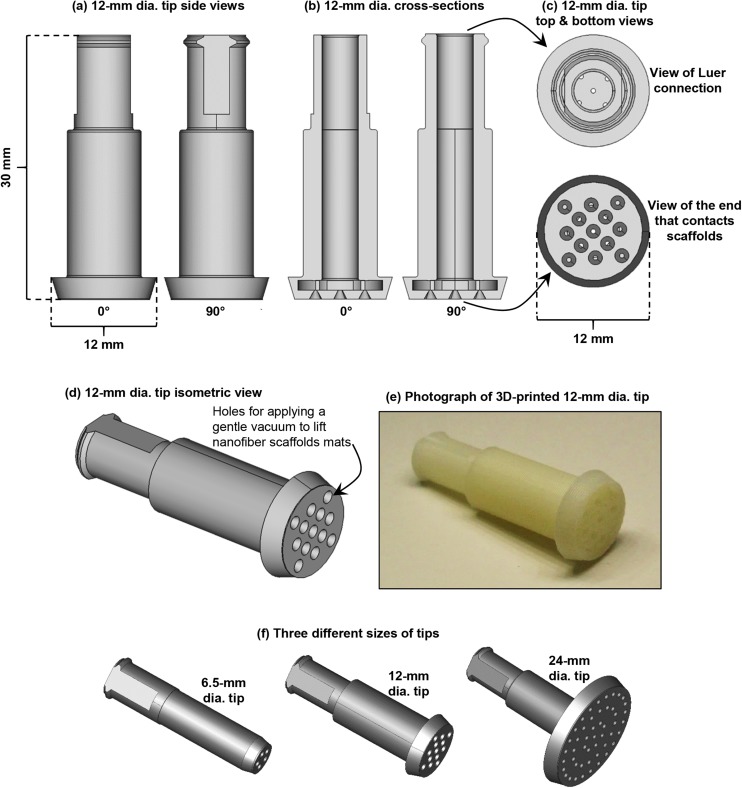
FIG. 2.
The 12-mm dia. tip of scaffold handling device (SHD). Images were generated with FreeCAD using the .stl file that is File S2 in the supplementary material. (a) Side views of the 12-mm dia. tip from two different angles. (b) Cross-sectional views of the 12-mm dia. tip from two different angles. (c) Views of the luer connection and the part of the 12-mm dia. tip that contacts the nanofiber scaffolds. (d) Isometric view of the 12-mm dia. tip. Air is gently sucked through the holes in the tip to lift the nanofiber scaffolds and then air is gently blown through the holes to “place” the scaffolds. (e) Photograph of a 3D-printed tip. The tip is nominally 30 mm on its longest axis. (f) FreeCAD images of the 3 tips of different sizes. All tips are nominally 30 mm long and tip sizes refer to the nominal distance across the face of the tip.
For the video demonstrations, three types of scaffolds were used. First, thicker nanofiber scaffold mats were used [Akron Biotech, AK-PolyFibers, poly(ε-caprolactone) (PCL), random orientation, catalog numbers AK9001-0006 (6-well plate inserts, cut into 12 mm dia. discs using a punch), and AK9001-0024 (24-well plate inserts, 14 mm dia. discs)]. A second set of thinner PCL scaffolds were also used (Stellenbosch Nanofiber Company, PCL, number average molecular mass 70 000–90 000 g/mol, 5 min spinning time, nominal fiber dia. range 300 nm–500 nm, nominal scaffold dia. 12 mm, nominal scaffold thickness range 25 μm–35 μm). Third, thin collagen nanofiber scaffolds were used (Akron Biotech, AK-PolyFibers, collagen, random orientation, 24-well plate inserts, catalog number AK9016-0024, 14 mm dia. discs). Color videos (.mp4 files, 1920 × 1080 pixels) were captured using a cell phone (Samsung S5) to demonstrate the use of the SHD (with 12 mm dia. tip) or plastic tweezers for loading the different types of scaffolds into 24-well cell culture plates. Videos were compressed using a web application (www.clipchamp.com) which resulted in a 5-fold reduction in file size.
For measurement of scaffold transfer times, the thicker PCL nanofiber scaffolds were transferred from a flat surface into a 24-well culture plate using either tweezers or the SHD (with 12 mm dia. tip). Transfer time for each of 9 scaffolds was recorded and statistical testing was completed in Minitab v17.
