Abstract
Background
Ventilator-associated pneumonia (VAP) is a nosocomial infection commonly seen in patients in intensive care units (ICU). This study aimed to analyze factors affecting prognosis of patients diagnosed with VAP.
Material/Methods
Critically ill patients with VAP were retrospectively evaluated between June 2002 and June 2011 in the ICU. VAP diagnosis was made according to 2005 ATS/IDSA (Infectious Diseases Society of America/American Thoracic Society) criteria. First pneumonia attacks of patients were analyzed.
Results
When early- and late-onset pneumonia causes were compared according to ICU and hospital admittance, resistant bacteria were found to be more common in pneumonias classified as early-onset according to ICU admittance. APACHE II score of >21 (p=0.016), SOFA score of >6 (p<0.001) on admission to ICU and SOFA score of >6 (p<0.001) on day of diagnosis are risk factors affecting mortality. Additionally, low PaO2/FIO2 ratio at onset of VAP had a negative effect on prognosis (p<0.001). SOFA score of >6 on the day of VAP diagnosis was an independent risk factor for mortality [(p<0.001; OR (95%CI): 1.4 (1.2–1.6)].
Conclusions
Resistant bacteria might be present in early-onset VAP. Especially, taking LOS into consideration may better estimate the presence of resistant bacteria. Acinetobacter baumannii, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA) were the most frequent causative microorganisms for VAP. SOFA score might be more valuable than APACHE II score. Frequently surveilling SOFA scores may improve predictive performance over time.
MeSH Keywords: Mortality; Organ Dysfunction Scores; Pneumonia, Ventilator-Associated; Risk Factors
Background
Nosocomial pneumonia is a definition used for pneumonias that begin in a non-intubated patient after 48 h of admission. It is the second most common hospital-acquired infection [1]. This common infection type causes extended length of hospitalization and increased mortality [2,3].
Ventilator-associated pneumonia (VAP) was defined as pneumonia that develops more than 48 h after patients are intubated and receive mechanical ventilation [1,4,5].
The mortality rate of VAP generally ranges between 25% and 50%; however, it may increase to 70% in some cases. According to the data of the National Nosocomial Infections Surveillance (NNIS) System, approximately 2.4–14.7 of pneumonia cases develop in 1000 ventilator days [1,6,7].
There are several risk factors affecting the development of VAP. Some of these risk factors may already be present at admission to the intensive care unit (ICU), such as advanced age, presence of a respiratory or cardiovascular system disease, organ failure, burns, trauma, acute respiratory distress syndrome (ARDS), gastric colonization, sinusitis, high-volume gastric aspiration, and seasonal changes [6,8]. Others include the risk factors that develop and can be changed during the diagnosis and treatment processes in the ICU. These risk factors influence development by impairing the defense mechanisms of the host.
The independent risk factors for mortality in VAP in the literature were found to be high Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Sequential Organ Failure Assessment (SOFA) scores; inappropriate antibiotic therapy; diabetes mellitus; tracheostomy; Confusion, Urea, Respiratory rate and Blood pressure (CURB) score ≥3; high C-reactive protein levels; high creatinine levels; VAP-related septic shock; and chronic respiratory disease [4].
Having information about bacteria, antibiotic susceptibilities, and prognostic risk factors in ventilator-associated pneumonia may play a significant role in reducing morbidity and mortality caused by VAP. Therefore, we aimed to identify the epidemiological characteristics and the factors affecting prognosis for patients being monitored or treated for VAP in the Department of Anesthesiology and Critical Care.
Material and Methods
This single-center study was conducted with approval (no; 2011-10/9 dated 10.05.2011) of the Medical Research Ethics Committee of Uludag University, Faculty of Medicine. Patients admitted to the ICU between June 2002 and June 2011 and who developed ventilator-associated pneumonia after admissions to the ICU were included in the study. The informed consent requirement was waived because this was a retrospective study. The records of the first pneumonia attacks of these patients were analyzed retrospectively.