A second test was performed where the thicker PCL scaffolds were transferred from a flat surface into a 24-well plate, and then moved back out of the plate onto the flat surface. Transfers were completed with either tweezers or with the SHD. The scaffolds were photographed to record the perturbations that occurred to the scaffolds during the transfer process.
III. ASSEMBLY
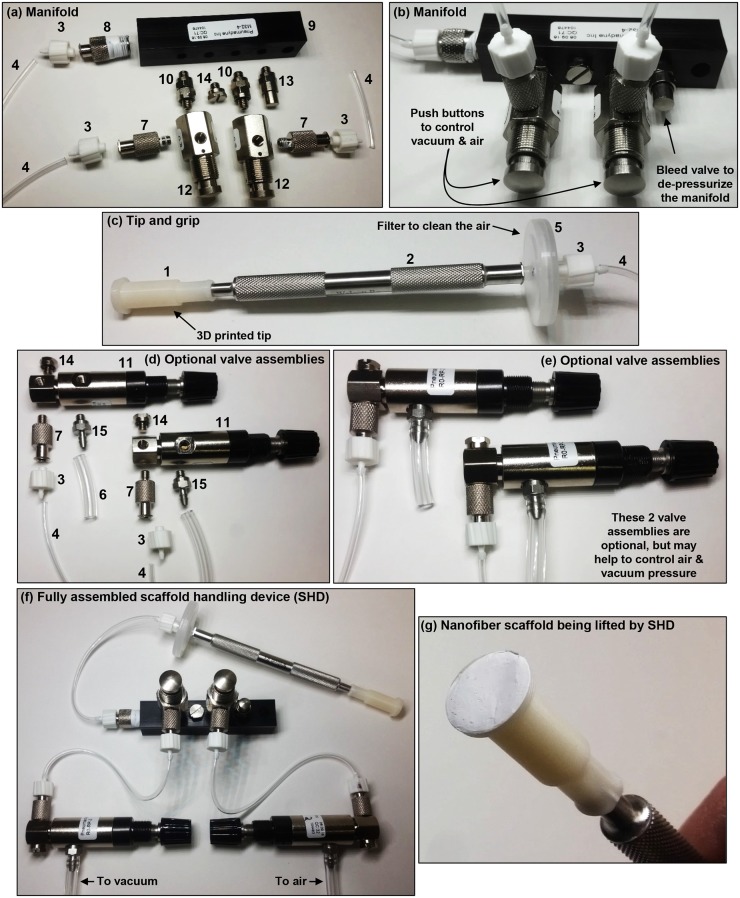
Parts should be assembled as shown in Figs. 2 and 3. Teflon tape can be used on the threads to reduce leakage.
FIG. 3.
Assembly of the scaffold handling device (SHD) with the 12-mm dia. tip. The numbers in the photos refer to part numbers in Table I. (a) Parts used to assemble the manifold are laid out to approximately indicate how they should be assembled. (b) Assembled manifold. (c) Assembled tip and grip. (d) Parts used to make the optional valve assemblies are laid out to approximately indicate how they should be put together. (e) Valve assemblies. The valve assemblies are optional and may be used to help control pressure for the air and vacuum for the SHD. (f) Fully assembled SHD with optional valve assemblies. Note that short sections of tubing were used for the images so that the connections between the parts would be clear. Longer sections of tubing are needed as appropriate for actual use of the SHD. Teflon tape may be used on threads to reduce leakage. (g) SHD with a nanofiber scaffold on the tip under gentle vacuum.
The manifold to control the vacuum and gas is shown in Figs. 3(a) and 3(b):
-
•
Parts 8, 10 (there are 2), 13, and 14 should be screwed into the manifold (part 9) in the arrangement shown in Figs. 3(a) and 3(b).
- •
-
•
Attach a part 12 to the two part 10s as shown in Figs. 3(a) and 3(b).
-
•
Attach one part 7 to each of the two part 12s as shown in Figs. 3(a) and 3(b).
-
•
Attach one part 3 to each of the two part 7s as shown in Figs. 3(a) and 3(b).
-
•
Attach the desired length (60 cm) of tubing (part 4) to each of the three part 3s as shown in Figs. 3(a) and 3(b).
The tip and grip are show in Fig. 3(c):
-
•
Attach part 1 to part 2 as shown in Fig. 3(c).