The study involved patients aged over 18 years old who underwent mechanical ventilation for more than 48 h. We recorded data on age, sex, location and cause of ICU admission, presence of chronic disease(s) [diabetes mellitus (DM), hypertension (HT), congestive heart failure (CHF), coronary artery disease (CAD), chronic renal failure (CRF), a regular dialysis program, chronic obstructive pulmonary disease (COPD), neurological disease, and malignancy], use of steroids and immunosuppressive therapy, and a history of surgery.
For the diagnosis of nosocomial pneumonia, aside from the development of pneumonia within at least 48 h after hospital admission and the presence of new or progressing infiltrates in the chest radiography, at least 2 of the following criteria should be present: (i) temperature >38°C; (ii) leukocytes>10000/mm3 or <4000/mm3; (iii) purulent bronchial secretion (i.e., leukocytes>25) and the presence of 10 or fewer epithelial cells in the gram-staining examination of deep endotracheal aspirate (ETA) (10x); and (iv) decline in oxygenation [4,5].
Ventilator-associated pneumonia was defined as pneumonia that developed more than 48 h after patients were intubated and received mechanical ventilation. Ventilator-associated pneumonia which developed within 4 days after mechanical ventilation was defined as early-onset ventilator-associated pneumonia, while those which developed after 4 days were defined as late-onset ventilator-associated pneumonia.
APACHE II score and Glasgow coma score (GCS) were calculated within 24 h of admission to the ICU. The SOFA scores of patients were calculated within 24 h following admission to the ICU, and another SOFA score was calculated on the day of the diagnosis of VAP.
Mechanical ventilation values on the day of VAP [tidal volume, positive end-expiratory pressure (PEEP), and partial oxygen pressure/administered oxygen% concentration (PaO2/FIO2)] were recorded for all patients.
„Appropriate therapy” and „inappropriate therapy” referred to empiric antibiotics. The use of ineffective antibiotic therapy according to in vitro results (resistant or intermediate) within the 24 h after the diagnosis of VAP was defined inappropriate, and the use of effective antibiotic therapy (in vitro sensitive) was defined appropriate [9,10]. Duration of mechanical ventilation, length of ICU and hospital stay, and 14-day and 28-day mortality rates were recorded for all patients.
Statistical analysis
In this study, median (Interquartile range) values were used as descriptive statistics of the continuous variables, while numbers and relevant percent values were used to express categorical variables. Normality of the continuous variables was assessed by Shapiro-Wilk test. According to normality, test results between mortality group comparisons of continuous variables were performed by using independent samples t test or Mann-Whitney test, while the Wilcoxon test was used for within-group comparisons of variables. Categorical variables were compared between mortality groups using the chi-square test. Logistic regression analysis was performed to identify the independent risk factors that were thought to affect mortality. Calibration was tested using the Hosmer-Lemeshow goodness of fit statistic. Regarding mortality, the ROC (receiver operating characteristic) curve analysis was performed to specify the cut-off points for the SOFA score and APACHE II score, and sensitivity, specificity, and AUC (area under the curve) values were identified. The measure of discrimination (Nagelkerke R2) was calculated. p<0.05 was considered as statistically significant. Study data were analyzed using SPSS version 14 (Chicago, IL, USA).
Results
The study population included 167 patients with the diagnosis of VAP who were followed at the ICU between June 2002 and June 2011. No statistically significant difference was observed between the deceased and surviving patients with respect to their age, sex, location before admission to ICU, reasons for mechanical ventilation, and underlying diseases (Table 1).
Table 1.
Baseline characteristics and Risk factors for mortality in patients with ventilator- associated pneumonia.
| Total (n=167) | Survivors (n=125) | Non survivors (n=42) | p-Value | |
|---|---|---|---|---|
| Age (year), median (IQR) | 58 (29) | 56 (27) | 60.5(33) | 0.743 |
|
| ||||
| Gender (F/M) (n) | 55/112 | 40/85 | 15/27 | 0.800 |
|
| ||||
| Admission place for ICU (n) | 0.237 | |||
| – Community | 54 | 37 | 17 | |
| – Wards | 78 | 64 | 14 | |
| – Other ICU | 24 | 17 | 7 | |
| – Other Hospital | 11 | 7 | 4 | |
|
| ||||
| Reason of ICU admission (n) | 0.512 | |||
| – Pulmonary | 52 | 42 | 10 | |
| – Cardiac | 11 | 7 | 4 | |
| – Neurologic | 25 | 19 | 6 | |
| – Sepsis | 21 | 11 | 10 | |
| – Trauma | 27 | 24 | 3 | |
| – Intoxication | 5 | 4 | 1 | |
| – Other | 13 | 12 | 1 | |
|
| ||||
| Underlying disease (n) | ||||
| – Hypertension | 59 | 43 | 16 | 0.805 |
| – Diabetes mellitus | 27 | 16 | 11 | 0.072 |
| – Neurologic disease | 28 | 23 | 5 | 0.462 |
| – Malignancy | 24 | 18 | 6 | 1.00 |
| – COPD | 21 | 14 | 7 | 0.512 |
| – CAD | 17 | 10 | 7 | 0.139 |
| – CHF | 11 | 8 | 3 | 1.00 |
| – CRF | 16 | 10 | 6 | 0.236 |
|
| ||||
| APACHE II scoreAdmission | 23 (9) | 21 (8.5) | 24 (6.3) | 0.016* |
|
| ||||
| SOFAAdmission median (IQR) | 7 (3) | 6 (4) | 7.5 (2.3) | <0.001* |
|
| ||||
| SOFAat VAP diagnosis | 7 (3) | 6 (4) | 8(2.3) | <0.001* |
|
| ||||
| GCSAdmission | 6 (6) | 7 (7) | 5 (4) | 0.053 |
|
| ||||
| PaO2/FIO2Admission | 247.4±114.1 | 253.6±116.7 | 228.8±105.3 | 0.241 |
|
| ||||
| PaO2/FIO2 at VAP diagnosis | 140.69±45.2 | 148.40±44.9 | 117.73±37.9 | <0.001* |
|
| ||||
| Duration of MV (day), median (IQR) | 7 (4) | 7 (3) | 6.5 (5) | 0.029* |
|
| ||||
| PEEP (>5 cmH2O) | 154 | 113 | 41 | 0.188 |
|
| ||||
| VAP | 1.00 | |||
|
| ||||
| Early (n) | 40 | 30 | 10 | |
| Late (n) | 127 | 95 | 32 | |
|
| ||||
| Empirical antibiotic treatment(n) | 167 | 125 | 42 | 0.449 |
| Appropriate | 82 | 64 | 18 | |
| Inappropriate | 85 | 61 | 24 | |
|
| ||||
| Secondary bacteremia (n) | 17 | 10 | 7 | 0.138 |
|
| ||||
| Use of corticosteroid (n) | 22 | 15 | 7 | 0.610 |
|
| ||||
| Dialysis (n) | 16 | 10 | 6 | 0.236 |
MV – mechanical ventilation; DM – diabetes mellitus; COPD – chronic obstructive pulmonary disease; CAD – coronary artery disease; CHF – congestive heart failure; CRF – chronic renal failure; APACHE – acute physiology and chronic health evaluation; SOFA – sequential organ failure assesment; PEEP – positive end-expiratory pressure; GCS – Glasgow coma score;
p<0.05 accepted as statistically significant.
IQR – interquantile range.
When the Glasgow Coma Score, PEEP values, (in)appropriateness of empirical therapy, development of secondary bacteremia, use of steroid, and dialysis were analyzed for each patient, there was no statistically significant difference between deceased and surviving patients (Table 1).
There was a statistically significant difference between surviving and deceased patients with respect to their APACHE II score, SOFA score, and PaO2/FIO2 ratio on admission to the ICU as well as the duration of mechanical ventilation before diagnosis of VAP (Table 1).