-
•
Attach part 5 to part 2 as shown in Fig. 3(c).
-
•
Attach part 3 to part 5 as shown in Fig. 3(c).
-
•
Attach the appropriate section of tubing (part 4) coming off the end of the manifold to part 3 on the “tip and grip” as shown in Fig. 3(f).
Two optional valve assemblies may be useful for controlling the gas and air pressure as shown in Figs. 3(d) and 3(e):
-
•
To each of the two part 11s, attach one part 14, one part 7, and one part 15 in the arrangement shown in Figs. 3(d) and 3(e).
-
•
Attach one part 3 to each of the two part 7s as shown in Figs. 3(d) and 3(e).
-
•
Attach the appropriate sections of tubing (part 4) coming off the manifold to part 3 on each of the two valve assemblies as shown in Fig. 3(f).
-
•
Connect part 15 on each valve assembly to air and vacuum using the desired length (>1 m) of tubing (part 6) according to the arrangement shown in Fig. 3(f). Note that very short sections of tubing were used in Fig. 3 so that the order of the connections between the parts was clear to the reader (longer sections of tubing are required in practice).
-
•
The final assembled device, with optional valve assemblies, should resemble the SHD shown in Fig. 3(f).
A gentle house vacuum, just below atmospheric pressure [nominally −1 to 0 gauge psi (pounds per square inch); absolute psi 13.7–14.7; absolute kPa 94 431–101 325] and a gentle house air just above atmospheric pressure (nominally 0–1 gauge psi; absolute psi 14.7–15.7; absolute kPa 101 325–108 220) were used to operate the SHD. Alternatively, a vacuum pump or a compressed gas cylinder may be used.
IV. RESULTS
The SHD has been used by the authors for several years and has been improved through several engineering design iterations. The SHD was designed to gently lift nanofiber scaffold mats and place them into cell culture plates in a GMP- (good manufacturing practice) or GLP-setting (good laboratory practice). File S4 in the supplementary material is a video showing the lifting and placement of nanofiber scaffolds with the SHD and with plastic tweezers. The video shows the difficulty that can be encountered when using tweezers to move the scaffolds. The delicate nature of the scaffolds makes them difficult to pickup with tweezers and shows one of the scaffolds crumpling during pickup. The video shows how the static charge makes it difficult to put the scaffolds down into the culture plates since the scaffolds cling to the tweezers and the sides of the culture well. Two sets of tweezers may be required to place the scaffolds, leading to increased handling times and perturbations that may alter the structure of the scaffolds. The video shows how the SHD is comparatively more efficient and less perturbing than tweezers.
In a second video (File S5 in the supplementary material), a different set of PCL scaffolds which are thinner are moved to a culture plate with the SHD and tweezers. These thinner scaffolds are weaker and more prone to deformation under the forces of static charge. After these scaffolds are loaded into the culture plate, the video shows that one of the scaffolds that was moved with tweezers is folded against the side of the culture well due to the static charge. The scaffolds that were moved with the SHD are lying flat in the bottom of the culture wells.
In a third video (File S6 in the supplementary material), a thin collagen scaffold was moved into a culture plate with the SHD and then with the tweezers. The video shows how the strong static adhesion between the collagen scaffold and the flat surface made it difficult to lift the scaffold with tweezers leading to crumpling of the scaffold and to the scaffold being scraped with the tip of the tweezers. These perturbations to the scaffold could be perceived as trivial. However, in a clinical setting, where the goal may be to implant the scaffold into the eye of a patient, it is desirable to minimize any perturbations that could alter the structure or properties of the scaffold.