The median time from hospital admission to occurrence of VAP was 9 days (range, 3–22). The median time from intensive care unit admission to occurrence of VAP was 9 days (range, 7–18).
Length of stay (LOS) in the ICU was 50 days (range, 9–272) in the surviving group and 16 days (range, 7–27) in the deceased group. LOS in the hospital was 53 days (range, 9–272) in the surviving group and 17.5 days (range, 7–52) in the deceased group. There was a statistically significant difference between surviving and deceased patients in length of stay in the ICU and in hospital days (p<0.001; p<0.001, respectively).
The distribution of the causative microorganisms on the day of admission to hospital and ICU is shown in Table 2. When early- and late-onset pneumonia causes were compared according to ICU and hospital admittance, resistant bacteria were found to be more common in pneumonias classified as early-onset according to ICU admittance (Table 2). Carbapenem resistance was 61.3% for Acinetobacter baumannii, 43.9% for Pseudomonas aeruginosa, and 50% for Klebsiella pneumonia.
Table 2.
Microorganisms causing early and late onset ventilator-associated pneumonia according to hospital and intensive care unit admission day.
| Total (n=167) | Hospital admission day | ICU admission day | |||
|---|---|---|---|---|---|
| Late (n=152) | Early (n=40) | Late (n=127) | Early (n=15) | ||
| A. baumannii | 77 | 8 | 69 | 18 | 59 |
| P. aeruginosa | 41 | 4 | 37 | 13 | 28 |
| MRSA | 21 | – | 21 | 4 | 17 |
| K. pneumoniae | 12 | 1 | 11 | 2 | 10 |
| MSSA | 5 | 1 | 4 | 1 | 4 |
| Enterobacter spp. | 3 | – | 3 | – | 3 |
| S. maltophilia | 3 | – | 3 | – | 3 |
| Serratia spp. | 2 | – | 2 | – | 2 |
| H. influenzae | 1 | – | 1 | 1 | – |
| E. coli | 1 | 1 | 1 | – | |
| Aspergillus spp. | 1 | – | 1 | – | 1 |
The cutoff point of the SOFA score for mortality was >6 while the cutoff point of APACHE II score was found to be >21(Table 3). Receiver operating characteristic (ROC) curves of APACHE II and SOFA scores was shown in the Figures 1 and 2.
Table 3.
ROC curve for APACHE II and SOFA score for 28th day mortality.
| Cutoff value | Sensivity | Specifity | AUC | p Value | |
|---|---|---|---|---|---|
| SOFA admission | >6 | 76.0% | 58.40% | 0.706 | <0.001* |
| SOFA at VAP diagnosis | >6 | 73.8% | 71.2% | 0.821 | <0.0001* |
| APACHE II admission | ≥21 | 71.43% | 50.40% | 0.624 | <0.016* |
AUC – area under the receiver operating characteristic curve; APACHE – acute physiology and chronic health evaluation; SOFA – sequential organ failure assesment; VAP – ventilator-associated pneumonia.
Figure 1.
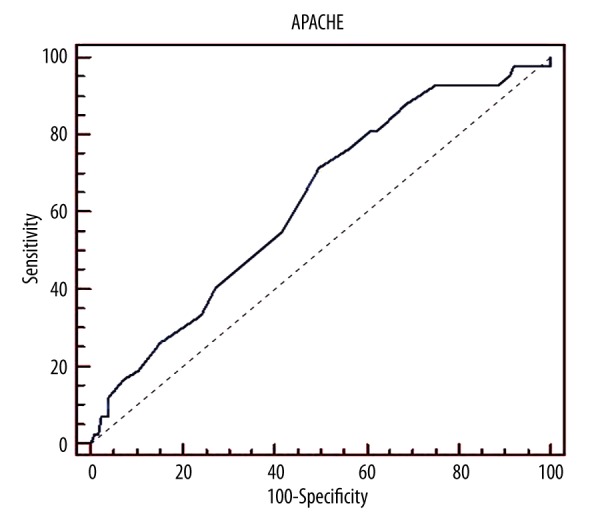
Apache II Score-Roc curves
Figure 2.
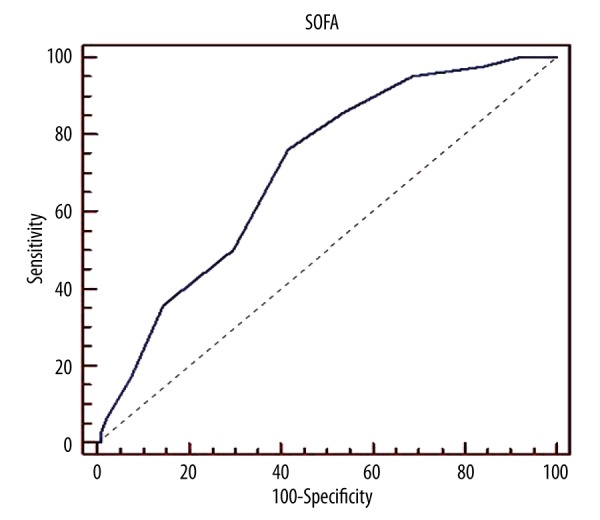
Sofa Score-Roc curves
To determine the risk factors affecting mortality, patients who died and those who survived at 28 days were compared. Univariate analysis showed that APACHE II scores on admission, SOFA scores on admission, and SOFA scores at VAP diagnosis were significant risk factors and were included in the multivariate logistic regression analysis. SOFA scores at VAP diagnosis were independent risk factor for mortality [(p<0.001; OR(95%CI): 1.4(1.2–1.6)], but APACHE II scores on admission [(p=0.661; OR(95%CI): 1.2(0.5–3.3)] and admission SOFA scores [(p=0.301; OR(95%CI): 2.1(0.5–7.6)] were not statistically significant. A 1-unit increase in SOFA score on diagnosis increased risk of 28-day mortality by 1.4 times.
The logistic regression model was significant (p>0.001) and compatible with the data (R2=0.15, p=0.432).
When mortality was analyzed for the 167 study patients, the 14-day mortality was 10.2% (n=17) and the 28-day mortality was 25.1% (n=42).
Discussion
In the present study, patients admitted to the ICU and who developed VAP were retrospectively analyzed. Acinetobacter baumannii, Pseudomonas aeruginosa, and MRSA were the most common causative bacteria. While the APACHE II score on admission was significantly associated with ICU mortality, SOFA score > 6 was an independent risk factor for mortality. On the other hand, inappropriateness of empirical antibiotic therapy, presence of secondary bacteremia, use of steroids, and dialysis were not found to be associated with increased mortality. The 14-day mortality after VAP diagnosis was 10.2% and the 28-day mortality was 25.1%.
When the etiology of VAP was analyzed, Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae, and MSSA were found to be more common in early-onset VAP, whereas Pseudomonas aeruginosa, Acinetobacter baumannii, and MRSA were more frequent in late-onset VAP. Many studies have reported that prolonged hospital stay and earlier use of antimicrobial therapy alters respiratory tract flora [11–13]. An earlier prospective study reported that the variety of microbiological oropharyngeal samples obtained on admission to the ICU varied greatly according to length of hospital stay before ICU admission.
Another study reported that patients staying in the hospital for more than 48 h are more likely to be colonized with Pseudomonas spp and MRSA [14]. In the present study, Acinetobacter. baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and MRSA were the most common microorganisms seen in early-onset VAP. Locations from which the patients came and earlier antibiotic use were analyzed for each patient, and most identified to have stayed in the hospital for longer than 48 h before admission to the ICU and to have received antibiotherapy. Therefore, this condition should be taken into consideration for antibiotic preference when planning treatment for VAP patients. Chastre et al. [6] demonstrated that LOS and prior antibiotic treatment were the major risk factors for resistant bacteria in VAP.