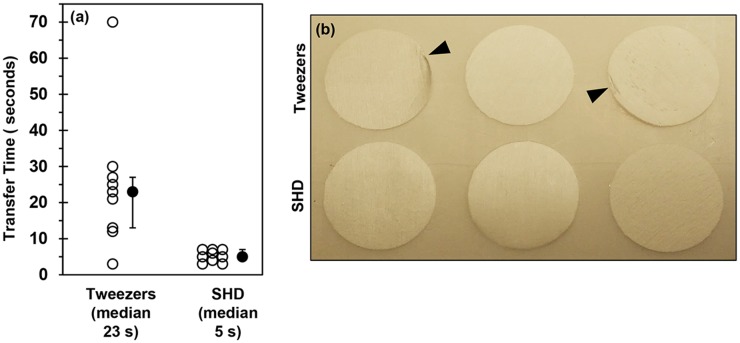
The time to transfer the thicker PCL nanofiber scaffolds from a flat surface into a culture plate was measured. Figure 4(a) indicates that the median transfer time was 4–5 times faster when using the SHD as compared to tweezers (P = 0.005, Mann-Whitney test). A second test was performed where the thicker PCL scaffolds were transferred from a flat surface into a 24-well plate, and then moved back out of the plate onto the flat surface. This is a notoriously difficult task for tweezers since it is hard to get the tweezers down into the wells to grasp the scaffolds. The image shows visible perturbations on 2 of the three scaffolds that were transferred with tweezers. The three scaffolds that were moved with the SHD do not have any visible perturbations. Note that these results do not suggest that a skilled technician could not effectively transfer the delicate scaffolds with tweezers. Further, it is not being suggested that scaffolds should not be handled with tweezers. Instead, the data suggest that use of the SHD may enable more rapid and consistent scaffold transfers with a lower prevalence of scaffold damage.
FIG. 4.
(a) Plot of transfer times for SHD vs. tweezers. The time to move the thicker PCL nanofiber scaffolds from a flat surface into a 24-well culture plate was measured. The 12-mm dia. tip was used for the SHD. Open circles are individual data points (n = 9) and black circles are the median with first and third quartiles. Nine scaffolds were moved by tweezers or SHD. The Anderson-Darling normality test indicated that the tweezer data may be non-normal (P = 0.04) and that SHD data may be normal (P = 0.24). A 2-sample non-parametric test was used to compare medians (Mann-Whitney) and indicated that there may be a significant difference between SHD and tweezers (P = 0.005). (b) Photograph of thicker PCL nanofiber scaffolds that were moved into and then out of a 24-well culture plate using either tweezers or the SHD. The top three scaffolds were moved with tweezers and the bottom three scaffolds were moved with the SHD. The arrowheads indicate perturbations to the scaffolds caused by tweezers. Scaffolds are 14 mm in dia.
V. DISCUSSION
The SHD has been used for several years by the authors in a laminar flow hood under aseptic conditions for transferring delicate nanofiber scaffolds into cell culture plates. The tip (part 1) can be sterilized by ethylene oxide. The filters (part 5) can be purchased sterile. The rest of the parts (parts 2–4 and parts 6–15) may be sterilized by autoclave. In some cases, the tip (part 1) was disinfected in 70% (by volume) ethanol followed by air drying for an hour. The tips may be treated as consumable since they can be 3D-printed for low cost. The SHD is equipped with a filter (part 5) to sterilize the air used for suction and for the positive pressure.
In addition to the SHD introduced in the current manuscript, there are a variety of pen-like devices with a finger-controlled vacuum that may be considered for handling fragile scaffolds (vacuum pick-up device). In different types of devices, the vacuum can be generated (I) by manually depressing a vacuum push bar on the pen, (II) by an onboard battery-powered, or (III) by attaching to an electric vacuum pump by tubing. These devices can be fit with a rubber cup on the tip that can interface with delicate scaffolds.
None of these three options was suitable for our applications due to the lack of a positive pressure option (blow out) for placing scaffolds that have acquired a static charge and are clinging to the tip of the device. During testing with a commercial device described in III above, the nanofiber scaffolds clung to the rubber cup on the tip and were challenging to place in the culture plates. Another consideration for our application was that the rubber cups have only 1-hole for application of the vacuum, which places stress on the scaffolds at the point of vacuum and may cause them to wrinkle. The 3D-printed tips for the SHD were designed with multiple holes so that vacuum could be more evenly applied across the surface of fragile scaffolds. For a device with onboard battery power, (item II above) sterilization could be an issue for a GMP/GLP setting. Devices with an onboard manual pump (item I above) would not be able to apply a constant vacuum to items that are porous. Nanofiber scaffolds are porous which may cause the vacuum from the hand pump to dissipate resulting in an untimely release of the scaffold from the tip. For these reasons, the SHD was required for our applications where it was necessary to transfer fragile nanofiber scaffolds between containers in a GMP/GLP-setting in an efficient, consistent manner with minimal perturbations.