The APACHE II score is frequently used for evaluation of the severity of the medical condition of patients admitted to the ICU and identification of ICU mortality. APACHE II, however, is a scoring system in which age and chronic diseases of the patients are analyzed, together with the acute physiological changes [15]. Scores calculated at the time of diagnosis of VAP were reported to be independent risk factors for mortality [16–19]. It has been reported that there may be a 75% increase in mortality rate in case of an APACHE II score of >15 calculated at the time of diagnosis of VAP [19]. The APACHE II value may change after improvement of the hemodynamical changes occurring at the time of admission to the ICU or on diagnosis of VAP. In this case, the scores are influenced by age and the severity of the underlying disease(s). In the present study, APACHE II score was used to identify the severity of the medical condition at admission to the ICU. Although patients with higher APACHE II scores had higher mortality rates and these were found to be statistically significant according to univariate statistical analysis, the logistic regression analysis performed for APACHE II scores over 21 revealed that it was not an independent risk factor for mortality. APACHE II scoring does not include the effects of mechanical ventilation and the use of vasopressor drugs, so it may be ineligible for identification of organ dysfunction and mortality caused by VAP.
It is known that the presence of infection further contributes to organ disfunction. The SOFA scoring system can distinguish disfunction of different organ systems and has a predictive value for survival. However, high SOFA scores (SOFA score>13) can predict severe organ failure and therefore high mortality. For this reason, SOFA cut-off points and organ dysfunctions may vary in different patient groups. In the present study, the SOFA score at the time of VAP diagnosis was higher in deceased patients. In ROC analysis, AUC was 0.821, similar to the values obtained in earlier studies (AUC ranging from 0.61 to 0.88) [20,21]. Gursel et al. [14] found a sensitivity of 70% and a specificity of 83% for mortality in their study involving patients with a SOFA score of >4. Boeck et al. [21] reported that the mean SOFA score was 9 in deceased VAP patients, and it significantly increased mortality. In the present study, the logistic regression analysis conducted for SOFA score of >6 was determined to be significant in the multivariate analysis [(p<0.001; OR (95%CI): 1.4(1.2–1.6)] (i.e., a 1-unit increase of diagnosis SOFA score increases risk of 28 days mortality by 1.4 times). Hence, calculating SOFA score at the time of diagnosis or taking serial measurements may provide valuable information for prognosis of VAP in the ICU [21].
There are some specific points to be taken into consideration before starting antibiotic therapy in patients diagnosed with VAP, because determining the empirical antibiotherapy is crucial in the treatment. While choosing the antibiotic to be commenced, local flora and previous antibiotic use should be taken into account. Studies have reported that appropriate antibiotherapy can decrease mortality by up to 38% while inappropriate antibiotherapy can cause a 90% increase in mortality [1,22]. Irequi et al. [23] found that despite selection of an appropriate antibiotic, the extended time used for prescribing and administering the medicine caused increased mortality. Porzecanski et al. [24] confirmed that early and accurate diagnosis and use of appropriate antibiotics decrease mortality. Nevertheless, the effect of inappropriate empirical antibiotherapy on mortality was not significant in the present study, possibly because this was a retrospective study, so the time of initial antibiotic therapy, diagnosis, and treatment could not be standardized.
Studies have affirmed the presence of 8–20% bacteremia in every episode of nosocomial pneumonia [25,26]. Agbaht et al. [27] reported the rate of secondary bacteremia development in their subjects was 17.6%. Development of secondary bacteremia is more commonly seen in late-onset VAP and in patients with a history of hospitalization. Mortality is reported to be up to 40.6%, depending on the causative microorganism and use of vasopressors in patients with secondary bacteremia [28]. In the present study, the rate of bacteremia was 10%. It was more common (64%) in late-onset infections, and bacteremia was not identified as a risk factor according to statistical analysis. This may have resulted from the diversity of the causative microorganisms, antibiotic susceptibility, and differences in underlying diseases.