Another relevant technology is an ionizer (static eliminators or static removal tools), which can blow charged air towards an item to reduce or remove a static charge. These were not tested for our application due to the challenges of sterilizing a powered device for use in a GMP/GLP setting and due to concern about the flow of ionized air compromising the air flow in the laminar flow hood.
The SHD was designed for the special case of lifting and placing thin, membranous, light, deformable scaffolds that are delicate and are prone to adhere to surfaces via mechanisms such as static charge. The SHD is equipped with positive pressure capability that enables the placement of items that adhere to the tip of the SHD. Tip adherence may not interfere with the placement of heavy scaffolds since they may fall off the tip upon release of the vacuum. Tip adherence may not interfere with the placement of rigid, sturdy scaffolds since they may not conform to the shape of the tip, which minimizes the contact area between the tip and scaffold to reduce the adhesion force. Nanofiber scaffolds can be both light and deformable (like plastic wrap), causing them to conform to the shape of the tip which increases the contact area between the tip and scaffold and increases the adhesion force. The positive pressure capability of the SHD can break the adhesion of nanofiber scaffolds to the tip to enable placement of the scaffolds.
Use of the SHD to transfer nanofiber scaffolds between containers may improve consistency in tissue engineering research or biomanufacturing. The SHD may find additional utility in moving other types of fragile membranes, such as other types of porous media or polymeric thin films.
SUPPLEMENTARY MATERIAL
See supplementary material: File S1: this is a file (.stl) that can be used to 3D-print the 6.5-mm dia. tip; File S2: this is a file (.stl) that can be used to 3D-print the 12-mm dia. tip; File S3: this is a file (.stl) that can be used to 3D-print the 24-mm dia. tip; File S4: this is a video (.mp4) showing the lifting and placement of nanofiber scaffolds with the SHD and with plastic tweezers; File S5: This is a video (.mp4) where a different set of PCL scaffolds which are thinner are moved to a culture plate with the SHD and tweezers; File S6: this is a video (.mp4) where a thin collagen scaffold was moved into a culture plate with the SHD and then with the tweezers.
ACKNOWLEDGMENTS
N.A.H. acknowledges support by the National Research Council Associateship Program. This article, a contribution of NIST, is not subject to U.S. copyright. Certain equipment and instruments or materials are identified in the paper to adequately specify the experimental details. Such identification does not imply recommendation by NIST nor does it imply that the materials are necessarily the best available for the purpose.
REFERENCES
- 1.Kumar G., Tison C. K., Chatterjee K., Pine P. S., McDaniel J. H., Salit M. L., Young M. F., and C. G. Simon, Jr., Biomaterials 32, 9188 (2011). 10.1016/j.biomaterials.2011.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson A. L., Bennett N. K., Francis N. L., Halikere A., Clarke S., Moore J. C., Hart R. P., Paradiso K., Wernig M., Kohn J., Pang Z. P., and Moghe P. V., Nat. Commun. 7, 10862 (2016). 10.1038/ncomms10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotaling N. A., Khristov V., Wan Q., Sharma R., Jha B. S., Lotfi M., Maminishkis A., C. G. Simon, Jr., and Bharti K., J. Ocul. Pharmacol. Ther. 32, 272 (2016). 10.1089/jop.2015.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Q., Cosme J. G., Xu T., Miszuk J. M., Picciani P. H., Fong H., and Sun H., Biomaterials 115, 115 (2017). 10.1016/j.biomaterials.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J., Jun Y., Qin J., and Lee S. H., Biomaterials 114, 121 (2017). 10.1016/j.biomaterials.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 6.Collette B. and Falck D., FreeCAD [How-to] (Packt Publishing Ltd., Birmingham, United Kingdom, 2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material: File S1: this is a file (.stl) that can be used to 3D-print the 6.5-mm dia. tip; File S2: this is a file (.stl) that can be used to 3D-print the 12-mm dia. tip; File S3: this is a file (.stl) that can be used to 3D-print the 24-mm dia. tip; File S4: this is a video (.mp4) showing the lifting and placement of nanofiber scaffolds with the SHD and with plastic tweezers; File S5: This is a video (.mp4) where a different set of PCL scaffolds which are thinner are moved to a culture plate with the SHD and tweezers; File S6: this is a video (.mp4) where a thin collagen scaffold was moved into a culture plate with the SHD and then with the tweezers.