We have estimated the 14-day mortality to be 10.2% and 28-day mortality to be 25.1% in VAP. Studies in the literature have generally examined the 28-day mortality, and their results agree with the findings of this study. We found that 14-day mortality was lower than 28-day mortality. Various factors may cause higher 28-day mortality, such as non-infectious causes, relapse of infection, and onset of a new attack or sepsis [29,30]. In addition, we think that high carbapenem resistance rates of bacterial flora of our ICU may have contributed to the higher mortality.
There were some limitations for our study: 1) Results may not be valid for all centers because the study was done in a single center and there may be local differences between institutions. 2) This was a retrospective study; therefore, a prospectively designed study may increase the strength of our findings. 3) This study included a heterogeneous population with different causes leading to ICU admission. Conducting studies on more specific groups may contribute to determining other predictive factors. Although antibiotherapy cannot be specified according to susceptibility of the isolated microorganism strain, it can make a significant contribution to forming local guidelines.
Conclusions
Although our study is limited due to its retrospective nature and the fact that VAP is very common, the results show that the causative microorganisms of VAP vary in patients according to length of hospital stay. Acinetobacter baumannii, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA) were the most common causative microorganisms for VAP. Information on the causative organisms of VAP in our hospital and their antibiotic susceptibility profiles, with the regular monitoring of resistance patterns, is important to make more effective antibiotic choices in initial empirical therapy.
Resistant bacteria may be present in early-onset VAP. Especially, taking LOS into consideration may improve estimates of presence of resistant bacteria. Every hospital should monitor its own microorganism flora and rates of resistance to antibiotics. The SOFA score might be more valuable than APACHE II score. Surveilling SOFA scores frequently improves predictive performance over time. Rigid application of infection control precautions and limitation of carbapenem use due to high resistance to carbapenem can prevent infections with resistant bacteria.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Craven DE, Kunches LM, Kilinsky V, et al. Risk factors for pneumonia and fatality in patients receiving continuous mechanical ventilation. Am Rev Respir Dis. 1986;133:792–96. [PubMed] [Google Scholar]
- 3.Fagon JY, Chastre J, Domart Y, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989;139:877–84. doi: 10.1164/ajrccm/139.4.877. [DOI] [PubMed] [Google Scholar]
- 4.Ozvatan T, Akalin H, Sinirtas M, et al. Nosocomial Acinetobacter pneumonia: Treatment and prognostic factors in 356 cases. Respirology. 2016;21(2):363–69. doi: 10.1111/resp.12698. [DOI] [PubMed] [Google Scholar]
- 5.Kwa AL, Low JG, Lee E, et al. Low the impact of multidrug resistance on the outcomes of critically ill patients with gram-negative bacterial pneumonia. Diagn Microbiol Infect Dis. 2007;58:99–104. doi: 10.1016/j.diagmicrobio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 7.Morehead RS, Pinto SJ. Ventilator-associated pneumonia. Arch Intern Med. 2000;160:1926–36. doi: 10.1001/archinte.160.13.1926. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH, Von Harz B, Prentice D, et al. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest. 1997;112:765–73. doi: 10.1378/chest.112.3.765. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Ellis P, Arabi Y, et al. Cooperative antimicrobial therapy of septic shock Database Research Group. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 10.Garnacho-Montero J, Gutierrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40:32–40. doi: 10.1007/s00134-013-3077-7. [DOI] [PubMed] [Google Scholar]
- 11.Drakulovic MB, Bauer TT, Torres A, et al. Initial bacterial colonization in patients admitted to a respiratory intensive care unit: Bacteriological pattern and risk factors. 2001;68:58–66. doi: 10.1159/000050464. [DOI] [PubMed] [Google Scholar]
- 12.De Latorre FJ, Pont T, Ferrer A, et al. Pattern of tracheal colonization during mechanical ventilation. Am J Respir Crit Care Med. 1995;152:1028–33. doi: 10.1164/ajrccm.152.3.7663779. [DOI] [PubMed] [Google Scholar]
- 13.Sirvent JM, Torres A, Vidaur L, et al. Tracheal colonisation within 24 h of intubation in patients with head trauma: Risk factor for developing early-onset ventilator-associated pneumonia. Intensive Care Med. 2000;26:1369–72. doi: 10.1007/s001340000611. [DOI] [PubMed] [Google Scholar]
- 14.Olsen B, Weinstein RA, Nathan C, et al. Epidemiology of endemic Pseudomonas aeruginosa: Why infection control efforts have failed. J Infect Dis. 1984;150:808–16. doi: 10.1093/infdis/150.6.808. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 16.Gursel G, Demirtas S. Value of APACHE II, SOFA and CPIS scores in predicting Prognosis in patients with ventilator-associated pneumonia. Respiration. 2006;73:503–8. doi: 10.1159/000088708. [DOI] [PubMed] [Google Scholar]
- 17.Gursel G, Aydogdu M, Ozyilmaz E, Ozis TN. Risk factors for treatment failure in patients with ventilator-associated pneumonia receiving appropriate antibiotic therapy. J Crit Care. 2008;23:34–40. doi: 10.1016/j.jcrc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Erbay RH, Yalcin AN, Zencir M, et al. Costs and risk factors for ventilator-associated pneumonia in a Turkish university hospital’s intensive care unit: A case-control study. BMC Pulm Med. 2004;4:3. doi: 10.1186/1471-2466-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meric M, Willke A, Caglayan C, Toker K. Intensive care unit-acquired infections: Incidence, risk factors and associated mortality in a Turkish university hospital. Jpn J Infect Dis. 2005;58:297–302. [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Boeck L, Eggimann P, Smyrnios N, et al. The Sequential Organ Failure Assessment score and copeptin for predicting survival in ventilator-associated pneumonia. J Crit Care. 2012;27(5):521e1–9. doi: 10.1016/j.jcrc.2011.07.081. [DOI] [PubMed] [Google Scholar]
- 22.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: A risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 23.Iregui M, Ward S, Sherman G, et al. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–68. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 24.Porzecanski I, Bowton DL. Diagnosis and treatment of ventilator-associated pneumonia. Chest. 2006;130:597–604. doi: 10.1378/chest.130.2.597. [DOI] [PubMed] [Google Scholar]
- 25.Visnegarwala F, Iyer NG, Hamill RJ. Ventilator-associated pneumonia. Int J Antimicrob Agents. 1998;10(3):191–205. doi: 10.1016/s0924-8579(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 26.Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312–17. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 27.Agbaht K, Diaz E, Muñoz E, et al. Bacteremia in patients with ventilator-associated pneumonia is associated with increased mortality: A study comparing bacteremic vs. nonbacteremic ventilator-associated pneumonia. Crit Care Med. 2007;35:2064–70. doi: 10.1097/01.CCM.0000277042.31524.66. [DOI] [PubMed] [Google Scholar]
- 28.Siempos II, Vardakas KZ, Kyriakopoulos CE, et al. Predictors of mortality in adult patients with ventilator-associated pneumonia: A meta-analysis. Shock. 2010;33:590–601. doi: 10.1097/SHK.0b013e3181cc0418. [DOI] [PubMed] [Google Scholar]
- 29.Vidaur L, Gualis B, Rodriguez A, et al. Clinical resolution in patients with suspicion of ventilator-associated pneumonia: A cohort study comparing patients with and without acute respiratory distress syndrome. Crit Care Med. 2005;33:1248–53. doi: 10.1097/01.ccm.0000165811.61232.d6. [DOI] [PubMed] [Google Scholar]
- 30.Chastre J, Wolff M, Fagon JY, et al. PneumA Trial Group. Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA. 2003;290:2588–98. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]


